1. The learnt lesson: protein corona governs the fate of nanoparticles in the systemic circulation
The idea of using nanotechnology as drug delivery tool has been around for decades, with the exciting – yet perhaps simplistic – perspective that we could control the targeting and pharmacokinetics of nanoparticles (NPs) by manipulating their size and surface properties. Nevertheless, only a dozen years ago the scientific community had to take a serious step backward (or forward) in this regard, based on the fact that NPs, as any other physical entities having an active surface area, can interact with the surrounding environment [Citation1,Citation2]. This means that when NPs enter into the bloodstream a complex interaction with the native proteins begins immediately. As protein corona (PC) builds up rapidly on the surface of NPs, their original size and surface properties change. Thus, all efforts to chemically engineer the surface characteristics of NPs and tune in vivo targeting and pharmacokinetics become transient and can be voided by the formation of PC. In fact, it is often the external PC that determines the true ‘biological identity’ and the in vivo fate of the NPs, including aggregation, circulation time, clearance rate and targeting [Citation3].
Figure 1. A realistic and evidence-based vision of the behaviour of orally administered nanomedicines. A simplistic (a) vs a realistic and evidence-based (b) vision of the behavior of orally administered nanomedicines.
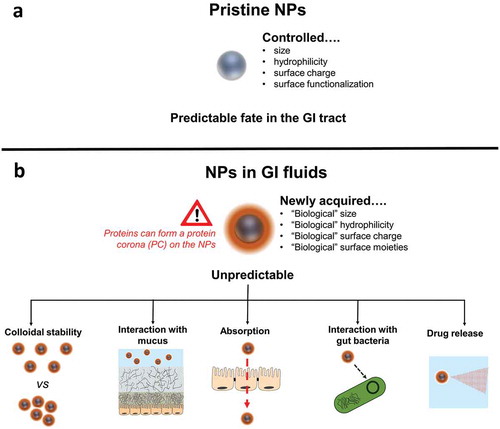
This is a learnt lesson and – as in many other cases – scientists have rapidly turned a challenge into an opportunity. For instance, not only formation, kinetics and dynamic nature of the PC have been investigated in the last few years, but also strategies to either minimize its building-up or exploit its use for targeting purposes have been developed [Citation4]. Nevertheless, the majority of studies on PC and colloidal stability of NPs are performed in blood or serum, thus only related to NPs administration by injection [Citation3,Citation5].
2. What about the protein corona in the gut?
The oral route is the preferred and so far most exploited modality of drug administration, considering that 70% of all medicines are given orally. Although many authors have studied the potential use of NPs in oral drug delivery, the complexity of the behavior of NPs in the gastrointestinal (GI) fluids has been massively underestimated. Orally delivered NPs need to cross: 1) the harsh GI environment (i.e. low pH and digestive enzymes); 2) the mucus barrier and then 3) the intestinal enterocyte lining before reaching the bloodstream [Citation5]. Scientists have focused so far on studying these three biological barriers only, without taking into consideration that NPs might completely change their physicochemical features even before reaching the mucosal and absorption sites in the gut. Indeed, in protein-rich GI fluids, a PC is likely to form on the NPs, affecting their surface characteristics (e.g. size and charge). This newly acquired biological identity of the NPs is expected to influence their colloidal stability and interaction with mucus and enterocytes. Thus, all efforts to understand the behavior of NPs downstream (i.e. NP-mucus interaction and intestinal absorption) are worthless if a prediction of what happens upstream has not been performed (i.e. formation of PC in GI fluids).
We believe that similar miscalculations done with parenteral delivery of NPs, i.e. to underestimate the complexity of NP-protein interaction in body fluids, should not be repeated. To predict the formation of PC on orally administered NPs and its impact on their in vivo performance could be even more challenging than for injected NPs. Indeed, while the composition of blood is quite homogenous, GI fluids are extremely complex and heterogeneous: pH ranging from 1 to 8, ionic strength between 10 and >200 mM, variable viscosity and presence of surface-active molecules such as bile components, digestive enzymes, food protein, lipids, and their degradation products. Moreover, the GI milieu is extremely dynamic and constantly changing in response to internal (i.e. secretions) and external stimuli (i.e. food/liquid ingestion) and subject to inter-individual and intra-individual variability [Citation6]. It is likely that the interaction of such complex fluids with NPs might result in PC formation and in many cases colloidal instability. Considering that PC and colloidal stability of NPs in oral fluids dictate their in vivo fate, this issue should be addressed seriously and appreciated in its complexity by nanotechnologists working in oral drug delivery. Please note that in this article we use the term ‘protein corona’ for simplicity; in fact, probably other biomacromolecules (i.e. peptides, bile salts, glycoproteins, etc.) get adsorbed on the surface of orally administered NPs.
A last point to consider is that it has been realized only recently that certain macroscopic oral dosage forms, i.e. tablets and capsules, do not actually perform as effectively as previously thought. This is because the complex physiology of GI tract has been underestimated and oversimplified [Citation7]. Let’s not repeat the same mistake with nanoscopic dosage forms.
2.1. What is it known so far?
Nanotechnologists in the field of food sciences have started to acknowledge the importance of PC on the fate and toxicity of ingested food-grade NPs [Citation8]. It has been shown that incubation of silver NPs in simulated gastric media containing pepsin can result in the formation of PC, which facilitates NP aggregation [Citation9,Citation10]. Similarly, PC can also build upon the surface of polystyrene NPs exposed to various digestive enzymes [Citation11]. Besides enzymes, food components can also become adsorbed on the surface of nanomaterials: indeed, Cao et al. have shown that complex protein-NP structures could be formed upon exposure of TiO2 NPs to casein [Citation12]. Moreover, Lichtenstein et al. highlighted that both in vitro digestion steps and presence of food components have an impact on PC formation, colloidal stability and in vitro intestinal uptake of silver NPs [Citation13]. Interestingly, even the interaction of nanosized food additives with GI bacteria can be influenced by the PC that forms in artificial GI fluids [Citation14].
In the field of nanomedicine and drug delivery, there is an even greater paucity of studies relative to the formation and impact of PC on the delivery of orally administered NPs. This is in stark contrast with the several hundreds of published articles relative to protein-NPs interactions in blood [Citation3]. It has been recently shown that high, but not low, pepsin concentrations can induce aggregation of lipid NPs, hindering the delivery of encapsulated siRNA to Caco-2 cells [Citation15]. Di Silvio et al. demonstrated that upon simultaneous in vitro digestion of magnetite NPs and bread, a PC builds upon the NPs. These PC-enriched NPs could be better absorbed by Caco-2 cells than bare NPs, demonstrating that the fate of ingested NPs is affected by the PC [Citation16]. Finally, we have shown in two recent works that viral NPs, a class of protein cages suitable for drug delivery, do not form a detectable PC upon incubation with pepsin enzyme, casein, and pig gastric and intestinal fluids, owing to the particular physicochemical features of these nanomaterials [Citation17,Citation18].
3. Expert opinion
The aim of this editorial is to encourage a more realistic and evidence-based vision of the behavior of orally administered nanomedicines ().
It should be highlighted that NPs are unlikely to exist in the GI fluids as bare; but rather NPs will rapidly acquire a new biological and physicochemical identity upon adsorption of biomolecules on their surface. With this vision in mind, scientists should consider that all biological responses of orally administrated NPs would be dictated by the PC imprint, rather than by the original features of the NPs. This also implies that all in vitro studies of colloidal stability, mucus-penetration and absorption of NPs will not be biologically relevant if performed using bare, i.e. corona-free, NP substrates. Thus, we stress on:
the need to systematically test colloidal stability of nanoformulations in bio-relevant GI fluids containing native biomolecules and/or food components. The formation of a PC in these oral media could promote stability or, on the contrary, aggregation of the NPs at the various pH of GI tract. Ideally, such tests of colloidal stability should be performed using animal (or human if possible) excised GI fluids as reference media rather than in vitro. If in vitro models are used, the choice of a single standardized medium is discouraged. Rather, a variety of media reflecting the heterogeneous composition of the fluids in vivo (with variable pH, qualitative and quantitative protein and amphiphilic molecules composition) should be employed.
the importance to study the drug release from oral nanodelivery systems in presence of PC. Indeed, it has been demonstrated that drug release from NPs can be modulated by the PC formed by serum protein [Citation19–Citation22]. This could also occur in the GI fluids.
the necessity to pre-incubate custom oral nanoformulations in bio-relevant media before testing their biological propensity to cross, for instance, GI mucus and epithelium barriers. This pre-incubation would render the size, shape and surface characteristics of NPs more similar to those that they have in vivo.
the recommendation to use approaches based on design of experiment (DoE) to unravel the complex relationships between 1) NPs physicochemical properties (i.e. size, shape, surface charge and hydrophilicity), 2) physiological parameters (pH, food matrix, and enzymatic and pancreatic secretions) and 3) PC formation and colloidal stability in GI fluids. DoE has already been successfully used to underpin the influence of NPs size and surface charge on the composition of PC in serum [Citation23].
We also envisage that a deeper understanding of the PC formed on NPs in GI fluids could open the venue for engineering new bioinspired NPs: for example, harnessing the surface of NPs with coatings of either food components or native GI proteins could improve their oral biocompatibility. In this regard, a recent study has shown that pre-coating of insulin-loaded liposomes with a corona of bovine serum albumin (BSA) could improve their mucus-penetration and intestinal absorption ability [Citation24].
Overall, a comprehension of the evolution of the NPs in terms of PC formation and colloidal stability upon their voyage through the GI tract will strongly support the development of effective nanotechnology systems for oral drug delivery.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewers Disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Norde W. My voyage of discovery to proteins in flatland …and beyond. Colloids Surf B Biointerfaces. 2008;61:1–9.
- Ke PC, Lin S, Parak WJ, et al.A decade of the protein corona. ACS Nano 2017;11:11773–11776.
- Docter D, Westmeier D, Markiewicz M, et al.The nanoparticle biomolecule corona: lessons learned–challenge accepted? Chem. Soc Rev 2015;44:6094–6121.
- Cai R, Chen C. The crown and the scepter: roles of the protein corona in nanomedicine. Adv Mater. 2018; 1805740.
- Malhaire H, Gimel J-C, Roger E, et al. How to design the surface of peptide-loaded nanoparticles for efficient oral bioavailability? . Adv. Drug Deliv Rev. 2016;106:320–336.
- McConnell EL, Fadda HM, Basit AW. Gut instincts: explorations in intestinal physiology and drug delivery. Int J Pharm [Internet]. 2008;364:213–226. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18602774
- Koziolek M, Grimm M, Schneider F, et al. Navigating the human gastrointestinal tract for oral drug delivery: uncharted waters and new frontiers. Adv Drug Deliv Rev. 2016;101:75–88.
- McClements DJ, Xiao H Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. Npj Sci Food 2017;1:6.
- Ault AP, Stark DI, Axson JL, et al.Protein corona-induced modification of silver nanoparticle aggregation in simulated gastric fluid. Environ Sci Nano 2016;3:1510–1520.
- Pinďáková L, Kašpárková V, Kejlová K, et al. Behaviour of silver nanoparticles in simulated saliva and gastrointestinal fluids. Int J Pharm. 2017;527:12–20.
- Wang Y, Li M, Xu X, et al. Formation of protein corona on nanoparticles with digestive enzymes in simulated gastrointestinal fluids. J Agric Food Chem. 2019;67:2296–2306.
- Cao X, Han Y, Li F, et al. Impact of protein-nanoparticle interactions on gastrointestinal fate of ingested nanoparticles: not just simple protein corona effects. NanoImpact. 2019;13:37–43.
- Lichtenstein D, Ebmeyer J, Knappe P, et al.Impact of food components during in vitro digestion of silver nanoparticles on cellular uptake and cytotoxicity in intestinal cells. Biol Chem 2015;396:1255–1264.
- Siemer S, Hahlbrock A, Vallet C, et al.Nanosized food additives impact beneficial and pathogenic bacteria in the human gut: a simulated gastrointestinal study. Npj Sci Food 2018;2:22.
- Ball RL, Bajaj P, Whitehead KA Oral delivery of siRNA lipid nanoparticles: fate in the GI tract. Sci Rep 2018;8:2178.
- Di Silvio D, Rigby N, Bajka B, et al. Effect of protein corona magnetite nanoparticles derived from bread in vitro digestion on Caco-2 cells morphology and uptake. Int J Biochem Cell Biol. 2016;75:212–222.
- Berardi A, Baldelli Bombelli F, Thuenemann E, et al. Viral nanoparticles can elude protein barriers: exploiting rather than imitating nature. Nanoscale. 2019; 11:2306–2316.
- Berardi A, Evans DJ, Baldelli Bombelli F, et al.Stability of plant virus-based nanocarriers in gastrointestinal fluids. Nanoscale. 2018;10:1667–1679.
- Behzadi S, Serpooshan V, Sakhtianchi R, et al. Protein corona change the drug release profile of nanocarriers: the “overlooked” factor at the nanobio interface. Colloids Surf B Biointerfaces. 2014;123:143–149.
- Chakraborty D, Mohan L, Alex SA, et al.Bimetallic gold nanorods with enhanced biocorona formation for doxorubicin loading and sustained release. Biomater Sci 2019;7:63–75.
- Peng Q, Wei X-Q, Yang Q, et al.Enhanced biostability of nanoparticle-based drug delivery systems by albumin corona. Nanomedicine. 2015;10:205–214.
- Al-Ahmady ZS, Hadjidemetriou M, Gubbins J, et al. Formation of protein corona in vivo affects drug release from temperature-sensitive liposomes. J Control Release. 2018;276:157–167.
- Mahmoudi M, Sheibani S, Milani AS, et al.Crucial role of the protein corona for the specific targeting of nanoparticles. Nanomedicine. 2015;10:215–226.
- Wang A, Yang T, Fan W, et al. Protein corona liposomes achieve efficient oral insulin delivery by overcoming mucus and epithelial barriers. Adv Healthc Mater. 2018;1801123.
