ABSTRACT
Objective: The main objective of this user experience testing study was to evaluate the impact of human factors on the use of a disposable pen containing follitropin alfa by patients and nurses with special focus on the convenience, safety and ease of use, in different types of stimulation protocols.
Methods: Infertile women trying to conceive, and specialist nurses were recruited across 6 European countries. In total 18 patients and 19 nurses took part in the testing, which included both nurse-patient pairings and in-depth interviews. A standardized list of expected and pre-defined critical steps according to the Instructions for Use (IFU), was used to assess the correct handling of the pen.
Results: During the user experience testing, no critical errors, related to the use of the pen, which could affect the success of the injection process were identified. In general, both nurses and patients found the pen very easy to learn, use and would be confident using the pen for self-injection. Nurses also found the pen very easy to train the patients.
Conclusions: The study provides valuable information on the pen from both patient and nurse perspectives in different simulated scenarios reflecting standard practice.
1. Introduction
During controlled ovarian stimulation (COS) for assisted reproductive technologies (ART) the use of several different medications and dosing regimens are frequently utilized [Citation1]. Fertility specialists are constantly in search of the ideal ovarian stimulation protocol, which will produce the ‘optimal’ number and quality of oocytes, while eliminating any increased risk of overstimulation resulting in ovarian hyperstimulation, for different types of patients [Citation2,Citation3]. Markers of ovarian reserve (anti-Müllerian hormone, AMH [Citation4]; antral follicle count, AFC [Citation5]), and hence indicators of the patients’ ability to respond to COS are routinely measured in order to assist the treating clinician in selecting the most appropriate follicle stimulating hormone (FSH) starting dose. Thus, based on ovarian reserve measurements, each subgroup of patients, e.g. normal, poor, or hyper-responders, will be treated using a protocol tailored to her own needs [Citation6].
The medications involved include gonadotropin treatment in the form of FSH which is self-injected generally over a period of up to 14 days (median of 11 Citation7, Citation8). FSH is available in many different forms and administered through different systems, such as vials and syringes, disposable prefilled pens and reusable pens with and without cartridges [Citation9–Citation11]. These different delivery systems can often be confusing for patients, resulting in unnecessary stress and leading to the possibility of medication errors during treatment. This may lead in the worst case a failed cycle [Citation7,Citation9] or for the patient to continue to be anxious during the rest of the treatment period [Citation12]. In a French [Citation13] as well as a multinational study [Citation12], over half the patients interviewed reported that ovarian stimulation had an impact on their day to day life style, in particular regarding whether the correct daily dose has been administered correctly [Citation9,Citation12–Citation14].
Many couples discontinue ART without achieving their goal of a life birth for many reasons other than a poor response or the cost of treatment [Citation15]. One of the most common reasons for discontinuation is the overall burden of disease, and this can be attributed to factors related to the patient, clinic or the actual treatment [Citation16,Citation17]. Therefore, any potential solutions to improve treatment for ART patients should be investigated.
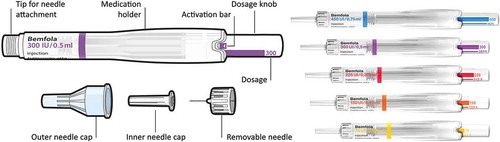
Follitropin alfa (α) has been available for subcutaneous self-injection since its commercialization in 1996. The first preparations however were only available in lyophilized vials. Subsequently, there was the introduction of pen devices, with the first two pen injectors being approved in the US in 2004 and 2007, respectively [Citation18]. The improvements in injection systems have generally increased the percentage of patients performing their own injections [Citation10,Citation19,Citation20]. The most recent follitropin alfa pen available on the market, approved by the EMA on 28 March 2014, is the Bemfola pen [Citation21] (Gedeon Richter, Budapest, Hungary) injector which was awarded the ‘Red Dot’ Design award in 2011 for high design quality.
Bemfola (follitropin alfa) is produced using recombinant DNA technology and is a formulation of the naturally occurring hormone FSH, which plays a key role in human reproduction. The product is the result of a targeted drug development process aimed to replicate as closely as possible the reference product, Gonal-f® (Merck, Germany). The brief to the development engineers when designing the injector pen was to produce a novel, state-of-the-art delivery system which minimized the number of steps a patient needed to take when preparing the injection and to ensure that the patient and physician had maximum control and the least chance of a patient error. The result is therefore a simple, single-use, once-a-day disposable device, which allows the patient to self-inject, subcutaneously. The pen has a clearly legible selected dose, as well as a click signal after successful completion of the injection, thus avoiding dosing errors, which in turn may improve therapy compliance. Given that non-compliance to treatment represents one of the most critical obstacles in a patient’s fertility journey, the availability of easy-to-use, simple injector systems, should increase the adherence to the prescribed dosage. This in turn should lead to higher compliance and ultimately success rates among patients. Studies comparing this pen with other established fertility treatment devices have shown a high level of patient preference (over 55%) and demonstrated significantly higher ratings for example, inconspicuousness, ease of use and dose adjustment for the Bemfola pen compared to other marketed pens [Citation22,Citation23]
The main objective of this user experience testing study was to evaluate the impact of human factors on the use of this disposable pen by patients and nurses with special focus on the convenience, safety and ease of use in particular for dose adaptations in multiple and dynamic stimulation protocols i.e. those protocols requiring both increases and decreases in dose within the same cycle.
Additional objectives were to evaluate the pen regarding, ease of learning, ease of use, ease of teaching and overall usability for the nurses, the risk of errors in administration, the understanding of the patient leaflet and other supportive documentation (colored patient leaflet and instruction video) and finally to assess the adequacy of the different pen strengths and doses delivered for the utilization on diverse possible scenarios as they occur in standard clinical practice.
2. Methods
2.1. Participants
During the routine clinical use of the follitropin alfa pen there are two main classes of users, both of which were included in this user experience testing. The first set of users is the fertility patients who are undergoing COS and who will use the pen to induce multiple follicular stimulation. The second group is made up of specialized fertility nurses who train and supervise the use of the pen by the patients.
Women with typical characteristics of fertility patients and having a range of ages, educational backgrounds and personal situations where recruited via the use of ART clinics and nurses. All patients recruited had to be a minimum of 18 years of age and having already a consultation by a health care professional who had prescribed the need for ART. At the time of testing, they were completely naïve to all pen systems containing gonadotropins for fertility treatment and be a native speaker or have a good level of understanding of the local language. All nurses recruited into the testing had to be experienced fertility nurses (be a maximum of 60 years of age and be a native speaker or have a good level of the local language), who trained patients on a daily basis in the use of the various medications available for the ART process. Additionally, they had to have a minimum of 2 years’ experience working in the fertility environment, be employed by a site which carried out at minimum 300 treatment cycles per year, undertook a minimum of 10 training sessions per month on fertility devices.
Participants provided written, informed consent. All patient simulations were video recorded, with the patients’ permission.
Evaluations were carried out in a simulated, non-interventional manner (i.e. no actual patient injections were performed), and thus did not require ethics committee approvals in any of the countries who participated. All testing, however, complied with all relevant codes of experimentation and legislation on human subjects and all patients freely participated in the testing.
Participants were recruited in cities from six European countries (Berlin, Amsterdam, Madrid, Stockholm, Copenhagen, and London). In each country two nurse–patient pairs were carried out, along with in-depth interviews (IDIs) with one nurse and one patient. In total 18 patients and 19 nurses took part in the testing (). The sample size for these evaluations was based on Health Authority requirements, which stipulate that a minimum of 15 individuals from each distinct user group (patients and nurses) should participate in a usability test [Citation24–Citation26]. It was decided that rather than recruit all patients and nurses from the same center/country, a spread across European countries would give a better overview of real-world user experience with the pen. Given that standard practices are very similar within countries, it was felt this would also allow any potential differences between countries to be identified, even with the small sample size.
Table 1. Nurse/patient split by country & type of test.
The interviews were all carried out by experienced and appropriately trained research professionals (research interviewer) at each of the country locations. All testing was carried out in the local language, and all questionnaires were completed on-site by the interviewers.
2.2. Materials
The materials used for the testing were the Bemfola pen, in several dose presentations, which was provided by Finox AG, Switzerland (which was fully acquired by Gedeon Richter in 2016 to become a member of the Gedeon Richter group). The pen administers a preset dose of follitropin alfa and is available in five dosage strengths (75 IU/0.125 ml, 150 IU/0.25 ml, 225 IU/0.375 ml, 300 IU/0.50 ml, and 450 IU/0.75 ml), which allows a fine-tuning dosing adjustment in 12.5 IU increments. Each dosage is differentiated by a different coloring on the dosage knob of the pen, making them easily identifiable for the user () [Citation21]. Each pen was provided as part of a training kit labeled as ‘demonstration pen, not for human injection’ but otherwise identical to the commercial version, along with needles for injection (Ypsomed Clickfine Type: 0.33 mm × 12 mm (29G × 1/2ʺ), an injection sponge; patient leaflet as per the standard packing; colored patient leaflet; patient instruction video; and a sharps container.
2.3. Testing methodology – usability testing
During routine clinical practice, most patients are trained by a nurse in the fertility center regarding how to handle the pen before they use it independently at home. The patient will then undertake the process at home. However, in some circumstances the pen may be used by the patient without any prior formal training. Therefore, to simulate these different types of scenarios, two types of testing were carried out.
For each type of testing, a standardized list of expected steps, according to the patient leaflet, was created, along with a subset of predefined critical steps, to assess the correct handling of the pen. The steps classified as major/critical for the correct use of the pen were as follows:
attaching the needle until it was fixed securely, to ensure that the dosage knob was pushed until it stopped in order to perform the ‘priming’ of the pen, and that a small amount of liquid had been expelled
ensuring that the dosage knob was turned until the desired dose was set in the display window and that at this point the dosage knob was not pushed inwards
during the injection process ensuring that the dosage knob was fully pushed in
finally, that the needle was left for approximately 5 s after the injection process before withdrawing from the skin.
The interviewer observed and recorded each step of the injection process, according to the patient leaflet, with particular focus on areas of usage and dosage errors, as well as critical errors and potential actions which could have resulted in misuse.
2.3.1. Paired testing
The first set of tests carried out was the ‘paired tests’ where a preinterview was firstly conducted with the nurse by the research interviewer to explain the handling of the pen and to allow a simulated 225 IU injection with the pen to be undertaken. Following this, and once the nurse was comfortable with the handling of the pen, the patient was subsequently trained by the nurse with the pen using a 225 IU dose pen. The objective of this session was to create a situation as close as possible to a ‘real’ training situation. During this process, the research interviewer played the role of a non-participant/non-interventional observer in order not to influence the interaction between the nurse and the patient.
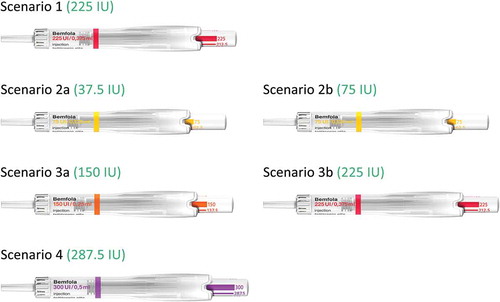
Once the patient had completed the initial simulation with the 225 IU dose, the injection process was simulated using a series of potential scenarios a patient might face during her treatment (). Each of these scenarios was created to assess the ability of the patient to adjust to different potential regimens she may receive during her treatment journey. The different scenarios were, firstly a standard 225 IU dose (red pen), during which the patient had to use and understand this was equivalent to a one pen, one dose, daily regimen, as is often used during ART stimulation. The second scenario undertaken was a regimen equivalent to that used if the patient were undertaking an ovulation induction (OI) type of stimulation [Citation27]. During this scenario, firstly an injection with a dose of 37.5 IU from a 75 IU pen (yellow pen) was simulated, followed by a simulation of a dose of 75 IU (yellow pen). This scenario was to show the patient the possibility of using the same pen, but with two different dosing regimens, and to show that with the 37.5 IU dosing, even if some liquid remained in the pen it should not be reused but the pen must be disposed of and a new pen used for the next dosing. The third scenario undertaken was a regimen equivalent to that often used during an ART stimulation cycle when the patient may need to increase the dose after several days due to a lower than expected response to the stimulation. During this scenario, an injection with a dose of 150 IU (orange pen) was simulated, followed by a simulation of a dose of 225 IU (red pen). This scenario was to show the patient the possibility of using two different pens but administering the maximum dose with each pen. The final scenario undertaken was a simulation of 287.5 IU using the 300 IU pen (purple pen). This is a dose not commonly used during ART but was undertaken to show the patient that even with unusual dosing regimens, the pen is easy to use and easy to adjust the dosing (). During each simulation by both nurse and patient, the research interviewer recorded the handling of each step of the injection process, to assess whether the step had been carried out correctly or not. Each paired interview lasted for approximately 90 min.
2.3.2. In-depth interviews
The second set of testings carried out was the IDIs. This type of testing was carried out to evaluate the individual understanding of the pen via the patient leaflet, with no formal training, in patients not involved in the paired testing. The patient leaflet is a document which is included as standard in the drug packaging and provides step-by-step instructions for the proper use of the pen. In order to simulate a ‘real-life’ situation the patients and the nurses had to use just the patient leaflet in an unassisted manner to carry out simulations of the injection process. In the case of the nurses this was one injection of 225 IU and for the patients they undertook the same scenarios as those undertaken during the paired tests (). Each IDI lasted for approximately 60 min.
During the IDI’s, in addition to the simulation of the injection process, patients were asked to identify sections in the patient leaflet which for them were unclear or would cause them confusion if they were at home alone carrying out the injection for the first time. They were then shown a colored version of the patient leaflet and finally a video version of the instructions (both materials created by the manufacturer) to assess if either of these would assist them with any unclear issues related to using the pen in a correct way.
2.4. Testing methodology – handling tests
The handling tests were all non-interventional and standardized across the different countries. These tests were carried out to assess both the patients and the nurses view of the ease-of-use of the pen, ease of learning, their confidence in using the pen correctly and from the perspective of the nurses the ease of teaching the use of the pen to the patients.
2.4.1. Paired testing
After completion of the paired testing, the nurses and patients were asked a series of questions to assess the use of the pen. Each question was assessed on a scale of 1 to 10, where 1 was the worst score e.g. Very Difficult and 10 was the best score e.g. Very Easy. Patients were asked to assess the following aspects of the pen; overall ease of use, ease of changing the dose, security/confidence using alone at home, identification of the different pens, and therefore doses. Nurses were asked to assess the following aspects; ease of use for the patients, ease of changing the dose, ease of training patients, time taken to train patients, identification of the different pens, confidence that patient uses pen correctly at home, confidence that patient changes dose correctly when required, and confidence that patient will inject correct full dose.
2.4.2. In-depth interviews
After completion of the IDIs, the nurses and patients were asked the same series of questions to assess the ease of use of the pen, as those used during the paired testing.
2.5. Data analysis
The sample size for this testing was based on Health Authority requirements, which stipulate that a minimum of 15 individuals from each distinct user group (patients and nurses) should participate in a usability test [Citation24,Citation25]. It has previously been shown that observing a minimum of 15 participants in each group of users will allow a usability practitioner to uncover 80% of a product’s usability problems. Secondly, observing additional participants will reveal fewer and fewer new usability problems, and finally more severe usability problems are easier to detect with the first few participants [Citation26]. It was therefore decided that given six countries would be used during the testing, 3 patients and 3 nurses would be recruited in each country, giving a total of 18 distinct users overall in each group of users. One extra nurse was recruited in the UK due to an error by the recruiting agency, but it was felt valuable to use her responses in the final analysis. Each step of the injection process was assigned a scoring value where 0 = operation missed/performed incorrectly and 1 = operation performed correctly. A sub-score was calculated for the steps in the process classified as major/critical. For this sub-score the number of patients assigned a value of 0 was calculated i.e. missed or performed incorrectly the step.
3. Results
3.1. Demographic characteristics
In total 18 patients and 19 nurses participated in the user experience testing (). The nurses who took part in the testing had on average 15 years of experience and normally trained approximately 25 patients per week regarding fertility products, such as pen devices. The corresponding patients during the testing had a mean (SD) age of 32 (+/−6.1) years.
3.2. Usability testing
3.2.1. Paired testing
During the paired testing, the nurses only undertook a simulation of a 225 IU injection, which is one of the most commonly used dosing regimens during ART stimulation. The patients undertook several different scenarios as described in Section 2.3.1.
The numbers of errors reported, by the nurses, for the major/critical steps are shown in . Overall very few errors were made by the nurses. In fact, only one nurse made any errors and these errors were during the priming of the pen regarding the pushing of the dosage knob until it stopped, the splashing out of some liquid and turning the dosage knob to the correct dose, and not holding the needle in the skin for 5 s after completion of the injection. For this nurse, she corrected her actions either manually or verbally for all steps after making the initial mistakes, and subsequently carried out the correct injection process. All other nurses completed all steps without any errors.
Table 2. Usability tests – paired testing.
The numbers of errors reported, by the patients, for the major/critical steps for each of the scenarios are shown in . Overall although some errors were observed, no errors caused any critical problem with the delivery of the required dose for each scenario. The two steps which caused patients to make the most errors where during the priming of the pen, firstly regarding the pushing of the dosage knob to activate the pen, and secondly the subsequent splashing out of liquid. In all cases the initial ‘failure’ to complete these steps were rectified to enable the correct injection process to be completed. One patient made one error at each scenario when trying to attach the needle, as she had been instructed to twist the needle on by the nurse rather than simply click. However, this did not cause any issue in completing the actual injection process correctly.
3.2.2. In-depth interviews
During the IDIs, the nurses and the patients undertook the same scenarios, as those undertaken for the paired testing (). The only difference being that no formal training was carried out and only the standard patient leaflet, included in the drug packaging, was used by both parties.
The numbers of errors reported, by the nurses, for the major/critical steps are shown in . In fact, all nurses completed all major/critical steps without any errors. The numbers of errors reported, by the patients, for the major/critical steps for each of the scenarios are shown in . Overall very few errors were made, and the patients in all scenarios completed nearly all major/critical steps without errors. All patients followed the patient leaflet very carefully and this corresponded to the low error rate seen during this type of interview. Similar to the paired testing, the data showed that no additional errors occurred, irrespective of the scenario.
Table 3. Usability tests – in-depth interviews (IDIs).
3.3. Handling testing
3.3.1. Paired testing
After completion of the usability paired testing, the nurses and patients were each asked a series of questions to assess the use of the pen. Each question was assessed on a scale of 1 to 10, where 1 was the worst score e.g. Very Difficult and 10 was the best score e.g. Very Easy.
The patients were asked to assess the following aspects of the pen; overall ease of use, ease of changing the dose, security/confidence using alone at home, identification of the different pens, and therefore doses.
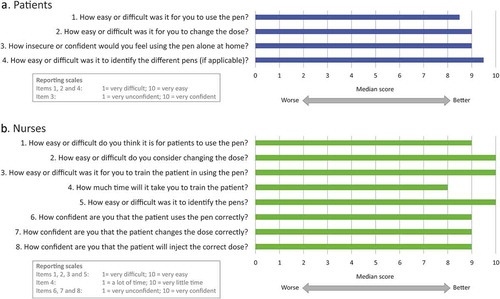
Overall for the paired tests the median rating for the patients regarding ease of use was 8.5, with a median of 9.0 regarding the ease to change the dose. Similarly, the median regarding the confidence in using the pen was 9.0 and the median regarding the ease of identification of the different pens was 9.5 (, ). These results showed that overall the patients found the pen very easy to use, easy to learn and would have a lot of confidence using it at home alone.
Table 4. Handling tests – paired testing.
The nurses were asked to assess the following aspects; ease of use for the patients, ease of changing the dose, ease of training patients, time taken to train patients, identification of the different pens, confidence that patient uses pen correctly at home, confidence that patient changes dose correctly when required, and confidence that patient will inject correct full dose.
For the nurses, the median regarding the ease of use was 9.0, with the easy of changing the dose and ease of training a patient both scoring medians of 10. The time taken to train the patient scored a median of 8, with several nurses reporting that the time taken to train a patient is very patient dependent. The nurses reported a median of 10.0 regarding ease of identification of the pens, showing that over 50% of nurses found this to be very easy, mainly due to the differing colors of the pens. Finally, regarding the confidence of the nurses in the patient using the pen correctly, changing the dose and administering the correct dose at home, all aspects scored medians of 9.0 (, ). These results confirmed that like the patients, the nurses found the pen very easy to use, easy to teach, and would have a lot of confidence in patients using it at home alone.
On viewing a colored, more pictorial version of the patient leaflet, and an instruction video, all patients and nurses agreed that the colored patient leaflet was clearer than the black and white package insert but that the demonstration video was the best supportive piece of information for a patient when they would be at home alone giving themselves the injection with the pen.
3.3.2. In-depth interviews
After completion of the IDIs, the nurses and patients were asked the same set of questions to assess the use of the pen.
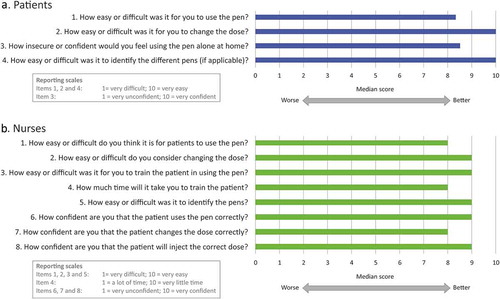
Overall for the IDIs the median rating for the patients regarding ease of use was 8.3, with a median of 10.0 regarding the ease to change the dose. The median regarding the confidence in using the pen was 8.5 and the median regarding the ease of identification of the different pens was 10.0 (, ). These results confirmed the findings from the paired testing in that the patients found the pen very easy to use and would have a lot of confidence using it at home alone.
Table 5. Handling tests – in-depth interviews (IDIs).
For the nurses, the median regarding the ease of use was 8.0, with the easy of changing the dose and ease of training a patient both scoring medians of 9.0. The time taken to train the patient scored a median of 8, with the nurses reported a median of 9.0 regarding ease of identification of the pens. Finally, regarding the confidence of the nurses in the patient using the pen correctly, changing the dose and administering the correct dose at home, the median scores were 9.0, 8.0, and 9.0 respectively (, ). These results also confirmed that like the patients, and in a similar fashion to the paired testing results the nurses found the pen very easy to use and would have a lot of confidence in patients using it at home alone.
On viewing a colored, more pictorial version of the patient leaflet, and an instruction video, all patients and nurses agreed, as in the paired testing, that the colored patient leaflet was clearer than the black and white package insert but that the demonstration video was the best supportive piece of information for a patient when they would be at home alone giving themselves the injection with the pen.
4. Discussion
During the current user experience testing, no critical errors, related to the use of the pen, which could affect the success of the injection process were identified. In general, both nurses and patients found the pen very easy to learn, use, and would be very confident using the product alone at home. Nurses also found the pen very easy to train the patients. The only area which caused some minor issues for the patients was related to the ‘priming’ of the pen, particularly the fear of pushing the dosage knob too hard and the concern about how much liquid should splash out of the pen, to show the pen is activated for use. These minor issues are related more to the ‘unknown’ for the patient, and given all patients recruited into this study were naïve to fertility treatment, this is not a surprising finding. Fertility treatment is a very emotional process, and patients want to ensure they are doing every step correctly to maximize their chance of getting pregnant [Citation17]. It was clear from the testing days that after one or two times of doing the injection, these ‘fears’ disappeared, and all processes where done correctly without any problems. It was also seen that the fact the patient had to use only one pen per day with the same process for all pens, reduced the complexity and in turn the stress of giving themselves the injection on a daily basis.
One interesting observation during this testing was the relationship between the nurse and the patient during the paired testing. It was clear to see that the patients valued highly and depended significantly on what the nurses were teaching them, even if this was slightly different compared to what was written in the patient leaflet. One example of this was the position into which the pen should be put to ‘splash out the liquid’ during the priming stage. Many nurses, although doing the step correctly themselves, for the purpose of the testing, instructed patients to hold the pen over a sink in a horizontal position, to avoid the liquid being splashed into the air or on the surface. This step in itself would not cause any problem with the validity of the injection process but showed that the role of the nurse is crucial to the final training of a patient. Patients value the training and support they receive from their health care professionals, and in turn are confident to follow their instructions, irrespective of the formal training materials. In fact, this training reassures the patient that they are carrying out the injection process correctly which in turn will increase their chances of having a successful outcome i.e. a live birth. Additionally, systematic reviews of randomized clinical trials and analytic studies of health care professional-patient communication have also confirmed a positive influence of quality communication on psychological and physical health outcomes [Citation28–Citation30]. Minimizing drug administration errors is one of the key steps to optimize ART treatment results. An ART cycles’ failure has important consequences in terms of economics, time, emotional, and even organizational costs for both the patients and the treating institutions. Additionally, some errors may put the patient at risk when the gonadotropin dosage exceeds the prescribed amount. The fact that many of the drug administration errors may be undetected is of special concern as the next cycle dose adjustment will be based on previous cycle response. For those reasons, an easy to use pen for both health care professionals and patients is highly recommended to avoid any drug administration mistakes.
The current testing, however, had some limitations. Firstly, this was a simulated scenario, during which no actual injections were given to patients which might had influenced the attitude of participants toward the injection process. Secondly, during the simulation of the different scenarios by the patient, the nurse only remained with the patient for the first scenario, leaving the patient alone with the moderator, who remained in the role of a non-participant/non-interventional observer in order not to influence the patient, for all remaining scenarios. In real life, the patient would always have the ability to call a nurse or experienced person, to answer any queries she had on the correct steps to carry out the injection. One final limitation to this testing was the sample size. Although the sample size used was aligned with the guidelines for such usability testing, a larger sample size within each country would have potentially given a wider overview of the user experience and allowed any intra-patient or intra-nurse variability to be further assessed.
The results of this testing showed that the current patient instructions had some sections which could be slightly confusing for the patients. Given the effect the burden of the disease can have on a successful treatment [Citation17], a decision was taken to make minor improvements to the patient leaflets, in order to simplify some of the instructions for the patients. Some examples of these minor improvements were to include a labeled diagram of all the pen parts, to enable the patient to better understand the instructions such as ‘push the dosage knob in until the activation bar with the small arrow disappears.’ With the diagram the parts of the pen corresponding to the ‘dosage knob’ and ‘activation bar’ are clearly identified for the patient. Another example of an improvement which was made was with regard to how the needle should be placed into the skin to make the injection. The previous version instructed the patient to ‘insert the injection needle completely at a 45–90º angle using a dart-like motion,’ this has been amended to say, ‘hold the pen at approximately a right angle and insert the needle completely in a steady movement.’ With these minor improvements, it is hoped that the utilization of the pen will be even easier for the patient, allowing them to go through their fertility treatment journey in a less stressful way, and to utilize the pen in the correct manner from the outset of treatment.
Overall, this testing showed that the pen is easy to learn, use, teach, change dose, and easy to adapt in diverse possible scenarios that a patient could encounter in standard clinical practice.
5. Conclusion
This study met its primary objective, which was to evaluate the impact of human factors on the use of the Bemfola pen injector with special focus on the convenience, safety, and ease of use (especially the adaptation of the dosage). Results showed that there was no significant risk of any critical/major errors occurring in any of the tests, by either nurses or patients, which could have in turn resulted in an incorrect injection process. Both patients and nurses rated highly the ease of use, ease of teaching, and easiness continuing with the same dose or of changing dose in diverse scenarios, which could occur during standard clinical practice. In addition, both patients and nurses would have felt very confident in using the pen alone at home.
Declaration of interest
H Saunders, P Arriagada and CM Howles are employed by PregLem S.A; J Glaser & T Hoja are employed by Point-Blank International. C Eftekhar is employed by CREATE Fertility, London and L De la Fuente Bitaine is employed by the Obstetrics and Gynaecology Service of Hospital Doce de Octubre, Madrid. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgment
The company would like to thank Jac Lee of Fourwave Communications for his assistance in the preparation of the figures for this manuscript.
Additional information
Funding
References
- Papanikolaou EG, Kolibianakis E, Devroey P. Emerging drugs in assisted reproduction. Expert Opin Emerg Drugs. 2005;10:425–440.
- Macklon NS, Stouffer RL, Giudice LC, et al. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27:170–207.
- Fauser BC, Diedrich K, Devroey P. Predictors of ovarian response: progress towards individualized treatment in ovulation induction and ovarian stimulation. Hum Reprod Update. 2008;14:1–14.
- Nelson SM, Yates RW, Lyall H, et al. Anti-Müllerian hormone-based approach to controlled ovarian stimulation for assisted conception. Hum Reprod. 2009;24:867–875.
- Broekmans FJ, de Ziegler D, Howles CM, et al. The antral follicle count: practical recommendations for better standardization. Fertil Steril. 2010;94:1044–1051.
- Allahbadia G. The Ideal Stimulation Protocol: Is There One? J Obstetrics Gynecol India. Nov–Dec 2015;65(6):357–361.
- Markle RL, King PJ, Martin DB. Characteristics of a successful human chorionic gonadotropin (hCG) administration in assisted reproduction. Fertility and Sterility. 2002;78(Suppl 1):S71–72.
- Howles CM, Saunders H, Alam V, et al. FSH Treatment Guidelines Clinical Panel. Predictive factors and a corresponding treatment algorithm for controlled ovarian stimulation in patients treated with recombinant human follicle stimulating hormone (follitropin alfa) during assisted reproduction technology(ART) procedures. An analysis of 1378 patients. Curr Med Res Opin. 2006;22:907–918.
- Weiss N. Gonadotrophin products: empowering patients to choose the product that meets their needs. Reprod Biomed Online. 2007;15:31–37.
- Schertz JC, Saunders H, Hecker C, et al. The redesigned follitropin alfa pen injector: results of the patient and nurse human factors usability testing. Expert Opin Drug Deliv. 2011;8:1111–1120.
- Rama Raju GA, Suryanarayana K, Jaya PG, et al. Comparison of follitropin-beta administered by a pen device with conventional syringe in an ART programme – a retrospective study. J Clin Pharm Ther. 2008;33:401–407.
- Huisman D, Raymakers X, Hoomans EH. Understanding the burden of ovarian stimulation: fertility expert and patient perceptions. Reprod Biomed Online. 2009;19(Suppl 2):5–10.
- Sedbon E, Wainer R. Perves C Quality of life of patients undergoing ovarian stimulation with injectable drugs in relation to medical practice in France. Reprod Biomed Online. 2006;12:298–303.
- Namdar A, Naghizadeh MM, Zamani M, et al. Quality of life and general health of infertile women. Health Qual Life Outcomes. 2017;15:139.
- Gameiro S, Boivin J, Peronace L, et al. Why do patients discontinue fertility treatment? A systematic review of reasons and predictors of discontinuation in fertility treatment. Hum Reprod Update. 2012;18:652–669.
- Cousineau TM, Domar AD. Psychological impact of infertility. Best Practice and Research. Clin Obstetrics Gynaecol. 2007;21:293–308.
- Boivin J, Domar AD, Shapiro DB, et al. Tackling burden in ART: an integrated approach for medical staff. Hum Reprod. 2012;27:941–950.
- FDA approved drug products. GONAL-f RFF. Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails
- Somkuti SG, Schertz JC, Moore M, et al. Patient experience with follitropin alfa prefilled pen versus previously used injectable gonadotropins for ovulation induction in oligoanovulatory women. Curr Med Res Opin. 2006;22:1981–1996.
- Abbotts C, Salgado-Braga C, Audibert-Gros C. A redesigned follitropin alfa pen injector for infertility: results of a market research study. Patient Prefer Adherence. 2011;5:315–331.
- Rettenbacher M, Andersen AN, Garcia-Velasco JA, et al. A multi-centre phase 3 study comparing efficacy and safety of Bemfola versus Gonal-f in women undergoing ovarian stimulation for IVF. Reprod Biomed Online. 2015;30:504–513.
- Imthurn B, McVeigh E, Stiller R, et al. Evaluation of the use and handling of three different pen systems considered for in vivo fertilization treatment. Expert Opin Drug Deliv. 2014;11:1859–1864.
- Quintero LA, Merino VV, Bitaine LDLF, et al. An evaluation by potential IVF/donor oocyte patients of the use and handling of the Bemfola® Pen compared with the Gonal-f® Pen and Puregon Pen® Revista Iberoamericana de Fertilidad y Reproducción Humana. 2016;33(3):42–51.
- Association for the Advancement of Medical Instrumentation (AAMI). Human factors design process for medical devices. Report No.: ANSI/AAMI HE 74. Arlington (VA): Association for the Advancement of Medical Instrumentation. 2001
- National Health Service. Design for patient safety: user testing in the development of medical devices. 2010. Available from: http://www.nrls.npsa.nhs.uk/resources/collections/design-for-patient-safety/?entryid45=74946
- Lewis JR. Sample sizes for usability studies: additional considerations. Human Factors. 1994;36:368–378.
- Homburg R, Howles CM. Low-dose FSH therapy for an ovulatory infertility associated with polycystic ovary syndrome: rationale, results, reflections and refinements. Hum Reprod Update. 1999;5:493–499.
- Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152:1423–1433.
- Faller H. Shared decision making: an approach to strengthening patient participation in rehabilitation. Die Rehabilitation. 2003;42: 129–135. (in German).
- Teutsch C. Patient-doctor communication. Med Clin North Am. 2003;87:1115–1145.