1. Microneedle patches for drug delivery to skin
Drug delivery is often limited by the need to cross a barrier to gain access to target tissues. In the case of skin, the main barrier is the skin’s outer layer of stratum corneum, which measures just 10–20 µm in thickness, but effectively blocks delivery of most molecules into the body [Citation1]. Microneedle patches (MNPs) have been developed to penetrate stratum corneum with micron-scale pores that are large enough to enable drugs, including macromolecules, to enter the skin while being small enough to avoid pain, irritation, and needle phobia () [Citation2,Citation3]. This review focuses on MNPs, which are composed of solid microneedles that either encapsulate or are coated with drug. This review does not include hollow microneedles that can be used like tiny hypodermic needles for injection [Citation4].
Figure 1. Microneedle patch (MNP) for transdermal drug delivery. (A) Representative MNP. (B) Magnified view of microneedles. (C) Image showing skin histology after puncture with a microneedle. Images reproduced with permission from JW Lee, Georgia Tech.
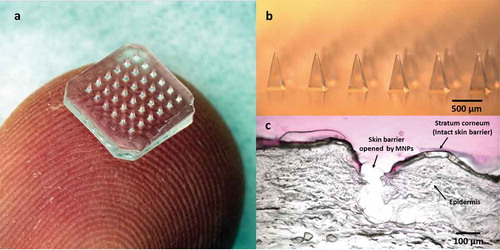
Based on this concept, many kinds of MNPs employing different delivery strategies have been developed for transdermal administration of drugs that would otherwise require injection. MNPs generally contain an array of 102–104 microneedles in an area of approximately 1 cm2. Each microneedle typically measures 100–1000 µm in length and is either (a) made of a non-water-soluble material like metal, polymer, or ceramic and often coated with a drug formulation or (b) made of a water-soluble material, often containing saccharides and/or polymers, to encapsulate drug within the microneedle matrix. Advantages of drug delivery with MNPs include (i) rapid onset of drug action avoiding first-pass metabolism by targeting delivery to dermal capillaries, (ii) simple local administration providing direct access to the tissue in need of treatment, and (iii) safe self-administration achieving therapy on demand in many cases. MNPs are already in advanced development for transdermal drug and vaccine delivery as well as cosmetic skin care. Below, we describe representative examples of MNPs that have reached the stage of clinical study or commercial introduction.
Metal MNPs coated with a drug have advanced furthest toward a licensed pharmaceutical product. While drugs, including human parathyroid hormone and glucagon, have been studied in clinical trials, an MNP administering zolmitriptan has already reached a phase 3 clinical trial designed to study fast onset of drug action for treating migraine attack rapidly. Pharmacokinetic analysis showed that zolmitriptan delivered by MNP can rapidly reach peak concentration, with a median tmax ≤ 20 min, which is much faster than oral delivery having tmax = ~1 h. Peak drug concentration, Cmax, was up to six times higher after MNP delivery compared to oral administration [Citation5]. In a recent Phase 2b/3 trial, the zolmitriptan MNP provided effective relief of migraine headache and was well tolerated by patients [Citation6]. These findings indicate that MNPs can be used to provide faster drug uptake and onset of action when required to treat patients urgently.
In addition to drugs, MNPs can also be used to deliver vaccines, which are especially appealing because the skin is abundant in antigen-presenting cells, which can improve the immune response to an antigen [Citation7]. A recent phase 1 clinical trial involving 100 subjects studied administration of influenza vaccine using MNPs and showed that vaccination by MNP, including a group that self-administered the vaccine patches, was at least as immunogenic as intramuscular vaccination, had an excellent safety profile, and was better accepted by study subjects [Citation8]. This study shows that MNPs can be used for simplified vaccination that is strongly immunogenic and well accepted by patients.
MNPs for cosmetics have already been commercialized by multiple companies and sold around the world with the purpose of improving skin appearance [Citation9]. MNPs made of hyaluronic acid have been developed to enable frequent self-administration to targeted skin sites without the need for hypodermic injections. This is appealing to customers because hyaluronic acid is known to stimulate collagen synthesis and increase skin volume to reduce the appearance of wrinkles [Citation10]. The many cosmetic MNPs available commercially show that MNPs can be widely accepted in many countries and cultures, and self-administered repeatedly in a home setting.
2. MNPs for drug delivery to other tissues
Inspired by successes with MNPs for drug delivery to the skin, researchers have started exploring other tissues that would benefit from local delivery using MNPs [Citation11]. Some of these additional tissues studied with MNPs are listed in .
Table 1. Tissues studied for drug delivery with microneedle patches.
The oral mucosa is an attractive site for vaccination, in part due to the presence of the mucosal-associated lymphoid tissue just below the epithelial surface. Ma et al. [Citation12] examined antigens delivered to the oral cavity using coated MNPs by breaking the barrier of the oral mucosa and found that MNP vaccination significantly stimulated antigen-specific IgG (serum) and IgA (saliva) responses, but intramuscular injection did not.
The vaginal mucosa may also be a useful site for vaccination, specifically to target delivery to antigen-presenting cells in vaginal tissue. Wang et al. [Citation13] administered antigen using MNPs into the vaginal cavity and found robust antigen-specific immune responses both systemically and in the reproductive tract mucosa.
Oral administration of biotherapeutics is known for very poor bioavailability because of enzymatic degradation in the gastrointestinal tract and low permeability of macromolecules through the epithelial barrier of the intestine. Traverso et al. [Citation14] developed an early prototype of a device that can deploy microneedles from within the intestinal lumen and can increase oral bioavailability of insulin. The cylindrical device was designed to use peristaltic action of the intestine to break microneedles off the device and/or force them to puncture the epithelial barrier of the gastrointestinal tract.
Ocular drug bioavailability is very low when using topical application of drugs (i.e. eye drops) due in part to the barrier imposed by corneal epithelium. Jiang et al. [Citation15] developed microneedles coated with fluorescein that increased fluorescein bioavailability by almost two orders of magnitude, and microneedles coated with pilocarpine that enabled greater and longer-lasting pupil constriction compared to topical drug treatment.
Drug delivery to the nail bed is notably difficult due to the barrier properties of the nail. Chiu et al. [Citation16] demonstrated sustained topical drug delivery into the nail after pretreatment with microneedles administered using a cylindrical roller. The microneedle-porated nail allowed rapid drug diffusion deep into the nail, while topical delivery without microneedles resulted in much slower diffusion of drug limited by the highly keratinized structure of the nail.
Fecal incontinence can be treated by local delivery of phenylephrine (PE) to the anal sphincter muscle. Because topical gel application often does not deliver a therapeutic PE dose, Baek et al. [Citation17] developed a MNP to increase PE bioavailability to the anal sphincter. Delivery in this way increased PE delivery 10-fold and thereby increased resting anal sphincter pressure significantly above that after topical PE gel treatment alone.
Treatment of alopecia is constrained by poor bioavailability of drug delivery to the hair follicle. Dhurat et al. [Citation18] studied the effect of microneedle pretreatment of the skin of the scalp on hair growth in human subjects by comparing minoxidil treatment with or without microneedling. The microneedle-treated group showed a significantly faster rate of hair growth, suggesting that microneedle pretreatment improved the permeation of minoxidil through the skin to the hair follicle, although the effect of microneedles on the drug transport process was not directly measured.
Drug delivery in cardiovascular disease can be limited by poor drug transport into vessel walls. Choi et al. [Citation19] designed MNPs with a curved backing layer to treat blood vessels undergoing atherosclerosis. They designed this system to address the limitations of drug-eluting stents (which injure the blood vessel endothelium) and cuff-style external devices (which suffer from slow drug diffusion and complex drug distribution in the tissue). The MNP with curved backing layer wrapped around the diseased blood vessel such that the microneedle tips were inserted into the barrier tunica adventitia, where they rapidly dissolved and released drug into the target vascular tissue.
3. Expert opinion
There is great interest in delivering drug directly to diseased tissue, thereby maximizing efficacy, minimizing side effects, and avoiding delivery hurdles encountered before reaching the target tissue after systemic drug administration. For tissues that can be accessed by a device and have a thin barrier layer on the surface (e.g. tissues covered by epithelium or endothelium), MNPs offer a simple yet powerful solution to make biological barriers temporarily permeable to drugs or releasing them after puncturing the barrier. MNPs have been extensively studied for transdermal drug delivery but can also be designed for delivery targeted to other tissues including, but not limited to, those in . Commercial translation of MNPs for transdermal delivery has largely been limited by the need for more study in humans through clinical trials and development and implementation of large-scale, cost-effective manufacturing. New issues arise when administering microneedles to other tissues, as they are often wet (which means microneedles should not dissolve or soften due to moisture before inserting into the tissue), soft (which may require different microneedle design and insertion techniques), and sensitive to pain and damage (which may require microneedle administration by trained professionals). While lessons from MNPs used on skin are valuable, developing MNPs for safe and effective use in other tissues will require novel designs that account for different tissue anatomy, physiology, and biomechanics, as well as the types and doses of drugs to be delivered and the types of pathologies to be treated.
Declaration of interest
M Prausnitz is an inventor of patents licensed to companies developing microneedle-based products, is a paid advisor to companies developing microneedle-based products, and is a founder/shareholder of companies developing microneedle-based products (Micron Biomedical, Clearside Biomedical). This potential conflict of interest has been disclosed and is managed by Georgia Tech and Emory University. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008 Nov;26(11):1261–1268.
- Quinn HL, Kearney MC, Courtenay AJ, et al. The role of microneedles for drug and vaccine delivery. Expert Opin Drug Deliv. 2014 Nov;11(11):1769–1780.
- Prausnitz MR. Engineering microneedle patches for vaccination and drug delivery to skin. Annu Rev Chem Biomol Eng. 2017 Jun;7(8):177–200.
- Pettis RJ, Harvey AJ. Microneedle delivery: clinical studies and emerging medical applications. Ther Deliv. 2012 Mar;3(3):357–371.
- Donald J, Kellerman MA, Tepper SJ. Rapid systemic delivery of zolmitriptan using an adhesive dermally applied microarray. Pain Management. 2017;7(6):559–567.
- Spierings EL, Brandes JL, Kudrow DB, et al. Randomized, double-blind, placebo-controlled, parallel-group, multi-center study of the safety and efficacy of ADAM zolmitriptan for the acute treatment of migraine. Cephalalgia. 2018 Feb;38(2):215–224.
- Marshall S, Sahm LJ, Moore AC. The success of microneedle-mediated vaccine delivery into skin. Hum Vaccin Immunother. 2016 Nov;12(11):2975–2983.
- Rouphael NG, Paine M, Mosley R, et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet. 2017 Aug 12;390(10095):649–658.
- McCrudden MT, McAlister E, Courtenay AJ, et al. Microneedle applications in improving skin appearance. Exp Dermatol. 2015 Aug;24(8):561–566.
- Hong JY, Ko EJ, Choi SY, et al. Efficacy and safety of a novel, soluble microneedle patch for the improvement of facial wrinkle. J Cosmet Dermatol. 2017 Oct 7.
- Rzhevskiy ASST, Donnelly RF, Anissimov YG. Microneedles as the technique of drug delivery enhancement in diverse organs and tissues. J Control Release. 2018;270:184–202.
- Ma YZ, Tao WQ, Krebs SJ, et al. Vaccine delivery to the oral cavity using coated microneedles induces systemic and mucosal immunity. Pharm Res-Dordr. 2014 Sep;31(9):2393–2403.
- Wang N, Zhen YY, Jin YG, et al. Combining different types of multifunctional liposomes loaded with ammonium bicarbonate to fabricate microneedle arrays as a vaginal mucosal vaccine adjuvant-dual delivery system (VADDS). J Control Release. 2017 Jan;28(246):12–29.
- Traverso G, Schoellhammer CM, Schroeder A, et al. Microneedles for drug delivery via the gastrointestinal tract. J Pharm Sci-Us. 2015 Feb;104(2):362–367.
- Jiang J, Gill HS, Ghate D, et al. Coated microneedles for drug delivery to the eye. Invest Ophthalmol Vis Sci. 2007 Sep;48(9):4038–4043.
- Chiu WS, Belsey NA, Garrett NL, et al. Drug delivery into microneedle-porated nails from nanoparticle reservoirs. J Control Release. 2015 Dec 28;220:98–106.
- Baek C, Han M, Min J, et al. Local transdermal delivery of phenylephrine to the anal sphincter muscle using microneedles. J Control Release. 2011 Sep 5;154(2):138–147.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013 Jan;5(1):6–11.
- Choi CK, Kim JB, Jang EH, et al. Curved biodegradable microneedles for vascular drug delivery. Small. 2012 Aug 20;8(16):2483–2488.
