1. Introduction
Rheumatoid arthritis (RA) is characterized by synovial inflammation and hyperplasia, autoantibody production, cartilage and bone destruction, and systemic features including cardiovascular, pulmonary, psychological, and skeletal disorders [Citation1]. Currently, small-molecule disease-modifying anti-rheumatic drugs still serve as the first-line therapy. Besides, advances in understanding the pathogenesis of RA reveal more promising biological targets such as antibodies, cytokines, growth factors, and intracellular signaling molecules [Citation2]. For instance, vobarilizumab, a nanobody consisting of an anti-IL-6R domain and an anti-human serum albumin domain, represented an exhilarating effect on RA in a phase 2b study [Citation3]. However, side effects, long-term administrations, unstable during the circulation hampered their clinical applications.
Nanomedicines, the combination of nanotechnology and medicine, take the advantages of nanotechnology to passively or actively deliver the drugs to the targeted tissues, cells, or subcellular domains. In passive targeting, abnormal vasculature and impaired lymphatic drainage occurred in both solid tumors and inflammatory diseases facilitate the penetration and accumulation of nano-sized materials within the neoplastic and inflammatory microenvironments. However, this so-called enhanced permeation and retention effect is restricted. Several factors associated with biology of tumor or inflammation (e.g. different type of tumors or inflammatory diseases, the degree of vascularization and angiogenesis, and the high interstitial fluid pressure) and also the properties of nanocarriers (e.g. size, shape, and surface charge) may influence the actual efficiency [Citation4]. In active targeting, on the other hand, the modification with appropriate ligands on the surface of nanocarriers leads to an efficient and specific binding with targeted cells. In this editorial, we will highlight the strategies and applications of active targeting nanomedicines for RA treatment.
2. How to design an active targeting nanomedicine for RA treatment
In general, active targeting nanomedicines need to overcome at least three barriers to achieve the optimal effects on abrogating RA (). Once nanomedicines were given intravenously, for instance, the first barrier they came across was the reticuloendothelial system. Short circulation times caused by opsonization decreased the efficiency of the delivery of nanomedicines to the inflamed sites. The second limiting factor was the inflamed vascular endothelium. Under inflammatory stimuli, endothelial cells were activated to release matrix-degrading enzymes to digest the basal membrane, then emigrated and divided to form primitive angiotubes, which eventually developed into angiogenesis [Citation5]. The circulating nanomedicine could either target to this abnormal vasculature to eliminate the angiogenesis or further transfer across the endothelium to enter the joint cavity and relieve other rheumatic symptoms such as inflammation. Thirdly, the ligands attached to the nanocarriers should be specific, i.e. the corresponding receptors were only expressed on the targeted cells for maximum decrease of side effects.
Figure 1. Schematic illustration of active targeting nanomedicine for RA treatment. (a) therapeutic drugs loaded-nanocarriers were modified with PEG and active ligands to form the active targeting nanomedicine; (b) PEGlyation prolonged the blood circulation time of nanomedicine; (c) modification with specific ligands facilitated the active delivery of nanomedicine to inflamed tissues and cells.
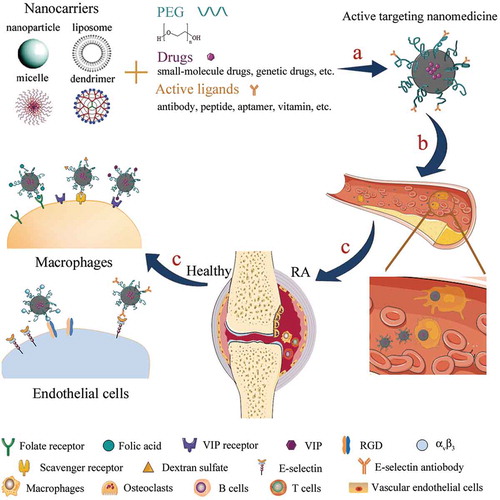
Taken together, an ideal active targeting nanomedicine for RA treatment need to meet the following requirements: (a) with a suitable size (ranging from 10 to 100 nm) and surface charge (neutral or anionic); (b) PEGylation to prolong the circulation time; (c) modification with proper ligands. Specifically, nanomedicines with a suitable size could avoid both the filtration by the kidneys (larger than 10 nm) and the capture by the liver (smaller than 100 nm) [Citation4]. Moreover, increased angiogenesis induced the inflamed endothelial cell layers became discontinuous and hyperpermeable, which enable nanocarriers with a size around 100 nm to, at least partially, permeate the inflamed endothelium by the passive way.
To date, nanocarriers modified with poly(ethylene glycol), i.e. PEGylation is still the most widely used method to efficiently prevent opsonization. Leaf Huang [Citation6] demonstrated that an ideal PEGylated nanocarrier should be modified with >8 mol% of PEG to form the brush conformation, which could provide complete coverage of the surfaces of nanocarriers. In reality, however, it is difficult to prepare stable, PEGylated nanocarriers with a brush conformation while maintaining the integrity of lipid membrane. Moreover, a high density of PEG may hinder the binding and uptake of nanocarriers by targeted cells with a contradictory effect. Apart from the density, different chain length or structures of PEG, as well as the coating strategies, e.g. covalent or physical adsorption, could also influence the PEGylation effects.
To choose ligands that specifically and efficiently target to RA need to combine with the pathology of disease. During the progress of RA, angiogenesis and inflammation are the two most distinguished characteristics. Numerous relevant mediators like growth factors, pro-inflammatory cytokines, chemokines, cell adhesion molecules, and proteases have participated in the development of angiogenesis. Among these, the hypoxia-vascular endothelial growth factors (VEGF)-angiopoietin system plays a central role [Citation5]. In addition, several adhesion molecules including αvβ3 integrins, E-selectin, vascular cell adhesion molecule-1 (VCAM-1), and intercellular cell adhesion molecule-1 are over-expressed on the surface of endothelial cells [Citation5,Citation7–Citation10]. Macrophages, abundant in the inflamed synovial membrane, possess broad pro-inflammatory potential and contribute considerably to inflammation and joint destruction. Due to their successful response to anti-rheumatic therapy, selective delivery of nanomedicine to active macrophages by binding to a specific receptor could switch off their complex connections with other cells to ameliorate the RA condition while maintaining the biological functions of the resting macrophages. Several receptors such as CD44, CD64, folate receptor-beta (FR-β), vasoactive intestinal peptide (VIP) receptor, scavenger receptor class A, toll-like receptors, transforming growth factor-beta receptors, etc. were over-expressed on activated macrophages [Citation1,Citation11–Citation16].
3. Applications of active targeting nanomedicines
During the last two decades, we have witnessed an explosive development of nanotechnology. Somehow limited efforts have been put on RA treatment using nanotechnology, especially active targeting methods. As seen in , we summarized the representative examples and further discussed in detail.
Table 1. Summary of active targeting nanomedicines for RA treatment.
Kyung-Hwa Yoo and co-workers [Citation8] prepared Methotrexate (MTX)-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles and deposited 15 nm Au film onto the nanoparticles monolayer resulting in a half-shell structure MTX-PLGA-Au and then cyclic Arginine-glycine-aspartate (RGD) peptide was conjugated on the Au surface as the active target. In a following study, the same group prepared a similar nanoparticle by replacing the Au film with Au/Fe/Au complex film onto nanoparticle monolayer [Citation9]. Collagen induced arthritis (CIA) mice were treated with these nanoparticles and the mass of Au in the inflamed joints was measured to quantify the accumulation of nanoparticles. Compared to non-RGD modified nanoparticle, larger amount of Au was detected in the inflamed joints of RGD modified group mainly due to the binding of RGD peptide to αvβ3 receptors. Since activated macrophages over-express scavenger receptors and dextran sulfate (DS) is a representative ligand for scavenger receptor class A, a self-assembled nanoparticles was developed basing on DS as a hydrophilic block and polycaprolactone (PCL) as a hydrophobic block [Citation11]. The following in vivo biodistribution study confirmed that DS-b-PCL Nanoparticles (NP) could selectively accumulate into the inflamed synovial of CIA mice. Of note, this article only provided a carrier and no therapeutic drug was encapsulated. Another example focused on the Fcγ receptors by utilizing anti-CD64-conjugated poly (lactic-co-glycolic acid) NPs to co-capsulate superparamagnetic iron oxide nanoparticles (SPIONs) and MTX [Citation12]. This multifunctional nanosystem contained one contrast agent for MRI detection (SPIONs), one therapeutic drug (MTX), and one active target (anti-CD64 antibody). Although this nanosystem was well characterized in vitro, yet the authors failed to provide in vivo results. Moreover, etoricoxib-loaded albumin nanoparticles conjugated with folic acid were successfully formulated to specifically target to activated macrophages [Citation13].
Koo et al. [Citation14] formulated sterically stabilized micelles (SSM) as nanocarries for camptothecin (CPT) and then surface-modified with vasoactive intestinal peptide for active targeting. The CPT-SSM with a small particle size (~13 nm) could selectively extravasate through leaky microcirculation of inflamed arthritis joint but not intact microvascular walls of healthy tissues, such as kidney and born marrow. Furthermore, the covalent conjugation of CPT-SSM with VIP facilitated the binding to activated macrophages over-expressed VIP receptors (mostly VPAG2 receptor) in arthritic joint. CIA mice were subcutaneously injected with a single dose of CPT-SSM-VIP composed with 0.1 mg/kg CPT and 0.05 nmol VIP. The results revealed complete abrogation of CIA-characteristic symptoms and significant reduction in macrophages.
Dendrimers, also called arborols or cascade molecules are well defined, regularly branched macromolecules with multifunctional surface to tailor-make their surface chemistry. Thomas et al. [Citation15] synthesized a folic acid- and methotrexate-conjugated poly(amidoamine) dendrimer (generation 5) to specifically target activated macrophages. Therapeutic effects were revealed by using CIA mice. However, in this study, MTX was conjugated to amino groups of dendrimers and exposed to the blood circulating system before delivering to the inflammatory sites, which could reduce the therapeutic efficiency. Likewise, the dendrimers exhibited a significant decrease of body weight of CIA mice probably due to their high cationic charge density.
Apart from small molecular drugs, selective delivery of gene drugs to the inflamed sites could be an alternative therapy. SAINT-liposomes based on cationic amphiphile SAINT-C18 (1-methyl-4-(cis-9-dioleyl)methyl-pyridinium-chloride) was prepared for encapsulating VE-cadherin specific siRNA [Citation7]. To achieve the specific targeting to inflamed endothelium, SAINT-liposomes were harnessed with antibodies against the VCAM-1 or E-selectin, respectively. Cell uptake and intracellular behaviors were subsequently investigated and all these nanomedicines could effectively down-regulate target genes in active Human umbilical vein endothelial cells (HUVEC) and Human aortic endothelial cells (HAEC) cells. Still, in vivo studies were absent. In another work, Nogueira et al. [Citation16] synthesized SP-DS3 peptide by conjugating surfactant protein D to folic acid and then prepared the SP-DS3 peptide modified liposome to target to FR-β receptor expressed on activated macrophages. Expectably, specific uptakes of liposomes were observed both in activated human primary macrophages (in vitro) and in inflamed ankles of CIA mice (in vivo).
Currently, gold nanoparticles (AuNPs) were exploited as therapeutic treatment for rheumatic diseases attributed to their anti-inflammatory and antiangiogenic actions [Citation17]. Inspired by this property, Lee et al. [Citation10] prepared hyaluronate-AuNPs/Tocilizumab (HA-AuNP/tocilizumab (TCZ)) complex with dual targeting effects. AuNP could actively bind to VEGF and TCZ, the first therapeutic antibody against IL-6 signaling, could target to IL-6R. The therapeutic effect of this complex was further confirmed by using CIA model mice.
4. Expert opinion
As outlined earlier, the development of active targeting nanomedicine for RA is still in its infancy with a number of unsolved issues: (i) the coexistence of PEG and ligands complicate the optimization of formulation. Nanocarriers with high density of PEG may gain a prolonged circulating time. However, increased PEG density also interferes with particle-cell interactions and targeted delivery. On the other hand, the active ligands are generally coated onto the surface of nanocarriers and preferably linked to the distal end of PEG chains, which could diminish the stealth efficiency of PEGylation [Citation4]. Accordingly, different parameters (e.g. density of PEG, choice of ligands, method of coupling, etc.) need to be considered to strike a balance between PEGylation and active targetability; (ii) theoretically, abnormal tissues or cells under RA condition (e.g. B cells and T lymphocytes) are potential targeted sites. Yet, to our best knowledge, all the reported works only focused on how to target to endothelial cells and macrophages. In addition, secondary osteoporosis is a well-known co-morbidity in RA and bone loss is a frequent and clinically serious event. Interference with osteoclasts using active targeting nanomedicine could thus be an important tool to combine the inhibition of inflammation and structural protection of joints during RA; (iii) last but not least, the biocompatibility and toxicity of nanocarriers should always attract our concerns. For example, adverse effects were observed in AuNPs associated with shape, size, coating, and dose range [Citation17]. Additionally, dendrimers such as polypropyleneimine (PPI), polyamidoamine (PAMAM) and poly-L-lysine (PLL) exert toxicity due to their high cationic charge density and multiple terminal amine groups. Although some of evidence regarding AuNPs and dendrimer safety is still conflicting, toxicity studies both in vitro and in vivo are necessary.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Nogueira E, Gomes AC, Preto A, et al. Folate-targeted nanoparticles for rheumatoid arthritis therapy. Nanomedicine. 2016;12(4):1113–1126.
- Yang M, Feng XR, Ding JX, et al. Nanotherapeutics relieve rheumatoid arthritis. J Control Release. 2017;252:108–124.10.1016/j.jconrel.2017.02.032.
- Dörner T, Weinblatt M, Durez P, et al. OP0098 Remission and maintenance of efficacy in a phase 2b study of vobarilizumab, an anti-interleukin 6 receptor nanobody, in patients with moderate-to-severe rheumatoid arthritis despite treatment with methotrexate. Ann Rheum Dis. 2017;76:92.
- Danhier F, Feron O, Préat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148(2):135–146. DOI:10.1016/j.jconrel.2010.08.027.
- Elshabrawy HA, Chen Z, Volin MV, et al. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis. 2015;18(4):433–448.
- Li SD, Huang L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J Control Release. 2010;145(3):178–181. DOI:10.1016/j.jconrel.2010.03.016.
- Kowalski PS, Lintermans LL, Morselt HW, et al. Anti-VCAM-1 and anti-E-selectin SAINT-O-Somes for selective delivery of siRNA into inflammation-activated primary endothelial cells. Mol Pharm. 2013;10(8):3033–3044.
- Lee SM, Kim HJ, Ha YJ, et al. Targeted chemo-photothermal treatments of rheumatoid arthritis using gold half-shell multifunctional nanoparticles. ACS Nano. 2013;7(1):50–57.
- Kim HJ, Lee SM, Park KH, et al. Drug-loaded gold/iron/gold plasmonic nanoparticles for magnetic targeted chemo-photothermal treatment of rheumatoid arthritis. Biomaterials. 2015 Aug;61:95–102. DOI:10.1016/j.biomaterials.2015.05.018
- Lee H, Lee MY, Bhang SH, et al. Hyaluronate-gold nanoparticle/tocilizumab complex for the treatment of rheumatoid arthritis. ACS Nano. 2014;8(5):4790–4798.
- Kim SH, Kim JH, You DG, et al. Self-assembled dextran sulphate nanoparticles for targeting rheumatoid arthritis. Chem Commun. 2013;49(88):10349–10351.
- Moura CC, Segundo MA, Neves JD, et al. Co-association of methotrexate and SPIONs into anti-CD64 antibody-conjugated PLGA nanoparticles for theranostic application. Int J Nanomedicine. 2014;9:4911–4922.
- Bilthariya U, Jain N, Rajoriya V, et al. Folate-conjugated albumin nanoparticles for rheumatoid arthritis-targeted delivery of etoricoxib. Drug Dev Ind Pharm. 2015;41(1):95–104.
- Koo OMY, Rubinstein I, Önyüksel H. Actively targeted low-dose camptothecin as a safe, long-acting, disease-modifying nanomedicine for rheumatoid arthritis. Pharm Res. 2011;28(4):776–787.
- Thomas TP, Goonewardena SN, Majoros IJ, et al. Folate-targeted nanoparticles show efficacy in the treatment of inflammatory arthritis. Arthritis Rheum. 2011;63(9):2671–2680.
- Nogueira E, Lager F, Le Roux D, et al. Enhancing methotrexate tolerance with folate tagger liposomes in arthritis mice. J Biomed Nanotechnol. 2015;11(12):2243–2252.
- Carneiro MFH, Barbosa JF Jr. Gold nanoparticles: A critical review of therapeutic applications and toxicological aspects. J Toxicol Environ Health B Crit Rew. 2016;19(3–4):129–148. DOI:10.1080/10937404.2016.1158762.
