ABSTRACT
Introduction: Ready-to-use prefilled syringes for drug delivery are increasingly used across a broad spectrum of clinical specialties. For patients with primary immunodeficiencies manifesting as antibody deficiencies, immunoglobulin G (IgG) replacement therapy (IgRT) by subcutaneous administration is an established treatment modality. Expanding IgRT administration options through the introduction of prefilled syringes may further improve its utility.
Areas covered: Here, we collate experience with prefilled syringes from other clinical settings to inform on their practicality and suitability for IgRT. In addition to discussing drug characteristics such as stability, pharmacokinetics, and efficacy, we focus on treatment delivery, physician/patient experience, costs, and the importance of education for the use of prefilled syringes.
Expert opinion: Perceived benefits of prefilled syringes include accurate dosing, sterility, and reduced treatment time, while offering patients greater choice, convenience, and ease-of-use. Our review of clinical experience with prefilled syringes supports this consensus. Relatively few studies directly compare prefilled syringes with conventional administration, and robust studies of cost-effectiveness and health-related quality of life are needed on a drug-by-drug basis. Growth in the availability of prefilled syringes will continue, encouraged by the importance of patient choice and treatment convenience, toward the goal of individualized treatment regimens and improved quality of life.
1. Introduction
Primary immunodeficiencies (PI) are a group of more than 350 disorders caused by poor or absent function of one or more components of the immune system [Citation1]. Approximately 70% of PI patients have primary antibody deficiency [Citation2,Citation3]; the prevalence of clinically significant antibody deficiency has been estimated at 1:25,000 to 1:111,000 persons [Citation4] and, for these patients, immunoglobulin G (IgG) replacement therapy (IgRT) is the standard of care. IgRT is also used in the treatment and management of some secondary immunodeficiencies and, at higher doses, for immunomodulation in a variety of inflammatory, autoimmune, and neurological conditions.
IgRT can be administered intravenously or subcutaneously, and a variety of IgG products are commercially available [Citation5]. Intravenous Ig (IVIG) administration is the traditional approach, having been successfully used for over five decades [Citation6]. However, over 25 years of accumulated treatment experience with subcutaneous (SC) Ig (SCIG) [Citation7] has established this modality as an effective, safe and well-tolerated alternative to IVIG that offers patients near steady-state IgG levels and the option to self-administer [Citation5,Citation7]. In addition, SCIG formulations, in particular those with higher IgG content, allow lower infusion volumes and shorter infusion durations relative to IVIG [Citation5,Citation7]. Despite the advancements provided by the SCIG route, further progress in administration logistics will improve its utility. This is particularly relevant for patients with poor dexterity, coordination issues or visual difficulties, such as the young and elderly, who may find it difficult or be unable to self-administer SCIG by the traditional vial and syringe method. Individualized IgRT regimens can offer improved convenience, flexibility, and quality of life for patients, and expanding available options through the use of prefilled syringes for SCIG administration is an interesting next step [Citation7,Citation8].
Prefilled syringes are already effectively used for the administration of blood-based products, such as coagulation factors VII (eptacog alfa) and VIII (moroctocog alfa and efmoroctocog alfa) for the treatment of bleeding disorders [Citation9–Citation11]. They are also a promising treatment modality for SC IgRT, particularly high-concentration SCIG products (i.e. 20% SCIG) as these can be filled into relatively small syringes that are convenient to use. They could potentially also be considered as an option for enzyme-facilitated SCIG products where prefilled syringes could help simplify the more complex infusion process of these products. To appraise the practicality of prefilled syringes for SC IgRT, we conducted a pragmatic literature review to collate the experience from other clinical settings and indications on the use of prefilled syringes. We present the findings of our literature review following a brief overview of the prefilled syringe.
It is important to note that this review includes information about prefilled syringes from a number of different disease conditions for which varying infusion paradigms are used. In several instances, there are important differences between SC IgRT and other products discussed here e.g., infusion volume (generally high for SC IgRT and lower for many other products) and infusion device (generally pump-based infusion for SC IgRT and pen-type autoinjectors for many other products). These have been included and discussed as there is a lack of literature on prefilled syringe products that are directly comparable to SC IgRT in terms of infusion volumes and device type. Nonetheless, keeping these differences in mind we still believe many valuable lessons can be learned from the use of prefilled syringes in other disease areas.
2. Prefilled syringes for drug delivery
Since the introduction of a prefilled syringe for heparin over three decades ago, their use for drug delivery has grown substantially and now encompasses a broad spectrum of clinical settings and indications. Prefilled syringes are now estimated to account for one quarter of all injectables [Citation12], a proportion that is expected to grow significantly. The benefits of ready-to-use prefilled syringes include provision of drugs in a closed system, accurate dosing, sterility assurance, and reduced risk of contamination [Citation13]. Moreover, they offer patients greater choice, convenience, and ease-of-use, particularly for self-administration. This may lead not only to improved adherence but greater patient satisfaction, independence, and quality of life. One of the primary disadvantages of prefilled syringes is the increased unit cost relative to traditional vials, although the price gap is narrowing with increasing demand and production. An overview of prefilled syringe characteristics relative to conventional methods is shown in [Citation11, Citation14–Citation63].
Table 1. Overview of prefilled syringes compared with conventional methods (vial/ampoule) for medication deliverya.
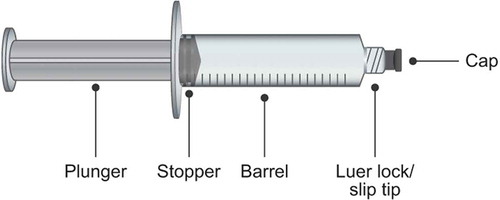
The overall design of prefilled syringes is generally consistent, comprising: the plunger, stopper, barrel, Luer lock or slip tip, and cap [Citation49] (). A variety of different manufacturing processes, materials, and drug formulation processes are used in their production (for a review, see [Citation12,Citation13,Citation49]). Dependent on purpose (including non-SC use), prefilled syringes are available in a range of sizes [Citation49] and are typically fabricated from glass or plastic. While most prefilled syringes in the US, European and global markets are made from glass [Citation13,Citation49], advances in polymer chemistry have led to an increase in the use of polymer-based syringes. Needles may be fixed to the syringe or attached to the barrel via a Luer lock screw cap mechanism or slip tip, as is typical in non-emergency settings and for self-administration [Citation12]. As mentioned previously, IgRT requires higher volumes (and consequently, larger prefilled syringes) than many other SC injections. Prefilled syringes that feature a retractable needle to increase safety are also available [Citation49]. The concept of the prefilled syringe has also been expanded in the form of prefilled pens/autoinjectors that are activated by a button press or as a result of pressure applied against the needle end upon administration. Examples include devices for the administration of insulin [Citation64,Citation65], and epinephrine [Citation66]. Further developments in prefilled syringe design are paralleled by continuing growth in their use across the healthcare sector, with marketing predictions estimating sales of prefilled syringes approaching 7 billion units in 2025 (from 2 billion in 2009) [Citation12,Citation67].
3. Prefilled syringes in clinical practice
3.1. Literature search results
Literature published on PubMed was searched to identify articles reporting on the use of prefilled syringes in clinical practice. No constraints on the publication type or year of publication were imposed. The literature search was performed in July 2017. The data presented should not be considered exhaustive, but instead as representative of principal findings from studies that evaluate clinical use and experience with prefilled syringes.
The primary search term was ‘(prefilled OR pre-filled) AND Syringe [MH],’ which identified 229 articles. Using the filters within PubMed, this list was initially refined to the 69 articles that were listed as a ‘clinical study’ (n = 37) or ‘comparative study’ (n = 46) (filtering on ‘clinical study’ also included all articles listed as a ‘clinical trial’ (n = 35), ‘controlled clinical trial’ (n = 23), or ‘randomized controlled trial’ (n = 21)). The title and abstract for each of the 69 articles was reviewed and a total of 31 relevant articles were retained. We also screened the titles of the remaining 160 articles to identify further articles of interest, and performed additional searches that incorporated more specific search terms (and derivatives), such as ‘efficacy,’ ‘safety,’ ‘convenience,’ ‘preparation,’ and ‘cost.’ This approach yielded a literature base encompassing a diverse spectrum of clinical settings, including anesthesiology, hemodialysis, and emergency medicine, and clinical indications, such as hemophilia, cystic fibrosis, psoriasis, allergy, multiple sclerosis, acromegaly, rheumatoid arthritis, and diabetes.
3.2. Drug characteristics
A principal consideration for the use of prefilled syringes is their potential impact on drug characteristics and performance, such as pharmacokinetics (PK), stability, efficacy, and safety/tolerability. Few studies have directly assessed bioequivalence relative to medication in the traditional vial and syringe format. Using a single-dose, randomized, cross-over study design, Shafer et al. [Citation11] reported comparable PK and bioequivalence (90% CI for ratio of mean values of Cmax and AUCinf within the bound of 0.8–1.25) of moroctocog alfa for the treatment of hemophilia A when administered by prefilled syringe compared with traditional vials. Comparable PK and bioavailability have also been shown for SC administration by prefilled syringe, but relative to an autoinjector, for drugs such as belimumab [Citation16] and ixekizumab [Citation14] used in the treatment of systemic lupus erythematosus and plaque psoriasis, respectively, the interleukin-6 inhibitor sirukumab [Citation18] and a pegylated form of recombinant granulocyte colony-stimulating factor, pegfilgrastim [Citation17].
The stability of medications when provided in prefilled syringes is also important. A variety of different drugs/agents have been evaluated, including epinephrine [Citation21], insulin [Citation23], penicillin [Citation20], epoetin alfa [Citation24], netilmicin [Citation26], fluconazole [Citation19], bevacizumab [Citation22,Citation25], and hepatitis E vaccine [Citation27]. While the clinical indications, conditions of use, and treatment periods differ for these agents, the consensus of these studies was of good drug stability in the prefilled syringe format (), and confirmed relative to traditional vials in some cases [Citation19,Citation22,Citation24,Citation25]. Furthermore, Garg et al. showed favorable stability data of penicillin-type antibiotics in prefilled syringes. Amoxicillin, benzylpenicillin, and flucloxacillin all showed stability at 2–7 days. This time is substantially longer than the current shelf-life of 24 hours and supports a change of clinical practice where these agents can be prepared in advance by pharmacies, improving safety and workflow [Citation20]. Good drug stability in prefilled syringes has also been reported for U-500 regular insulin (≥ 28 days when refrigerated) [Citation23], epoetin alfa (up to 6 weeks when refrigerated) [Citation24], netilmicin (300 days at room temperature) [Citation26], fluconazole (77 days at room temperature) [Citation19], and bevacizumab (6 months when refrigerated) [Citation22]. Demonstration of a good drug stability profile also led some investigators to raise additional potential benefits from the use of prefilled syringes, including cost [Citation19,Citation21,Citation24], convenience/ease-of-use [Citation19,Citation23], and reduction in drug dosing errors [Citation24]. However, in the case of bevacizumab, there are contrasting reports on drug stability/quality in prefilled syringes relative to vials, with one study [Citation22] reporting no significant differences, and another study [Citation25] an increase in particle density that was not observed with vials. Although individual drugs/agents must each be appropriately evaluated, current experience supports the stability of drugs in prefilled syringes and their potential application for other therapeutics.
Table 2. Studies evaluating the chemical and/or physical stability of medications supplied in prefilled syringes.
Our review of the literature did not identify any relevant articles directly comparing drug efficacy between administration by prefilled syringe and traditional injection methods. However, several studies have reported efficacy data for treatments administered by prefilled syringe (compared with a comparator). Although these studies do not provide a direct comparison with traditional injection methods, they do demonstrate the general viability of use of prefilled syringes across several disease areas. For instance, in a phase IV, randomized, double-blind, controlled trial in patients with rheumatoid arthritis, significantly more patients treated with SC methotrexate by prefilled syringe than with oral methotrexate achieved a clinical response according to American College of Rheumatology (ACR) 20 (78% vs. 70%, p < 0.05) and ACR70 (41% vs. 33%, p < 0.05) criteria [Citation29]. Similarly, drug efficacy following SC injection by prefilled syringe has been shown for secukinumab relative to placebo (~70% vs. 0% showing a clinical response according to the Psoriasis Area Severity Index [PASI] 75 criterion, p < 0.0001) [Citation28], for ixekizumab compared with an autoinjector (mean PASI improvement 89.3% and 86.9%, respectively) [Citation14] in the treatment of plaque psoriasis, and for belimumab versus placebo in patients with systemic lupus erythematosus (Systemic Lupus Erythematosus Responder Index clinical response in 61.4% vs. 48.4%, odds ratio 1.68 [95% CI 1.25–2.25], p < 0.0006) [Citation33]. Studies of patients with neuroendocrine tumors, acromegaly, and those on dialysis as a result of chronic kidney disease further show the efficacy of therapeutic agents (e.g. somatostatin analogs and erythropoiesis-stimulating agents) delivered by SC or IV injection with prefilled syringes [Citation30–Citation32].
In addition to confirming efficacy, regulators require that comparable safety and tolerability relative to known drug safety profiles are demonstrated for drugs administered by prefilled syringe. The study by Braun et al. [Citation29] evaluating SC methotrexate administered by prefilled syringe found similar tolerability relative to oral methotrexate (adverse events in 66% vs. 62%, respectively). Reported safety data for prefilled syringe administration of belimumab [Citation16,Citation33], ixekizumab [Citation14], secukinumab [Citation28] and omalizumab [Citation35], an anti-IgE antibody used to treat asthma, and a liquid formulation of interferon beta-1a for the treatment of multiple sclerosis [Citation34], were consistent with the known safety profiles for these agents. Studies have also examined the immunogenicity of liquid drug formulations delivered by prefilled syringe. No psoriasis patients treated with secukinumab by prefilled syringe developed treatment-emergent antidrug antibodies in the work by Blauvelt et al. [Citation28], a finding that was also reported by Somerville et al. [Citation35] for patients with allergic asthma treated with SC omalizumab.
The use of prefilled syringes, which have a simplified process and reduced number and associated risk of system vulnerabilities compared with traditional self-filled syringes, has also been proposed as a measure to improve safety for perioperative anesthesiology medication [Citation36].
3.3. Treatment delivery
Data from studies in different fields highlight that prefilled syringes can help improve the speed of infusion [Citation37–Citation44] and the number of dosing errors [Citation37,Citation45,Citation46] when compared with infusions done using traditional ampoules. It should be noted that many of these studies were performed in the critical care field, where reducing infusion time and dosing errors is of key importance. Moreover, the preparation steps required for time-critical injections such as those employed in the context of critical care, make up a larger amount of the total infusion time when compared with prolonged infusions applicable to SC IgRT. Although the reduction of administration time may be less critical for patients on SC IgRT compared with patients in an acute care setting, employing prefilled syringes to reduce the number of preparation steps and total time spent on the infusion could also conceivably reduce dosing errors and drug waste while improving patients’ satisfaction with treatment and adherence.
One consideration is the increased packaging used for prefilled syringes. Relative to packaged vials, packaged prefilled syringes are typically more voluminous and require larger storage space. These issues may be particularly pertinent in the clinical setting where transportation and storage of large numbers of prefilled syringes is necessary (with potential implications on costs).
3.4. Patient experience
Improving patients’ treatment experience is perhaps one of the single most important goals in the development of new approaches for drug delivery. Prefilled syringes are recognized to offer several potential benefits, including improved choice, convenience, ease-of-use, and satisfaction with treatment. For many patients, the benefits of prefilled syringes also support the transition from hospital-based treatment to home-based self-administration, which can have a positive impact on quality of life, a foremost concern of patients. presents an overview of findings regarding patient and physician/nurse experience with prefilled syringes [Citation16,Citation28,Citation50–Citation56].
Table 3. Summary of patient and healthcare professional experience with the use of prefilled syringesa.
In an early open-label study of patients with diabetes treated with insulin, patients switching to administration by prefilled syringe (n = 64) almost uniformly reported that the prefilled syringe was convenient and easy to use (98%), required little time to use at home (95%) and away from home (89%), and wished to continue using a prefilled syringe (91%) [Citation52]. Furthermore, significantly more patients were not worried about self-injecting after using the prefilled syringe for 4 weeks compared with baseline (87.5 vs. 70.3%, p < 0.05). Similarly, patient-reported acceptability determined by a modular self-injection assessment questionnaire was high at the initiation of self-injecting with prefilled syringes (mean score 7–8 out of 10) and remained high but with an increasing trend (mean score 8–9) through 12–48 weeks of treatment in patients with plaque psoriasis [Citation28,Citation51]. Overall, patients were confident using prefilled syringes, found them easy to use, and were satisfied with administration by prefilled syringe [Citation51]. Reported acceptability of the prefilled syringe format was also high for subjects self-administering belimumab (n = 81), with device handling acceptable in 98%, the effort required to use the device acceptable in 90%, and injection time acceptable in 100% of subjects [Citation16]. In the setting of surgical anesthesia, Webster et al. [Citation56] reported that clinical usability of a new safety-orientated drug administration system utilizing prefilled syringes was significantly higher relative to conventional administration methods (8.5 [5.9–9.4] vs. 7.5 [3.2–9.8], median [range] on a scale of 0–10; p = 0.027). Clinical acceptability also favored the prefilled syringe (8.3 [6.0–9.9], p = 0.001, where a score > 5.0 favored the prefilled syringe system).
Further data support the positive patient perspective on the use of prefilled syringes (). Using a treatment device scenario design, Cimino et al. [Citation50] conducted a cross-sectional study of patients with hemophilia to evaluate patient preference for five different coagulation factor VIII reconstitution systems: four using a traditional vial and syringe approach, and one using a single dual-chambered prefilled syringe. Not only was patient preference for the prefilled syringe significantly higher than for the other systems/scenarios (71 vs. ≤ 50 for other systems, median preference on a scale 0–100; p < 0.001), but in practical testing, the prefilled syringe was preferred over current treatment in terms of ease of coagulation factor preparation (8.4 [1.6] vs. 6.5 [2.1], mean [standard deviation (SD)] rating on a scale 0–10, p < 0.001) and ease of device handling (7.8 [1.8] vs. 7.2 [2.1], mean [SD], p = 0.007) [Citation50]. Positive responses to the use of prefilled syringes have also been reported for patients with arthritis, highlighting the potential value of prefilled syringes in patients with manual dexterity issues [Citation55], as well as in patients with high levels of low-density lipoprotein cholesterol [Citation53]. Furthermore, these and other studies have also examined the perspectives of healthcare professionals (physicians and nurses) and carers; overall, responses to the use of prefilled syringes were positive, in terms of preference, usability, acceptability, and willingness to self-inject [Citation53–Citation55]. Self-administration by prefilled syringe was also reported to lead to a feeling of more independence and improved quality of life by 89.1% and 83.6% of patients, respectively, in the study by Striesow and Brandt [Citation55]. The incorporation of prefilled syringes into advanced delivery devices such as autoinjectors and autoinjection systems [Citation13,Citation68] offers further improvements in patient experience, making injections easy, safe, and convenient, while ensuring that the correct dose is always administered.
3.5. Economic impact
The overall economic impact and cost-effectiveness of introducing prefilled syringes as a replacement for traditional vials has been evaluated in a variety of clinical settings (). Relative to drug administration with a traditional vial and syringe, prefilled syringes are typically associated with a higher cost per unit [Citation41,Citation42,Citation56,Citation57,Citation62,Citation63] (), which may lead to increased treatment costs. For example, in the setting of anesthesia, switching from standard ampoules to commercial ephedrine prefilled syringes was estimated to increase drug costs by over €50,000 annually in a retrospective, observational study in 32 operating theaters [Citation57], and more than double weekly costs (£274.32 vs. £115.72/week) in a small, retrospective study in the United Kingdom [Citation62]. The increased unit cost of prefilled syringes represents a primary barrier to their utilization and widespread provision.
Table 4. Cost of administration by prefilled syringe compared with conventional methods (vial/ampoule).
Unlike the higher direct cost per unit versus vials, prefilled syringes may provide cost savings to the healthcare provider due to a number of factors. First, with prefilled syringes there is an expected cost saving due to the elimination of ancillary equipment required for drug preparation with vial-based injections, although this was not specifically assessed in the articles identified in our literature search, so more data are needed to assess contribution of ancillary supplies to the budget impact of prefilled syringes. Second, several studies have shown that prefilled syringes may result in less drug waste. Wazny et al. [Citation63] conducted a retrospective analysis of epoetin alfa drug costs for patients in Manitoba, Canada, after a province-wide switch from multidose vials to administration using prefilled syringes. Despite a higher unit cost ($15.68 vs. $14.73/1000 units), the average weekly drug cost per patient was 6.4% lower with prefilled syringes ($183.23 vs. $195.71). When extrapolated to the total study population, the annual cost saving was estimated to be over $500,000, which was attributed to lower drug wastage with prefilled syringes [Citation63]. Lower costs relative to vials, through a reduction in drug wastage, were also reported for the use of thiopental prefilled syringes in one UK hospital (£780 vs. £2036/year) [Citation61], and for ephedrine prefilled syringes for obstetrical anesthesia (€2.6 vs. €3.1/patient, over a two week period) [Citation58]. Similarly, a budget impact analysis by Benhamou et al. [Citation59] revealed that use of more costly atropine prefilled syringes in French operating theaters resulted in a potential annual budget saving of over €5 million (approx. €9 million vs. €14.3 million), which was attributed in part to a reduction in drug wastage [Citation59]. Third, the use of prefilled syringes may result in cost savings due to less nursing time being required. For example, in a French observational study [Citation60], the use of ephedrine prefilled syringes in a single operating theater reduced overall annual costs by approximately €2800 and was associated with saved nursing time, as well as improved safety. Savings in nursing time were also cited as a main contributor to the 50–70% reduction in costs reported with the use of heparin prefilled syringes for the treatment of deep vein thrombosis relative to traditional IV infusion (£4.80 vs. £9.52–16.81/day) [Citation38], and in large-scale vaccination programs (25–75% reduction in nursing cost) [Citation41,Citation42]. Finally, prefilled syringes may be associated with a reduction in medication errors and their associated costs. This was a key contributing factor to the significant cost savings (€9 million per year) reported by Benhamou et al. [Citation59] in their budget impact analysis of atropine prefilled syringes. Also, a small, prospective study by Webster et al. [Citation56] discussed that while drug costs for surgical anesthesia delivered using a prefilled syringe-based system were greater than with conventional methods (€178 [102–428] vs. €155 [111–390], median [range], p = 0.041), the higher cost may be offset by the expected reduction in number of medication errors [Citation56].
An additional reported cost associated with prefilled syringes is the increased packaging relative to packaged vials (approximately 20 times the volume), which necessitates substantially greater storage capacity and impacts directly on cost, especially where refrigeration is required [Citation42]. Relative to vials, increased costs associated with prefilled syringes, which include those contingent on bulkier packaging, have been reported in the setting of large-scale vaccination programs. This is illustrated by findings from a controlled, comparative study of influenza vaccination in two Canadian clinics that reported prefilled syringes increased overall costs by $709–$926, including $260 for refrigerated storage, per 1000 individuals immunized [Citation42]. Also, a time-motion study in the United States reported a $324 higher cost (of which $11.4 was attributed to storage) per 1000 immunizations with the use of prefilled syringes [Citation41]. However, the authors of both studies point out that the increased costs would be offset by significant economic savings in a pandemic situation.
Overall, the increased unit cost of prefilled syringes should not alone be a deterrent to their introduction as an alternative drug delivery option. Cost effectiveness and cost savings can be achieved. Several factors may influence this, including the clinical setting, scale of need, and expected benefits from the introduction of prefilled syringes. Importantly, the reduced risk of human dosing errors and drug contamination relative to reconstitution from vials may provide considerable benefit, both in terms of cost and patient well-being.
4. Role of education and training
While the studies discussed above present data on different aspects of drug administration using prefilled syringes, no studies were identified that specifically investigated the role of patient education and training in this area. In general, however, there is good evidence in the literature to support the overall importance of patient education, particularly in the context of treatment adherence [Citation69,Citation70].
The training required for administration using prefilled syringes will likely show similarities to existing training for vial-based products, as several steps required to perform the infusion (e.g. identifying and cleaning a suitable infusion site) will be identical. Specific differences would include the omission of steps such as transferring fluid from vials to syringes and the inclusion of instructions for performing syringe-to-syringe transfers (e.g., using a tip-to-tip connector) in case prefilled syringes do not match infusion pump requirements. Instructions highlighting the differences between the two methods could be valuable for patients switching to prefilled syringes from vial-based administration. Patients should work with their physicians to determine the best way to make dose adjustments using prefilled syringes (e.g. combining multiple syringes).
In most cases, fewer steps will be required for administration with prefilled syringes, which could simplify teaching and increase ease of use for patients [Citation71–Citation73], factors that are likely to have a positive impact on adherence and patient satisfaction with treatment. With a simpler infusion option for patients and a decreased number of follow-ups, this is likely to impact less on nurses’ time, allowing nurses to assist in other areas of their place of work.
5. Considerations for use of prefilled syringes for IgG replacement therapy
Medications provided in traditional vials require manual handling to prepare the drug for injection using a separate syringe. In the setting of SCIG self-administration, patients may be required to pool multiple vials or draw up IgG into a larger syringe prior to use with a pump. These tasks require confidence and dexterity and may be difficult for some patients, especially the young and elderly. Furthermore, IgRT is indicated in patients with secondary immunodeficiency diseases and other conditions, such as the autoimmune neuropathy chronic inflammatory demyelinating polyneuropathy, the manifestations of which may compromise dexterity. Prefilled syringes, in contrast, are easier to hold and eliminate the manual handling associated with drug preparation, simplifying the administration process, and requiring fewer supplies. As such, training patients in self-administration by prefilled syringe is also simplified. These factors are important considerations when a significant number of patients requiring IgRT will opt for home-based self-administration. A large number of patients indeed appear to opt for this form of administration, with data from the United Kingdom and Sweden showing approximately 80% of newly diagnosed patients opting for home-based IgRT [Citation7].
6. Conclusion
Administration of medications by prefilled syringe is an established and important treatment option that can offer significant advantages over the more traditional treatment administration methods (). Recently, there has been significant growth in the availability and use of prefilled syringes across a broad spectrum of clinical settings. This growth is predicted to continue and accelerate. While the literature describing direct clinical comparisons with traditional vial and syringe is not extensive, evidence from studies investigating a variety of drugs indicate that drug stability, efficacy, and safety are maintained when administered by prefilled syringe. Also, and despite potential cost implications in some settings, prefilled syringes require less drug preparation and nursing time, and may reduce drug-dosing errors. Moreover, clinical experience with prefilled syringes indicates they are preferred by patients, physicians, and nurses as an acceptable, convenient, easy to use administration system. Ultimately, this has the potential to improve patient satisfaction, independence, and quality of life. We anticipate that the benefits of prefilled syringes will also apply for patients who require IgRT.
7. Expert opinion
The perceived benefits of prefilled syringes compared with conventional administration methods are generally well established. In addition to device characteristics such as accurate dosing, sterility, and reduced contamination risk, prefilled syringes reduce treatment time and offer patients greater choice, convenience, and ease-of-use. Our review of the available evidence encompasses a variety of therapeutic indications describing clinical experience with prefilled syringes and supports this consensus. It was noteworthy, however, that despite their widespread clinical use we found a limited number of clinical studies in the literature reporting direct comparisons of prefilled syringes with more conventional administration approaches (e.g. vial and syringe).
For patients requiring IgRT, SC administration has improved available treatment options (). SCIG is a safe and well-tolerated alternative to IVIG with comparable efficacy. However, improvements can be made. Advances are needed to widen the accessibility of SCIG as a treatment option in patients who may find SC administration by traditional vial and syringe challenging. Supported by their widespread use in other clinical settings, the introduction of prefilled syringes represents an attractive alternative to the vial-based packaging for SCIG therapy. This is an important step toward the goal of individualized IgRT treatment regimens that can be tailored according to individual patient circumstances, with the goal of enhancing quality of life.
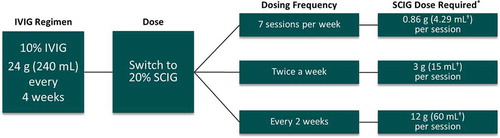
Figure 2. An example of a dosing regimen for PI switching from IVIG to SCIG based on a 1:1 dose conversion ratio.
A 60 kg patient currently on 24 g IVIG (0.4 g/kg) every four weeks equating to an infusion volume of 240 mL. A conversion rate of 1.37 should be applied when converting from IVIG to SCIG in the United States.*SCIG dose can be adjusted based on clinical response and serum IgG trough levels.†Volumes should be rounded to the nearest available syringe size or combination of syringes.
Abbreviations: IVIG: intravenous immunoglobulin; PI: primary immunodeficiency; SCIG: subcutaneous immunoglobulin.
Of significance, reductions in medication errors and drug wastage were reported with prefilled syringes. This is important for patients and healthcare providers alike. For the latter, these reductions have implications for overall costs and on the decision-making process for the provision of treatment in the prefilled syringe format. In our opinion, the higher unit cost of prefilled syringes (relative to vials) is mostly responsible for the widely held belief that treatments administered via prefilled syringes are more expensive than conventional approaches. While cost is a key consideration and may be a barrier to more widespread introduction of prefilled syringes, unit cost of prefilled syringes continues to fall with increasing demand. Moreover, evidence from various clinical settings outlined here demonstrates that the introduction of prefilled syringes cannot only be cost effective, but cost saving. However, this is not the case for all clinical settings examined and serves to illustrate the need for robust cost-effectiveness studies on a case-by-case level to convince healthcare providers and payers of the added value provided by prefilled syringes, such as SC IgRT.
Studies identified in this review show that drugs delivered by prefilled syringe are comparable with those delivered by conventional administration methods for parameters such as stability, pharmacokinetics, efficacy, and safety. Indeed, this is mandated by regulatory agencies before approval of alternative devices for drug delivery/administration. One of the key factors supporting the use of prefilled syringes for treatment administration is the perceived impact on the drug delivery process. We found evidence from a variety of clinical settings confirming the anticipated reductions in drug preparation/treatment time with prefilled syringes. Notably, this included significant savings in nursing time, and associated cost benefits. Studies evaluating SCIG will be needed to confirm that such savings can be achieved in IgRT. From the stability data for monoclonal antibodies [Citation19,Citation22,Citation25], the use of prefilled syringes was not detrimental to the shelf life of these products. Similarly, current data suggest prefilled SCIG syringes have the same shelf-life as the vial-based product (e.g. IgPro20 has a shelf life of 30 months at 20–25°C in both vials and prefilled syringes) [Citation74]. The similar stability and increased convenience of prefilled syringes represents a significant advantage for Ig therapy, allowing patients increased convenience regarding their therapy.
The lack of studies investigating the impact of prefilled syringes specifically on health-related quality of life was unexpected. Rather, the studies we identified in our search were concerned more with patient/physician experience with prefilled syringes. Health-related quality of life has previously been investigated in the context of IVIG and SCIG therapy for IgRT; improvements in several important aspects have been observed for home-based SCIG, relative to hospital-based IVIG. This supports steps, for example the introduction of prefilled syringes that can help make SCIG more widely accessible. A simpler IgRT infusion method could benefit patients with low dexterity and help patients administer this at home, reducing time spent at hospital. This could also result in fewer patient follow-ups with nurses, leading to reduced healthcare costs. There is a need, therefore, for robust studies evaluating health-related quality of life relating to the use of prefilled syringes in SCIG. The outcomes of such studies, and further developments in the area of SC administration, will be of significant interest.
Moving forward, we envisage continued and significant growth in the availability of prefilled syringes for an increasing number of treatments and therapeutic areas. The past few years alone has seen the introduction of many new entrants in the prefilled syringe market. In part, this will be driven by the growing demand for, and importance placed on, patient choice that is evident across the whole healthcare sector. Convenience, in particular, is also becoming a key attribute of treatments; this is what patients are asking for and can help to improve independence, satisfaction and quality of life. Such improvements are also likely to foster a positive impact on patient adherence.
Article highlights
Immunoglobulin G replacement therapy (IgRT) is the standard of care for patients with primary immunodeficiencies manifesting as antibody deficiencies, and subcutaneous administration is now an established and preferred treatment modality.
Prefilled syringes for drug administration are estimated to account for over one quarter of all injectables, with their use across a broad spectrum of clinical indications and settings increasing.
Available evidence demonstrates comparable stability, pharmacokinetics, bioequivalence, efficacy, and safety of drugs delivered by prefilled syringe relative to conventional vials.
Clinical experience with prefilled syringes shows reductions in preparation time, medication errors, and drug wastage; moreover, prefilled syringes are generally preferred by patients and healthcare professionals as an acceptable, easy to use, and convenient administration system.
Higher costs associated with prefilled syringes remain a potential barrier to their introduction in some clinical settings, but cost effectiveness/savings analyses are required to determine their practicality for defined conditions.
Prefilled syringes for IgRT have the potential to offer patients improved choice and satisfaction, and ultimately impact positively on patient independence and quality of life.
This box summarizes key points contained in the article.
Declaration of interest
AR Kafal is an employee of CSL Behring LLC. DC Vinh has served as an advisory board member for CSL Behring Canada, Astellas Canada, and Shire Canada; has received research support from CSL Behring Canada; and has received clinical trial support from CSL Behring Canada, Shire, and Cidara. MJ Langelier has served as an advisory board member for CSL Behring Canada. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they are a consultant for evolve, Korean Green Cross, Shire; an investigator for evolve, Korean Green Cross, Shire, Prometic, Octapharma, CSL Behring. Another reviewer on this manuscript has disclosed that they received an investigation grant from Grifols and a travel grant from CSL, LFB, biotest.
Additional information
Funding
References
- Picard, C., Bobby GH, Al-Herz W, et al. International union of immunological societies: 2017 primary immunodeficiency diseases committee report on inborn errors of immunity. J Clin Immunol. 2018;38(1):96–128.
- Albin S, Cunningham-Rundles C. An update on the use of immunoglobulin for the treatment of immunodeficiency disorders. Immunotherapy. 2014;6(10):1113–1126.
- Champi C. Primary immunodeficiency disorders in children: prompt diagnosis can lead to lifesaving treatment. J Pediatr Health Care. 2002;16(1):16–21.
- Wood P, UK Primary Immunodeficiency Network. Primary antibody deficiencies: recognition, clinical diagnosis and referral of patients. Clin Med (Lond). 2009;9(6):595–599.
- Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: A review of evidence. J Allergy Clin Immunol. 2017;139(3s):S1–S46.
- Koch F. [1st experiences with gamma-venin, an intravenously injectable gamma globulin preparation, in pediatrics]. Dtsch Med Wochenschr. 1963;88:282–285.
- Jolles S, Orange JS, Gardulf A, et al. Current treatment options with immunoglobulin G for the individualization of care in patients with primary immunodeficiency disease. Clin Exp Immunol. 2015;179(2):146–160.
- Chapel H, Gardulf A. Subcutaneous immunoglobulin replacement therapy: the European experience. Curr Opin Allergy Clin Immunol. 2013;13(6):623–629.
- Croom KF, McCormack PL. Recombinant factor VIIa (eptacog alfa): a review of its use in congenital hemophilia with inhibitors, acquired hemophilia, and other congenital bleeding disorders. BioDrugs. 2008;22(2):121–136.
- Frampton JE. Efmoroctocog Alfa: a review in haemophilia A. Drugs. 2016;76(13):1281–1291.
- Shafer, F., Charnigo RJ, Plotka A, et al. Assessment of relative bioavailability of two presentations of moroctocog alfa (AF-CC) in subjects with moderately severe or severe hemophilia A. Clin Pharmacol Drug Dev. 2015;4(3):237–241.
- Ingle RG, Agarwal AS. Pre-filled syringe - a ready-to-use drug delivery system: a review. Expert Opin Drug Deliv. 2014;11(9):1391–1399.
- Jezek J, Darton NJ, Derham BK, et al. Biopharmaceutical formulations for pre-filled delivery devices. Expert Opin Drug Deliv. 2013;10(6):811–828.
- Callis Duffin, K., Bagel J, Bukhalo M, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017;31(1):107–113.
- Chioato A, Noseda E, Colin L, et al. Bioequivalence of canakinumab liquid pre-filled syringe and reconstituted lyophilized formulations following 150 mg subcutaneous administration: a randomized study in healthy subjects. Clin Drug Investig. 2013;33(11):801–808.
- Struemper H, Murtaugh T, Gilbert J, et al. Relative bioavailability of a single dose of belimumab administered subcutaneously by prefilled syringe or autoinjector in healthy subjects. Clin Pharmacol Drug Dev. 2016;5(3):208–215.
- Yang BB, Morrow PK, Wu X, et al. Comparison of pharmacokinetics and safety of pegfilgrastim administered by two delivery methods: on-body injector and manual injection with a prefilled syringe. Cancer Chemother Pharmacol. 2015;75(6):1199–1206.
- Zhuang Y, de Vries DE, Marciniak SJ, et al. Absolute bioavailability and pharmacokinetic comparability of sirukumab following subcutaneous administration by a prefilled syringe or an autoinjector. Clin Pharmacol Drug Dev. 2017;6(6):570–576.
- Dentinger PJ, Swenson CF. Stability of reconstituted fluconazole oral suspension in plastic bottles and oral syringes. Ann Pharmacother. 2009;43(3):485–489.
- Garg A, Chan D, Ambados F, et al. Penicillin stability in prefilled syringes for the purpose of skin testing for drug allergy. J Allergy Clin Immunol Pract. 2015;3(4):599–601.
- Kerddonfak, S, Manuyakorn W, Kamchaisatian W, et al. The stability and sterility of epinephrine prefilled syringe. Asian Pac J Allergy Immunol. 2010;28(1):53–57.
- Khalili H, Sharma G, Froome A, et al. Storage stability of bevacizumab in polycarbonate and polypropylene syringes. Eye (Lond). 2015;29(6):820–827.
- Lull ME, Piacentino JJ, Traina AN. Stability of U-500 regular insulin in prefilled syringes. J Am Pharm Assoc. 2003;53(3):304–306.
- Naughton CA, Duppong LM, Forbes KD, et al. Stability of multidose, preserved formulation epoetin alfa in syringes for three and six weeks. Am J Health Syst Pharm. 2003;60(5):464–468.
- Palmer JM, Amoaku WM, Kamali F. Quality of bevacizumab compounded for intravitreal administration. Eye (Lond). 2013;27(9):1090–1097.
- Rigge DC, Jones MF. Shelf lives of aseptically prepared medicines–stability of netilmicin injection in polypropylene syringes. J Pharm Biomed Anal. 2004;35(5):1251–1256.
- Zhang X, Wei M, Sun G, et al. Real-time stability of a hepatitis E vaccine (Hecolin(R)) demonstrated with potency assays and multifaceted physicochemical methods. Vaccine. 2016;34(48):5871–5877.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172(2):484–493.
- Braun, J., Kastner P, Flaxenberg P, et al. Comparison of the clinical efficacy and safety of subcutaneous versus oral administration of methotrexate in patients with active rheumatoid arthritis: results of a six-month, multicenter, randomized, double-blind, controlled, phase IV trial. Arthritis Rheum.. 2008;58(1):73–81.
- Caron P, Beckers A, Cullen DR, et al. Efficacy of the new long-acting formulation of lanreotide (lanreotide autogel) in the management of acromegaly. J Clin Endocrinol Metab. 2002;87(1):99–104.
- Johanson V, Wilson B, Abrahamsson A, et al. Randomized crossover study in patients with neuroendocrine tumors to assess patient preference for lanreotide autogel((R)) given by either self/partner or a health care professional. Patient Prefer Adherence. 2012;6:703–710.
- Spinowitz B, Coyne DW, Lok CE, et al. C.E.R.A. maintains stable control of hemoglobin in patients with chronic kidney disease on dialysis when administered once every two weeks. Am J Nephrol. 2008;28(2):280–289.
- Stohl W, Schwarting A, Okada M, et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 2017;69(5):1016–1027.
- Phillips, JT, Rice G, Frohman E, et al. A multicenter, open-label, phase II study of the immunogenicity and safety of a new prefilled syringe (liquid) formulation of avonex in patients with multiple sclerosis. Clin Ther. 2004;26(4):511–521.
- Somerville L, Bardelas J, Viegas A, et al. Immunogenicity and safety of omalizumab in pre-filled syringes in patients with allergic (IgE-mediated) asthma. Curr Med Res Opin. 2014;30(1):59–66.
- Yang Y, Rivera AJ, Fortier CR, et al. A human factors engineering study of the medication delivery process during an anesthetic: self-filled syringes versus prefilled syringes. Anesthesiology. 2016;124(4):795–803.
- Adapa RM, Mani V, Murray LJ, et al. Errors during the preparation of drug infusions: a randomized controlled trial. Br J Anaesth. 2012;109(5):729–734.
- Barber ND, Hoffmeyer UK. Comparison of the cost-effectiveness of administering heparin subcutaneously or intravenously for the treatment of deep vein thrombosis. Ann R Coll Surg Engl. 1993;75(6):430–433.
- Elsey L. Service evaluation of a cystic fibrosis home intravenous antibiotic service provided by a NHS foundation trust. Arch Dis Child. 2016;101(9):e2.
- Helm C, Gillett M. Adrenaline in cardiac arrest: prefilled syringes are faster. Emerg Med Australas. 2015;27(4):312–316.
- Pereira CC, Bishai D. Vaccine presentation in the USA: economics of prefilled syringes versus multidose vials for influenza vaccination. Expert Rev Vaccines. 2010;9(11):1343–1349.
- Scheifele, DW, et al. Evaluation of ready-to-use and multi-dose influenza vaccine formats in large clinic settings. Can J Public Health. 2000;91(5):329–332.
- Souied E, Nghiem-Buffet S, Leteneux C, et al. Ranibizumab prefilled syringes: benefits of reduced syringe preparation times and less complex preparation procedures. Eur J Ophthalmol. 2015;25(6):529–534.
- Subhi Y, Kjer B, Munch IC. Prefilled syringes for intravitreal injection reduce preparation time. Dan Med J. 2016;63(4):A5214.
- Moreira ME, Hernandez C, Stevens AD, et al. Color-coded prefilled medication syringes decrease time to delivery and dosing error in simulated emergency department pediatric resuscitations. Ann Emerg Med. 2015;66(2):97–106.e3.
- Stevens AD, Hernandez C, Jones S, et al. Color-coded prefilled medication syringes decrease time to delivery and dosing errors in simulated prehospital pediatric resuscitations: A randomized crossover trial. Resuscitation. 2015;96:85–91.
- Makwana, S, Basu B, Makasana Y, et al. Prefilled syringes: an innovation in parenteral packaging. Int J Pharm Investig. 2011;1(4):200–206.
- Prentiss AS, Cockerel A, Butler E. Nurse perceptions and safety practices of the carpuject cartridge system. J Nurs Care Qual. 2016;31(4):350–356.
- Sacha G, Rogers JA, Miller RL. Pre-filled syringes: a review of the history, manufacturing and challenges. Pharm Dev Technol. 2015;20(1):1–11.
- Cimino E, Linari S, Malerba M, et al. Patient preference and ease of use for different coagulation factor VIII reconstitution device scenarios: a cross-sectional survey in five European countries. Patient Prefer Adherence. 2014;8:1713–1720.
- Gottlieb, AB, Blauvelt A, Prinz JC, et al. Secukinumab self-administration by prefilled syringe maintains reduction of plaque psoriasis severity over 52 weeks: results of the feature trial. J Drugs Dermatol. 2016;15(10):1226–1234.
- Plevin S, Sadur C. Use of a prefilled insulin syringe (Novolin prefilled) by patients with diabetes. Clin Ther. 1993;15(2):423–431.
- Roth EM, Bujas-Bobanovic M, Louie MJ, et al. Patient and physician perspectives on mode of administration of the PCSK9 monoclonal antibody alirocumab, an injectable medication to lower LDL-C levels. Clin Ther. 2015;37(9):1945–1954.e6.
- Seidl T, Trouillier HH. [Are 55 cents always better value than 90 cents for venous thromboembolism prophylaxis? Comparison of three low-molecular-weight heparins with regard to economics and guideline compliance]. Z Orthop Unfall. 2013;151(5):520–524.
- Striesow F, Brandt A. Preference, satisfaction and usability of subcutaneously administered methotrexate for rheumatoid arthritis or psoriatic arthritis: results of a postmarketing surveillance study with a high-concentration formulation. Ther Adv Musculoskelet Dis. 2012;4(1):3–9.
- Webster, CS, Merry AF, Gander PH, et al. A prospective, randomised clinical evaluation of a new safety-orientated injectable drug administration system in comparison with conventional methods. Anaesthesia. 2004;59(1):80–87.
- Armoiry X, Carry PY, Lehot JJ, et al. Estimated economic impact of pre-filled ephedrine syringes in the operating room. Acta Anaesthesiol Scand. 2016;60(7):917–924.
- Bellefleur, JP, Milhaud Y, Beconcini G, et al. [Use of ephedrine prefilled syringes reduces anesthesia costs]. Ann Fr Anesth Reanim. 2009;28(3):211–214.
- Benhamou D, Piriou V, De Vaumas C, et al. Ready-to-use pre-filled syringes of atropine for anaesthesia care in French hospitals - a budget impact analysis. Anaesth Crit Care Pain Med. 2017;36(2):115–121.
- Cregut-Corbaton, J, Malbranche C, Guignard MH, et al. [Economic impact of strategies using ephedrine prefilled syringes]. Ann Fr Anesth Reanim. 2013;32(11):760–765.
- Murdoch H, Jordan L, Tuckey J. Pre-filled thiopental syringes reduce cost and wastage whilst improving safety. Int J Obstet Anesth. 2012;21(4):384–385.
- Vipond A, de Mello W. Drugs used in anaesthetic emergencies: current practice and a cost analysis of prefilled syringes. Anaesthesia. 2000;55(3):303–304.
- Wazny, LD, Raymond CB, Do MK, et al. Reduced drug costs from switching hemodialysis patients from epoetin alfa in multidose vials to pre-filled syringes. Cannt J. 2009;19(3):39–41.
- Cuddihy RM, Borgman SK. Considerations for diabetes: treatment with insulin pen devices. Am J Ther. 2013;20(6):694–702.
- McCoy EK, Wright BM. A review of insulin pen devices. Postgrad Med. 2010;122(3):81–88.
- Guerlain S, Hugine A, Wang L. A comparison of 4 epinephrine autoinjector delivery systems: usability and patient preference. Ann Allergy Asthma Immunol. 2010;104(2):172–177.
- Visiongain. Prefilled syringe: world market outlook to 2025. London, UK: Visiongain; 2010.
- Kivitz A, Cohen S, Dowd JE, et al. Clinical assessment of pain, tolerability, and preference of an autoinjection pen versus a prefilled syringe for patient self-administration of the fully human, monoclonal antibody adalimumab: the TOUCH trial. Clin Ther. 2006;28(10):1619–1629.
- van Dulmen S, Sluijs E, van Dijk L, et al. Patient adherence to medical treatment: a review of reviews. BMC Health Serv Res. 2007;7:55.
- Devine EC. Meta-analysis of the effects of psychoeducational care in adults with asthma. Res Nurs Health. 1996;19(5):367–376.
- Ignaut DA, Schwartz SL, Sarwat S, et al. Comparative device assessments: humalog KwikPen compared with vial and syringe and FlexPen. Diabetes Educ. 2009;35(5):789–798.
- Lajara R, Guerrero G, Thurman J. Healthcare professional and patient perceptions of a new prefilled insulin pen versus vial and syringe. Expert Opin Drug Deliv. 2012;9(10):1181–1196.
- Shogbon AO, Ngo D, Jacob B, et al. Nurses’ perceptions and satisfaction with the use of insulin pen devices compared with insulin vial and syringes in an inpatient setting. Diabetes Technol Ther. 2014;16(11):742–746.
- CSL Behring. Data on file. 2018 [cited 2018 Oct 18]. Available from: https://www.ema.europa.eu/documents/product-information/hizentra-epar-product-information_en.pdf.