ABSTRACT
Background: This study examined the patient handling experience and self-injection success of patients with rheumatoid arthritis (RA) administering BI 695501 using an AI.
Methods: This Phase II, 7-week, open-label, interventional study (NCT02636907) included adult patients with moderately to severely active RA not adequately controlled by DMARDs, with no experience of self-injecting with AI/pen. Patients self-injected BI 695501 via AI every 2 weeks in the AI Assessment Period (AAP). Training was given on first injection; AI handling events were recorded. Percentage of self-injection success was the primary end point. Patients could enter a 42-week pre-filled syringe (PFS) safety extension.
Results: The AAP was completed by 73/77 patients. In total, 216/218 (99.1%) self-injections on Days 15, 29, and 43, were successful. Nine (11.7%) patients had drug-related adverse events (AEs). Two patients reported four serious AEs (SAEs), none drug-related. Overall (in the AAP and PFS extension), 28 (36.4%) patients had drug-related AEs; nine patients had SAEs, one was considered drug-related. Five (6.5%) patients reported injection-site reactions in the AAP; 13 (18.1%) in the PFS extension.
Conclusions: After training, almost all patients were successfully able to self-administer BI 695501 using an AI. BI 695501 via AI (and via PFS in the extension) was well tolerated.
Clinical Trial Registration: NCT02636907
1. Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that can adversely affect movement in the joints, particularly those of the hand [Citation1]. Therefore, it is important that injectable medications for self-administration can be routinely injected without failures or complications, despite the physical limitations of the patient.
Draft guidance from the US Food and Drug Administration on developing drug products for the treatment of RA mandates that the approval of an autoinjector (AI) presentation requires additional studies, including human factor studies and a pharmacokinetic (PK) bridging study [Citation2].
BI 695501 is a self-injectable adalimumab biosimilar that has demonstrated PK bioequivalence [Citation3] and similar clinical efficacy, safety, and immunogenicity [Citation4] with its reference product, Humira® (Abbvie Inc., North Chicago, IL, USA). This study examined the real-life patient handling experience and self-injection success of BI 695501 using an AI.
2. Patients and methods
2.1. Patients and study design
Male and female adult patients (aged 18–80 years) with moderately to severely active RA for ≥6 months that was not adequately controlled by nonbiologic disease-modifying antirheumatic drugs (DMARDs) were enrolled across 19 trial sites in Poland and the USA. Patients could be biologic naïve or biologic experienced, but were required to have no prior history of self-administration of medications using an AI or pen.
The study was a Phase II, 7-week, open-label, single-arm, interventional clinical trial (NCT02606903) to assess real-life patient handling of the BI 695501 AI, followed by an optional 42-week extension phase during which BI 695501 was administered via pre-filled syringe (PFS). The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the institutional review boards of all participating centers. All patients provided written, informed consent prior to any trial procedures.
Patients self-injected four subcutaneous (SC) doses of BI 695501 (one injection every other week, including one training injection) with an AI in the AI Assessment Period (AAP).
Patients were provided with detailed training and instruction on self-injection with the AI, based on the steps that are listed in the Product Information for Use, including a step-by-step explanation on the correct method for preparing and self-injecting BI 695501. This training was provided by qualified trial site personnel during the first self-injection by the patient. Success of the training injection was not included in the end point evaluation.
The three subsequent injections were administered by the patient at the trial site, under the supervision of the trial site personnel. Both trial site personnel and the patients recorded their observations of AI handling events and experience during the injection independently, using questionnaires (provided in the supplemental materials). AI handling events were any of the following preventing the patient from successfully self-injecting the full content of the AI: failure to remove the cap of the AI; failure to press the injection button of the AI; failure to hold the AI down against the skin until the injection was completed.
The 7-week AAP was followed by an optional 42-week extension phase, in which patients self-injected up to 22 injections of BI 695501 (one injection every other week) using a PFS.
Patients were permitted to receive methotrexate or any other conventional DMARD therapy as part of their standard of care throughout the trial.
2.1.1. Description of drug presentation
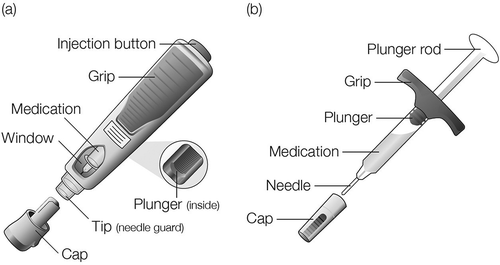
The AI ()) was a disposable presentation intended for SC injection of a single fixed dose of BI 695501. It was preassembled and ready to be used by the patient. The firm outer casing contained a PFS with the drug product, a top subassembly, and a lower subassembly, which were spring-powered. Dose setting, priming, or loading are not needed to administer the injection at the point of use. After letting the AI warm up to ambient temperature and disinfecting the skin, the user removed the cap and firmly pressed the AI against the skin at a 90° angle. Pushing the injection button was confirmed by a “click” sound. The duration of the injection was 10 s and its completion could be confirmed by inspecting the plunger in a window. As the AI was removed from the skin, a needle guard automatically covered the needle for protection.
The BI 695501 PFS ()) consisted of a 1 mL PFS with a staked needle and rigid needle shield, and was sealed with a plunger stopper. The PFS was preassembled with an extended finger flange and a plunger rod to enable injection of the BI 695501 drug product (ready-to-use PFS presentation).
2.2. Study end points
The primary end point was the percentage of successful self-injections as reported in the questionnaires completed by both patients and trial site personnel among the three planned self-injections (occurring after the training self-injection). Where unsuccessful injections were followed by a second attempt, those second attempts were not part of the analysis. Injections were considered successful if the full content of the AI was injected into the body. Where there were inconsistent answers between the site personnel and the patient, the attempt was determined as unsuccessful.
Where applicable, the causes of failure to self-inject by the patient were recorded and the proportion of patients with local injection-site reactions (ISRs) and drug-related treatment-emergent adverse events (AEs) per investigator assessment were assessed.
A technical robustness assessment was performed on the first 109 used, normally functioning AIs received by the Sponsor. The returned AIs were inspected for any signs of damage, malfunctioning, or injection incompleteness, identified through visual inspection by the Sponsor device engineer.
Safety data for the AAP were collected up to Day 50 (end of treatment visit), and up to Week 60 for the extension phase. Efficacy was not assessed during this trial.
2.3. Statistical methods
Analyses were based on descriptive statistics for the outcome of all questionnaires, ISRs, and safety assessments.
The percentage of successful self-injections was analyzed descriptively by visit (Day 15, Day 29, and Day 43) and overall. The 95% Wilson score confidence interval was calculated for the primary end point (percentage of successful injections).
The frequency of AI handling events was reported overall and by visit (Day 15, Day 29, and Day 43).
Frequency, severity, and causal relationship of AEs were tabulated by system organ class and preferred term (SOC/PT) after coding according to the current version of MedDRA (v19.0). All AEs were summarized overall and by treatment period and were also presented by SOC/PT, by severity, and by relationship to trial drug. Serious AEs, all AEs leading to trial drug discontinuation, and nonserious AEs were also summarized. ISRs and drug-related AEs were also summarized for the extension phase. Data were analyzed using SAS software (SAS, Cary, NC, USA).
3. Results
3.1. Patients
A total of 103 patients were screened at 19 trial sites, and 77 patients were included and treated with BI 695501. Patient demographics and baseline characteristics are presented in . Seventy-three patients (94.8%) completed the AAP. Four patients prematurely discontinued the trial after Day 1 (due to AEs and withdrawal by patient [two patients each]), and one patient missed the Day 15 visit. Seventy-two patients entered the PFS extension phase and 62 completed it.
Table 1. Baseline patient demographics and characteristics.
3.2. Injection success
Of 218 self-injections with the AI performed by 77 patients on Days 15, 29, and 43 of the AAP, 216 (99.1%) were reported by both the trial site personnel and the patients to be successful (primary end point, ). There were no discordant results; patients and trial site staff agreed on injection success in all cases.
Table 2. Primary end point: successful self-injections with AI, by visit.
In both cases of unsuccessful initial injection attempts, the reported cause was that the AI button could not be pressed by the patient. Both patients were able to successfully administer a second injection immediately after the first failed attempt. Both AIs involved in the unsuccessful self-injections were returned to the Sponsor for technical inspection and no technical issues were found. The unsuccessful attempts were attributed to potential user error.
The first 109 used AIs were returned to the Sponsor for robustness testing by visual and manual inspection. No signs of damage, malfunctioning, or injection incompleteness as per the test protocol were found after patient usage.
3.3. Safety
Thirty patients (39.0%) reported at least one AE during the AAP, and in nine patients (11.7%) at least one AE was considered by the Investigator as related to the trial drug (). Four serious AEs were reported by two patients during the AAP (depression and drug hypersensitivity to Duloxetine in one patient; anemia and esophageal carcinoma in one patient), none of which were considered to be related to the study drug by the Investigator. Five patients (6.5%) reported ISRs during the AAP, including injection-site bruising by two patients (2.6%), and erythema, pruritus, ISR, and swelling by one patient (1.3%) each, all of which were mild in severity and resolved without any treatment. One patient discontinued the trial drug before receiving a second injection due to an ISR and rash reported on Day 12 ().
Table 3. Overview of all AEs in the Autoinjector Assessment Period.
ISRs were reported by 13 patients (18.1%) in the PFS extension phase: nine patients (12.5%) reported injection-site bruising; four patients (5.6%) reported injection-site erythema; induration, pain, pruritus, swelling, and warmth were reported by two patients (2.8%) each; ISR was reported by one patient (1.4%).
Overall, during the AAP and PFS extension phase, 56 patients (72.7%) reported at least one AE, with 28 (36.4%) of these patients reporting at least one AE related to the trial drug (). Nine patients reported serious AEs during the whole trial, one of which (a case of autoimmune hepatitis) was considered related to the study drug by the Investigator.
Table 4. Overview of all AEs (Day 1 to Week 60, including the events described in ).
4. Discussion
Reduced manual dexterity can make self-injection of medication for RA challenging, and this may lead to reduced injection success or adherence to therapy [Citation5]. In the present study, 99.1% of self-injections were successful, suggesting that the AI presentation tested was generally easy to use and may not lead to unintentional nonadherence among patients [Citation5]. The two unsuccessful attempts (both with a first injection) were immediately followed by successful attempts, demonstrating that even in cases of difficulty, the patients were ultimately able to successfully self-administer BI 695501 via AI.
In a recent study on an AI presentation for ixekizumab [Citation6], 99.7% of self-injections (in 49 patients with a range of autoimmune conditions including RA) with and without training were successful, similar to the rate in the present study. It should be noted that the ixekizumab AI is not clinically used for patients with RA, and the additional handling difficulties that such patients experience may influence the extent of training required in clinical practice. The present study did not compare the patients who received training with those who did not, and this may be a worthwhile addition to the body of data, to determine the training requirements for a patient population who may experience difficulty in handling AIs.
5. Conclusions
After training by site personnel, almost all patients with moderately to severely active RA without prior experience of self-injections with AIs were able to successfully self-administer BI 695501 SC using an AI. BI 695501 via AI was well tolerated, and no new safety information was identified in this trial.
Declaration of interest
S Cohen is a consultant for Boehringer Ingelheim and has received research grants as an investigator from Boehringer Ingelheim. T Krahnke undertakes work for Boehringer Ingelheim as an external contractor. D Assudani was previously an employee of Boehringer Ingelheim. Writing assistance provided by Kate Booth of Watermeadow, and Christina Jennings of GeoMed, Ashfield companies, part of UDG Healthcare plc., was utilized in the production of this manuscript and funded by Boehringer Ingelheim. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. A reviewer on this manuscript has disclosed that they were an author on one of the papers referenced in the authors bibliography.
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344(12):907–916.
- FDA. Guidance for industry. Rheumatoid arthritis: developing drug products for treatment. [Internet]. US: Food and Drug Administration; 2013 [cited 2017 May 30]. Available from: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM354468.pdf
- Wynne C, Altendorfer M, Sonderegger I, et al. Bioequivalence, safety and immunogenicity of BI 695501, an adalimumab biosimilar candidate, compared with the reference biologic in a randomised, double-blind, active comparator phase I clinical study (VOLTAIRE®-PK) in healthy subjects. Expert Opin Investig Drugs. 2016;25(12):1361–1370.
- Cohen SB, Alonso-Ruiz A, Klimiuk PA, et al. Biosimilar Candidate BI 695501 and adalimumab reference product have similar efficacy and safety in patients with moderately-to-severely active rheumatoid arthritis (RA): 1-Year results from a Phase III study [abstract 2442]. Arthritis Rheumatol. 2017;69(suppl 10).
- Van den Bemt BJ, Zwikker HE, van den Ende CH. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol. 2012;8(4):337–351.
- Duffin KC, Bukhalo M, Bobonich MA, et al. Usability of a novel disposable autoinjector device for ixekizumab: results from a qualitative study and an open-label clinical trial, including patient-reported experience. Med Devices (Auckl). 2016;9:361–369.