ABSTRACT
Background: This study compared preference ratings of women with infertility and nurses before and after simulated injection, and handling errors, with the GONAL-f®, Bemfola®, Ovaleap® and Rekovelle® pen injectors.
Research design and methods: Injector-naïve women and injector-experienced fertility nurses tested injectors with masked labels in a randomized testing order. Injections were made into a foam pad and injectors were rated before and after use. Handling errors were recorded during the study. Ratings and errors were compared between pen injectors using ordinal or Poisson linear mixed models adjusted for testing order.
Results: 120 women and 60 nurses participated. All participants tested GONAL-f and Bemfola injectors. Because of their similarity, participants tested either Rekovelle (71 women; 30 nurses) or Ovaleap (49 women; 30 nurses) injectors. The ratings from women were higher for GONAL-f vs other injectors after simulated use (p < 0.001 for all comparisons). Fertility nurses rated GONAL-f injector higher than other injectors both before and after use (p < 0.01 for all comparisons). Adjusted rates of total handling errors were lower with the GONAL-f vs other injectors (p < 0.001 for all comparisons) for both groups.
Conclusions: GONAL-f injector was rated significantly higher than other injectors, which may be related to less handling errors observed with the GONAL-f injector.
1. Introduction
An essential part of controlled ovarian stimulation (COS) is administration of gonadotropins such as recombinant human follicle-stimulating hormone (r-hFSH). Women undergoing COS as part of assisted reproductive technology (ART) treatment are generally required to self-inject gonadotropins using an injection device. Pen injectors are commonly used for delivery of gonadotropins in ART treatment; however, self-injection and fear of potential errors are often causes of anxiety and emotional distress and might even cause treatment discontinuation [Citation1,Citation2]. Furthermore, handling errors (such as wrong dose injected) can lead to over- or under-medication, which may result in adverse events or delays in treatment. Therefore, it is important that treatment delivery method is patient-friendly and safe/easy to use.
There are a lot of factors that contribute to the safety of treatment delivery that need to be considered when selecting or using an injection device (i.e. accuracy, ease of use, handling errors, preference, etc.). Currently available r-hFSH pen injectors are not identical and there are functional differences between them. For example, the GONAL-f® (Merck KGaA, Germany) pen injector is multi-use, whereas the Bemfola® (Gedeon Richter PLC, Hungary) pen is a single-use disposable device. Ovaleap® (Theramex, UK) is a reusable pen injector with cartridge. The Rekovelle® (Ferring Pharmaceuticals Ltd, UK) pen injector tested in this study was also reusable and contained a cartridge (a new multi-dose Rekovelle pen injector has since been released in some markets; however, it was not available at the time of testing). In addition, the injection button for the GONAL-f and Bemfola injectors is on the top of the pen and the same button is also used to set the dose, while for the Rekovelle and Ovaleap pens the injection button is on the side with a separate dose-setting knob on the top of the device.
The first GONAL-f pen injector was approved in Europe in 2011 and since then it has been redesigned based on feedback from human factors engineering evaluations to improve robustness, handling, and readability [Citation3]. The redesigned GONAL-f pen injector was evaluated by women with recent or current infertility requiring ART or in-vitro fertilization (IVF) and fertility nurses, and the device was considered easy to use [Citation4,Citation5,Citation6]. Furthermore, an estimated 16,525,975 treatment cycles have been conducted since GONAL-f r-hFSH first gained marketing authorization for the stimulation of multifollicular development in patients undergoing medically assisted reproduction. Assuming a conservative live-birth rate estimate of around 20% per cycle, this represents 3.3 million babies born with the support of GONAL-f [Citation4,Citation7].
Previous studies comparing pen injectors (including GONAL-f, Bemfola and Puregon® [Merck Sharp & Dohme Ltd, UK]), assessed patient preferences using a questionnaire-based evaluation and reported that the Bemfola pen injector received the highest rating [Citation8,Citation9]. A questionnaire-based study is a widely used assessment method for patient-centered evaluation of devices and can be based either on patient recall of previous handling experience or on the actual use of the device during the study [Citation10].
Patient preference is of growing importance for fertility treatment owing to the emphasis on patient-centered care and treatment individualization [Citation11,Citation12,Citation13,Citation14,Citation15,Citation16]. Studies evaluating which aspects of pen injectors are most important to patients, and also how patients approach processes related to the use of pen injectors, are of interest and should employ the most optimal methods to compare different devices from the patient perspective. The opportunity to handle pen injectors during the study, as well as evaluation of errors that occur during the handling, should be an integral part of any study evaluating and comparing different pen injectors.
No comparative evaluations including the Rekovelle and Ovaleap pen injectors have been published to our knowledge. This study compared handling errors and preference ratings before and after use of the GONAL-f, Ovaleap, Bemfola, and Rekovelle pen injectors tested by women with infertility and fertility nurses in Germany, Poland, and the UK. Such evaluation of preferences and assessment of potential errors should aid in selecting the optimal pen injector for administration of r-hFSH.
2. Materials and methods
2.1. Study design
This was a simulated-use study conducted in Germany, Poland, and the UK between November 2017 and February 2018. Three research companies were involved in conducting this study: Leyhausen (Leverkusen, Germany), Medicys (Sittingbourne, UK) and Inquiry Market Research (Warsaw, Poland). The survey and testing of the pen injectors took place over 2 days in a central location in each country: 28–29 November 2017 in Frankfurt (Germany), 6–7 February 2018 in London (UK) and 15–16 February 2018 in Warsaw (Poland). The whole process of the study, including the research companies in each country, was supervised by a fourth independent market research company, Ifop (France). The moderators of the study were independent market researchers from the research companies. The sponsoring company was not involved in the moderation.
Testing was performed in specially equipped market research facilities in each country. The protocol was approved internally by the sponsors before the study started. Institutional Review Board or Independent Ethics Committee approval was not required because this was a nonclinical, simulated-use study, with all injections performed into injection pads. The participants were not aware of the company sponsoring the study, or of the drugs with which the fertility pen injectors were intended for use. The study was supervised by a qualified nurse (different to the nurses participating in the study) to ensure that all the procedures were carried out safely. Prior to the testing of the pen injectors, all participants signed a consent form to take part in the study.
2.2. Study population
Overall, 120 women with infertility and 60 fertility nurses were recruited: 40 women and 20 nurses per country. Women with infertility and fertility nurses were recruited separately: women were recruited through consumer panels and also by snowball sampling and nurses were recruited through specialized health-care professional panels. There was no interaction between women with infertility and fertility nurses during the study and both groups were interviewed separately. Women with infertility were eligible if they intended to undergo ovarian stimulation and had no previous experience of pen injectors, whether for fertility or for other disorders. Nurses were eligible if they had at least 1 year of experience in training women with infertility on pen injectors for fertility treatments.
2.3. Materials
Participants received pen injectors with masked labels in a random order to limit selection bias and prevent accidental bias. Brand names were also blinded on the instruction for use (IFU) of the corresponding pen injectors; however, other visual differences could not be masked. All of the participants received the GONAL-f and Bemfola pen injectors; however, as the Rekovelle and Ovaleap pen injectors were similar in their appearance and handling, a 50:50 allocation was planned so that half of the participants evaluated the Rekovelle pen injector and the other half evaluated the Ovaleap pen injector.
2.4. Testing procedure
Upon receiving the pen injector, participants first evaluated the device without testing it and completed a close-ended questionnaire with ratings from 1 to 10 (Likert 10-point scale) regarding initial impressions of the pen injector (Questionnaire 1, Supplementary Appendix 1 and 2). Participants were then instructed to test the pen injectors by preparing them for use and performing a single injection into a foam pad following the IFU. The moderator was only allowed to give instructions if the participant had made mistakes that made continuing the test impossible. The use steps (based on the respective IFUs) conducted by the participants during the testing of the pen injectors were: Step 1 – Preparing the pen injector for use, Step 2 – Inserting/changing the cartridge (only for the Rekovelle and Ovaleap pen injectors), Step 3 – Attaching the needle, Step 4 – Priming, Step 5 – Setting the dose, Step 6 – Giving the injection, and Step 7 – Removing the needle after the injection.
The doses the participants were asked to inject were 62.5 IU for the GONAL-f, Bemfola and Ovaleap pen injectors and 9.66 μg for the Rekovelle pen injector. The difference between doses with the Rekovelle injector versus the other pen injectors were due to different dial settings: graduations on the Rekovelle injector were expressed in µg, whereas for the other three pen injectors graduations were expressed in IU.
During the testing of the pen injector, participants verbally commented on their actions and reported any issues they were encountering. They were given approximately 45 min to test and compare three pen injectors (~10 min per pen injector and ~10 min for comparison). The moderator made a detailed note of the entire process on an observation sheet and recorded any handling errors that participants made. After testing the pen injector, participants completed a close-ended questionnaire with ratings from 1 to 10 regarding impressions of the pen injector after the testing (Questionnaire 2, Supplementary Appendix 1 and 2).
For the women with infertility, the pre-test questionnaire consisted of nine questions and the post-test questionnaire consisted of ten questions, of which seven were the same for both questionnaires (‘Overall impression/evaluation,’ ‘Ease to prepare the device for injection,’ ‘Ease to select the right dosage,’ ‘Confidence of delivering the correct dose,’ ‘Ease to perform the actual injection,’ ‘Confidence that the user can inject without injury,’ ‘Is a device I would like to use’). Two questions were only asked before testing (‘Overall appearance’ and ‘Intuitiveness of overall use’) and two questions were only asked after testing (‘Ease to prime the pen, i.e. to chase air bubbles’ and ‘Ease to correct the dosage in case of need’; Supplementary Appendix 1). For one question (‘Easy to learn to use’) asked only after the testing, no data were collected; therefore, this question was not included in the analysis. For nurses, the questions were the same as for the women with infertility, except two additional questions (‘Seems easy to teach to patients’ and ‘Is a device I would recommend to patients’) that were asked both before and after testing. Once participants had tested three pen injectors they were asked to rank their preferred pen injector (first, second or third choice) for the following questions: ‘Overall handling,’ ‘Overall feeling on safe handling,’ ‘Dosage selection and correction,’ ‘Performing the injection’ and ‘Overall preference’ (Questionnaire 3, Supplementary Appendix 1 and 2). A three-point ordinal scale for first, second, and last choice was used and the rank score was constructed (1 for third choice, 2 for second choice, and 3 for first choice).
2.5. Assessments
According to the protocol, moderators noted at which use step(s) each individual participant made an error. Categorization of the handling errors (performed retrospectively; not part of study protocol) was based on the internal Merck risk management reports (Human Factors Risk Management Report for Fertility Family of Pens and Assessment of Harms and Severity Ratings for Fertility Family of Pens). For the purpose of the post-hoc statistical analysis, errors were categorized according to their severity (ranging from Type-1 [inconvenience] to Type-10 [death] [Citation17]) and also analyzed per use step (Step 1–Step 7). Severity rating and the corresponding harm description of the different types of errors are presented in .
Table 1. Severity ratings and harm description for the handling errors [Citation17].
2.6. Statistical methods
All statistical analyses were post-hoc since no statistical plan was predefined prior to conducting the study. Statistical analyses were conducted using R version 3.5.1 and were run separately for women with infertility and fertility nurses. To compare the preference ratings before (Questionnaire 1) and after (Questionnaire 2) the testing of the pen injector within each participant group, a Cumulative-Link Mixed Model (CLMM) was applied (‘clmm’ function from R package ‘ordinal’ version 2018.8–25). Model specification was as follows: random effect was individual nested within pen testing sequence (i.e. which pen injector was tested first, second or third for each individual) and an unstructured correlation matrix was used for the repeated measurements within individuals; fixed effects terms included the randomized block (i.e. testing order of pens to which different individuals were assigned), pen, time (as a class effect: before vs after testing) and time-by-pen interaction. Testing order was considered as a fixed effect as all possible order combinations of pens were included in the study design (e.g. GONAL-f, Bemfola, Rekovelle; GONAL-f, Bemfola, Ovaleap; etc.). The model was run using a logit link function, flexible thresholds, and the Laplace approximation. Estimated marginal mean scores and associated 95% confidence intervals (CIs) were calculated from the CLMM model using the ‘emmeans’ function from the R package ‘emmeans’ version 1.2.4. To analyze the rank scores after testing (Questionnaire 3, 3-point ordinal scale for first, second and third choice) the same CLMM model specification as above was applied, except that fixed effects included only randomized block and pen.
To evaluate handling errors with each pen injector, a Poisson Generalized Linear Mixed Effects Model (GLMM) was applied (‘glmmTMB’ function from the R package ‘glmmTMB’ version 0.2.2.0). Model specification was as described previously. Fixed effects terms were randomized block and pen. Adjusted error incidence rates, incidence rate differences (IRDs, GONAL-f vs other pens) and associated 95% CIs were calculated from the GLMM using the ‘emmeans’ function from the R package ‘emmeans’ version 1.2.4. Comparisons were reported as IRDs rather than incidence rate ratios if there were zero errors in at least one comparator arm. In the event that mixed-models failed to converge, back-up unadjusted analyses were computed: descriptive medians and Wilcoxon Signed-Rank tests for GONAL-f versus other pen comparisons were planned for questionnaire measures; error incidence rates and exact 95% CIs assuming a Poisson distribution (‘pois.exact’ function from R package ‘epitools’ version 0.5–10), IRDs, 95% CIs, and p-values for the differences vs GONAL-f using the normal approximation to the Poisson distribution (‘ratedifference’ function from R package fmsb version 0.6.3) were planned for the handling error measures. All analyses were exploratory meaning that reported p-values were nominal and unadjusted for multiple comparisons.
3. Results
3.1. Women with infertility
From the 120 women enrolled in the study, all of them tested the GONAL-f and Bemfola pen injectors, 71 tested the Rekovelle pen injector and 49 tested the Ovaleap pen injector. While 50:50 allocation split was achieved in Poland, it was not achieved in Germany and the UK due to an allocation error (26 women with infertility tested the Rekovelle and 14 tested the Ovaleap pen injectors in Germany and 25 women with infertility tested the Rekovelle and 15 the Ovaleap pen injectors in the UK). The majority (92/120, 77%) of women participating in this study were 31–45 years old.
3.1.1. Preference ratings
Before testing, mean ratings for the ‘Overall impression’ from women with infertility were similar between the pen injectors (adjusted mean [95% CI] ratings were 7.00 [6.61, 7.39] for the GONAL-f pen injector, 6.80 [6.39, 7.21] for the Bemfola pen injector; 7.24 [6.72, 7.76] for the Rekovelle pen injector and 6.79 [6.13, 7.45] for the Ovaleap pen injector; p > 0.05 for all comparisons vs GONAL-f; , Supplementary Table 1). After testing, the GONAL-f pen injector received significantly higher ratings for overall impression compared with the other three pen injectors (adjusted mean [95% CI] ratings were 7.85 [7.51, 8.20] for the GONAL-f pen injector; 6.14 [5.68, 6.60] for the Bemfola pen injector, 5.44 [4.76, 6.11] for the Rekovelle pen injector and 4.06 [3.23, 4.89] for the Ovaleap pen injector; p < 0.001 for all comparisons vs GONAL-f; , Supplementary Table 1).
Figure 1. Adjusted mean ratings before and after testing the pen injector – women with infertility.
Data presented are adjusted marginal means derived from a cumulative-link mixed model with logit link function and flexible thresholds. Fixed effects were pen type, time, and testing order of pens (as covariate) with interaction term of pen-by-time. Random effects were by pen testing sequence within individual participant.
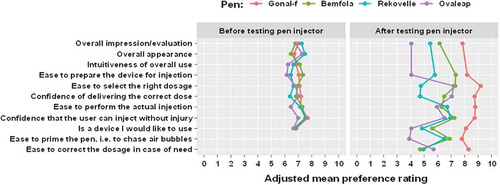
For the questions asked before the testing only (‘Overall appearance’ and ‘Intuitiveness of the overall use’), the ratings were similar across all the pen injectors (Supplementary Table 1). For the questions asked after the testing only (‘Ease to prime the pen, i.e. chase air bubbles’ and ‘Ease to correct the dosage in case of need’), the GONAL-f pen injector received significantly higher ratings compared with the other pen injectors (adjusted mean [95% CI] ratings for the question ‘Ease to prime the pen, i.e. chase air bubbles’ were 7.78 [7.37, 8.19] for the GONAL-f pen injector, 6.90 [6.42, 7.38] for the Bemfola pen injector, 6.51 [5.84, 7.18] for the Rekovelle pen injector and 3.87 [3.05, 4.69] for the Ovaleap pen injector; p < 0.01 for all comparisons vs GONAL-f; adjusted mean [95% CI] ratings for the question ‘Ease to correct the dosage in case of need’ were 8.28 [7.75, 8.81] for the GONAL-f pen injector, 4.67 [3.85, 5.49] for the Bemfola pen injector, 4.92 [3.85, 5.98] for the Rekovelle pen injector and 5.71 [4.57, 6.85] for the Ovaleap pen injector; p < 0.001 for all comparisons vs GONAL-f; Supplementary Table 1).
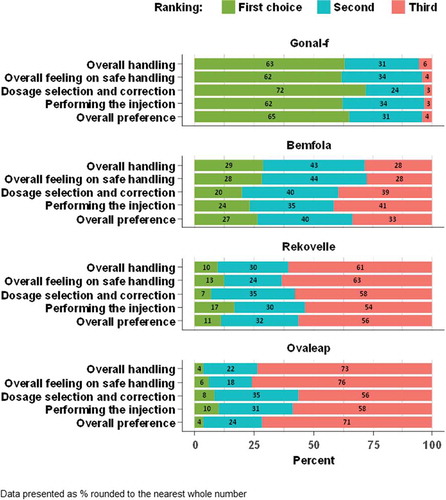
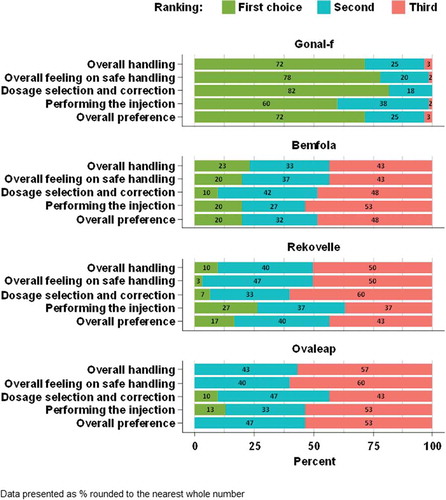
After the testing of the pen injectors, the GONAL-f pen was ranked as the first choice by the highest proportion of women with infertility for all questions (). For the overall preference, 78/120 (65%) of women ranked the GONAL-f pen as the first choice, 32/120 (27%) ranked the Bemfola pen as the first choice, 8/71 (11%) ranked the Rekovelle pen as the first choice and 2/49 (4%) ranked the Ovaleap pen as the first choice. On the basis of the adjusted mean rank score after testing, the GONAL-f pen injector was ranked significantly higher than other pen injectors for all questions (p < 0.001 for the comparisons on the ‘Overall handling,’ ‘Overall feeling on safe handling,’ ‘Dosage selection and correction,’ ‘Performing the injection,’ and ‘The overall preference’ for all pens vs GONAL-f; Supplementary Table 2).
3.1.2. Handling errors
Rates of total handling errors were significantly lower for the GONAL-f pen injector compared with the other three pen injectors (adjusted rate [95% CI] of total handling errors per woman were 1.02 [0.84, 1.20] for the GONAL-f pen injector, 1.64 [1.41, 1.87] for the Bemfola pen injector, 2.07 [1.68, 2.45] for the Rekovelle pen injector and 3.16 [2.50, 3.81] for the Ovaleap pen injector; p < 0.001 for all comparisons vs GONAL-f; ).
Table 2. Handling errors – women with infertility.
Similar trends were observed after a separate analysis was conducted for only Type-1 errors (defined as errors causing inconvenience but not disrupting the treatment) (). The most frequent individual Type-1 errors for each pen injector were: ‘Opening the pen injector’ with the GONAL-f pen injector (i.e. removing the cap; 56/120, 47%), ‘Unsure whether the dose has been delivered/unsure about the dose bar disappearing’ (as per IFU, the dosage knob had to be pushed slowly until it stopped and the grading on the dosage knob was not visible anymore) with the Bemfola pen injector (43/120, 36%), ‘Difficulties with instructions for inserting cartridge’ with the Rekovelle and Ovaleap pen injectors (19/71, 27% and 14/49, 29%, respectively), ‘Difficulties extending plunger’ with the Rekovelle and Ovaleap pen injectors (24/71, 34% and 14/49, 29%, respectively) (Supplementary Table 3).
Regarding Type-2 errors (defined as causing delay of the treatment), only one error was observed which was ‘Needle too tight’ for a woman using the GONAL-f pen injector (Supplementary Table 3). There were no Type-2 errors recorded with the other pen injectors; therefore, a separate analysis was not conducted for Type-2 errors.
For Type-4 errors (defined as incidents requiring self-medication or medical surveillance), the rates for the GONAL-f pen injector were similar to those for the Bemfola pen injector, but significantly lower than the other two pen injectors (adjusted rates [95% CI] were 0.09 [0.04, 0.14] for the GONAL-f pen injector, 0.10 [0.05, 0.16] for the Bemfola pen injector, 0.75 [0.42, 1.08] for the Rekovelle pen injector and 1.01 [0.43, 1.60] for the Ovaleap pen injector; p = 0.683 for Bemfola vs GONAL-f; p < 0.01 for the Rekovelle and Ovaleap vs GONAL-f; ). The most frequent individual Type-4 handling errors for each pen injector were: ‘Injecting insufficient amount of product due to not holding the injection button down long enough’ with the GONAL-f pen injector (10/120, 8%) and Rekovelle (5/71, 7%) pen injectors, ‘Accidentally triggering the injection too soon and not delivering the dose’ with the Bemfola pen injector (4/120, 3%), ‘Selecting wrong dose (9 μg or 9.33 μg instead of 9.66 μg) and injecting insufficient product’ with the Rekovelle pen injector (30/71, 42%), ‘Using the wrong button to trigger the injection and not delivering the dose’ with the Rekovelle and Ovaleap pen injectors (15/71, 21%; 16/49, 33%, respectively) (Supplementary Table 3). No other types of handling errors were recorded for women with infertility.
In total, 68% (81/120) of women with infertility made at least one handling error while using the GONAL-f pen injector, 84% (101/120) while using the Bemfola pen injector, 87% (62/71) while using the Rekovelle pen injector and 100% (49/49) while using the Ovaleap pen injector (Supplementary Table 4). The use step for which the most errors were recorded was ‘Giving the injection’ (). In total, 221 errors were recorded during this use step: 34 errors were recorded for the GONAL-f pen injector, 85 errors for the Bemfola pen injector, 65 for the Rekovelle pen injector and 37 for the Ovaleap pen injector (). The rates for handling errors recorded during the ‘Giving the injection’ use step were significantly lower for the GONAL-f pen injector compared with the other pen injectors (adjusted rates [95% CI] were 0.28 [0.18, 0.37] for the GONAL-f pen injector, 0.70 [0.55, 0.85] for the Bemfola pen injector, 0.87 [0.61, 1.13] for the Rekovelle pen injector and 0.79 [0.49, 1.08] for the Ovaleap pen injector; p < 0.01 for all comparisons vs GONAL-f, Supplementary Table 5).
Table 3. Handling errors by use step – women with infertility.
‘Priming’ was another use step during which a large number of errors was recorded (160 errors), especially for the Bemfola pen injector (69 errors) (). The rates for errors recorded during the ‘Priming’ use step were also significantly lower for the GONAL-f pen injector compared with other pen injectors (adjusted error rates [95% CI] were 0.19 [0.11, 0.26] for the GONAL-f pen injector, 0.56 [0.42, 0.69] for the Bemfola pen injector, 0.38 [0.23, 0.53] for the Rekovelle pen injector and 0.89 [0.52, 1.25] for the Ovaleap pen injector; p < 0.05 for all comparisons vs GONAL-f; Supplementary Table 5).
In addition, women with infertility had difficulties ‘Inserting/changing cartridge’ for the Rekovelle pen injector (45 errors) and the Ovaleap pen injector (54 errors) (). This step was not required for the GONAL-f and Bemfola pen injectors. There was also a large number of errors recorded with the GONAL-f pen injector while preparing the pen injector for use (56 errors; ). All of these errors were due to difficulties removing the cap from the pen injector (Supplementary Table 3).
3.2. Fertility nurses
Of the 60 nurses enrolled in the study, all of them tested both the GONAL-f and Bemfola pen injectors. Thirty (50%) nurses tested the Rekovelle pen injector and 30 (50%) the Ovaleap pen injector (9 nurses tested Rekovelle in Germany, 10 in Poland and 11 in the UK; 11 nurses tested Ovaleap in Germany, 10 in Poland and 9 in the UK). Thirty (50%) nurses had >10 years of experience as fertility nurses. Fifty-two (87%) nurses had previous experience training patients on the use of the GONAL-f pen injector, 26 (43%) on the Bemfola pen injector, 15 (25%) on the Rekovelle pen injector and 22 (37%) on the Ovaleap pen injector.
3.2.1. Preference ratings
The GONAL-f pen injector received significantly higher ratings than the other pen injectors for the ‘Overall impression’ both before and after the testing of the pen injector (adjusted mean [95% CI] ratings for the GONAL-f pen injector were 8.44 [8.06, 8.83] before the testing and 9.17 [8.85, 9.49] after the testing; for the Bemfola: 7.36 [6.85, 7.87] before the testing and 6.86 [6.25, 7.46] after the testing; for the Rekovelle: 7.28 [6.51, 8.05] before the testing and 6.88 [6.02, 7.74] after the testing and for the Ovaleap: 6.35 [5.34, 7.36] before the testing and 5.27 [4.07, 6.47] after the testing; p < 0.01 for all comparisons vs GONAL-f before testing and p < 0.001 for all comparisons vs GONAL-f after testing; , Supplementary Table 6).
Figure 3. Adjusted mean ratings before and after testing the pen injector – nurses.
Data presented are adjusted marginal means derived from a cumulative-link mixed model with logit link function and flexible thresholds. Fixed effects were pen type, time, and testing order of pens (as covariate) with interaction term of pen-by-time. Random effects were by pen testing sequence within individual participant.
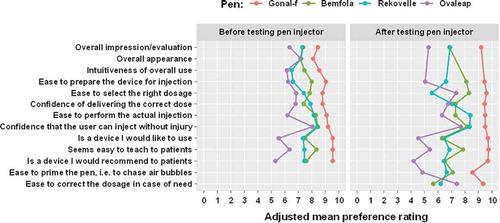
The ratings for the statement ‘Is a device I would recommend to patients’ were significantly higher for the GONAL-f pen injector compared with the other pen injectors both before and after the testing (adjusted mean [95% CI] ratings for the GONAL-f pen injector were 9.57 [9.25, 9.89] before the testing and 9.70 [9.44, 9.96] after the testing; for the Bemfola: 7.61 [6.88, 8.34] before the testing and 6.47 [5.55, 7.39] after the testing, for the Rekovelle: 7.43 [6.31, 8.54] before the testing and 6.45 [5.11, 7.80] after the testing and for the Ovaleap: 5.32 [3.77, 6.87] before the testing and 4.16 [2.59, 5.74] after the testing; p < 0.001 for all comparisons vs GONAL-f both before and after testing; Supplementary Table 6).
The GONAL-f pen injector received significantly higher ratings for both statements evaluated before the testing only (‘Overall appearance’ and ‘Intuitiveness of the overall use’) and after the testing only (‘Ease to prime the pen, i.e. chase air bubbles’ and ‘Ease to correct the dosage in case of need’), compared with the other pen injectors (adjusted mean [95% CI] ratings for ‘Overall appearance’ were 8.13 [7.65, 8.60] for the GONAL-f pen injector, 7.15 [6.55, 7.74] for the Bemfola pen injector, 7.19 [6.36, 8.03] for the Rekovelle pen injector and 7.18 [6.31, 8.05] for the Ovaleap pen injector; p < 0.05 for all comparisons vs GONAL-f; adjusted mean [95% CI] ratings for ‘Intuitiveness of the overall use’ were 8.54 [8.11, 8.97] for the GONAL-f pen injector, 7.44 [6.86, 8.02] for the Bemfola pen injector, 6.49 [5.55, 7.43] for the Rekovelle pen injector and 6.13 [5.11, 7.15] for the Ovaleap pen injector; p < 0.01 for all comparisons vs GONAL-f; adjusted mean [95% CI] ratings for ‘Ease to prime the pen, i.e. chase air bubbles’ were 8.53 [8.01, 9.06] for the GONAL-f pen injector, 7.08 [6.33, 7.83] for the Bemfola pen injector, 6.66 [5.56, 7.76] for the Rekovelle pen injector and 4.83 [3.54, 6.13] for the Ovaleap pen injector; p < 0.01 for all comparisons vs GONAL-f; adjusted mean [95% CI] ratings for ‘Ease to correct the dosage in case of need’ were 9.32 [8.97, 9.67] for the GONAL-f pen injector, 5.64 [4.66, 6.63] for the Bemfola pen injector, 6.18 [4.87, 7.48] for the Rekovelle pen injector and 7.40 [6.22, 8.58] for the Ovaleap pen injector; p < 0.01 for all comparisons vs GONAL-f; Supplementary Table 6).
In addition, after testing, the GONAL-f pen injector was ranked as the first choice by the highest proportion of fertility nurses (72% [43/60 nurses]; ). Based upon the adjusted mean rank score, the GONAL-f pen injector was ranked significantly higher after the testing than other pen injectors for all questions (p < 0.01 for all comparisons vs GONAL-f; Supplementary Table 7)
3.2.2. Handling errors
For nurses, error rates for total handling errors were significantly lower for the GONAL-f pen injector compared with the other pen injectors (adjusted rates [95% CI] of total handling errors per participant were 0.31 [0.16, 0.45] for the GONAL-f pen injector, 1.30 [1.00, 1.60] for the Bemfola pen injector, 1.19 [0.71, 1.66] for the Rekovelle pen injector and 1.64 [1.06, 2.21] for the Ovaleap pen injector; p < 0.001 for all comparisons vs GONAL-f; ).
Table 4. Handling errors – nurses.
For Type-1 errors, rates were significantly lower for the GONAL-f pen injector compared with the Bemfola and Ovaleap pen injectors (adjusted rates for Type-1 errors [95% CI] were 0.31 [0.17, 0.46] for the GONAL-f pen injector, 1.30 [0.99, 1.60] for the Bemfola pen injector, 0.61 [0.29, 0.93] for the Rekovelle pen injector and 1.01 [0.59, 1.42] for the Ovaleap pen injector; p < 0.01 for the Bemfola and Ovaleap vs GONAL-f comparisons; ). The most frequent individual Type-1 errors for each pen injector were (Supplementary Table 3): ‘Difficulties removing the cap from the pen injector’ with GONAL-f (13/60, 22%), being ‘Unsure whether the dose has been delivered/unsure about dose bar disappearing’ with the Bemfola pen injector (18/60, 30%), ‘Confused that needle doesn’t seem to lock’ with the Rekovelle pen injector (4/30, 13%), ‘Difficulties with instructions for inserting the cartridge’ and ‘Difficulties extending plunger’ with the Ovaleap pen injector (6/30, 20% for both errors).
One nurse made a Type-2 error which was ‘Needle breaks in the safety cap’ with the Rekovelle pen injector (Supplementary Table 3). No Type-2 errors were recorded with the other pen injectors; therefore, the separate analysis was not conducted for Type-2 errors.
Because the Poisson GLMM for Type-4 errors among nurses failed to converge, unadjusted error rates and unadjusted IRDs are provided for Type-4 errors (). Rates for Type-4 errors were significantly lower for the GONAL-f pen injector compared with the Rekovelle and Ovaleap pen injectors, but no significant difference was found between the GONAL-f and Bemfola pen injectors (unadjusted rates for Type-4 handling errors [95% CI] were 0.00 [0.00, 0.06] for the GONAL-f pen injector, 0.02 [0.00, 0.09] for the Bemfola pen injector, 0.53 [0.30, 0.87] for the Rekovelle pen injector and 0.63 [0.38, 0.99] for the Ovaleap pen injector; p < 0.001 for Rekovelle and Ovaleap vs GONAL-f; p = 0.317 for Bemfola vs GONAL-f; ). The most frequent individual Type-4 handling errors were: ‘Insufficient product injected because wrong dose selected (9 or 9.33 μg instead of 9.66 μg)’ with the Rekovelle pen injector (6/30, 20%), ‘Dose not delivered because used wrong button to trigger injection’ with the Rekovelle (6/30, 20%) and Ovaleap pen injectors (7/30, 23%) (Supplementary Table 3). No other types of handling errors were recorded for nurses.
Overall, 27% (16/60) of nurses made at least one handling error while using the GONAL-f pen injector, 72% (43/60) while using the Bemfola pen injector, 73% (22/30) while using the Rekovelle pen injector and 80% (24/30) while using the Ovaleap pen injector (Supplementary Table 8).
The use step during which the most errors were made was ‘Giving the injection’ (). Overall, 64 errors were recorded during this use step: 3 errors were recorded for the GONAL-f pen injector, 36 for the Bemfola pen injector, 15 for the Rekovelle pen injector and 10 for the Ovaleap pen injector. The error rates for handling errors recorded during the ‘Giving the injection’ use step were significantly lower with the GONAL-f pen injector compared with the other pen injectors (adjusted error rates [95% CI] were 0.05 [−0.01, 0.10] for the GONAL-f pen injector, 0.58 [0.38, 0.78] for the Bemfola pen injector, 0.39 [0.17, 0.61] for the Rekovelle pen injector and 0.42 [0.10, 0.74] for the Ovaleap pen injector; p < 0.05 for all comparisons vs GONAL-f; Supplementary Table 9).
Table 5. Handling errors by use step – nurses.
A large number of errors were also reported during the ‘Priming’ use step, especially for the Bemfola pen injector (26 errors) (). The rates for handling errors recorded during the ‘Priming’ use step were significantly lower with the GONAL-f pen injector compared with the Bemfola and Ovaleap pen injectors, but no significant difference was found between the GONAL-f and Rekovelle pen injectors (adjusted error rates [95% CI] were 0.04 [−0.01, 0.09] for the GONAL-f pen injector, 0.46 [0.28, 0.65] for the Bemfola pen injector, 0.30 [0.02, 0.58] for the Rekovelle pen injector and 0.36 [0.13, 0.59] for the Ovaleap pen injector; p < 0.05 for Bemfola and Ovaleap vs GONAL-f; p = 0.073 for Rekovelle vs GONAL-f; Supplementary Table 9). In addition, 13 errors were recorded for the GONAL-f pen injector while ‘Preparing the pen injection for use’ (). All of these errors were due to the difficulties removing the cap from the pen injector (Supplementary Table 3).
4. Discussion
This simulated-use study included a representative sample of women with infertility and fertility nurses from three European countries. Among women with infertility, the preference ratings for the different aspects of the pen injectors changed after the actual handling of the device when compared with the initial evaluation prior to handling. Before testing the pen injectors, there were no differences in mean ratings; however, after handling, the GONAL-f pen injector received significantly higher ratings compared with the other three pen injectors. In addition, the overall impressions for handling and using the GONAL-f pen injector were higher after the test than before the test, whereas for the other pen injectors these ratings were lower or similar after the test when compared to before the test. Such changes in ratings observed in this study may be because of difficulties using the devices that were not anticipated during the initial evaluation.
Among nurses, the preference ratings for the different aspects of the pen injectors also changed after handling when compared with an initial evaluation; however, the ratings for the GONAL-f pen injector remained higher than other pens after handling. The higher ratings observed with the GONAL-f injector may be because 87% of nurses were already familiar with the GONAL-f pen injector and had previously trained patients on its use. The nurse ratings for the GONAL-f pen injector were significantly higher after using the pen injector when compared with the ratings before using the pen injector. Conversely, ratings for the other pen injectors were lower after handling when compared to before the handling, including the rating for whether nurses would recommend the pen injector to their patients. This may be related to the significantly higher incidence of handling errors observed with the Bemfola, Rekovelle and Ovaleap pen injectors compared with the GONAL-f pen injector.
In both our analyses (women with infertility and nurses), the Rekovelle and Ovaleap pen injectors were associated with a significantly higher rate of Type-4 errors compared with the GONAL-f pen injector. Type-4 errors represented the most severe errors in the study. In actual clinical practice, this type of error could impact the outcome of treatment or could potentially result in an incident requiring medical surveillance. At least one Type-4 error occurred with all of the pens amongst women with infertility; however, the proportion of patients with at least one handling error was highest with the Bemfola pen injector. There was a large number of Type-4 errors recorded during the ‘Giving the Injection’ step, especially with the Rekovelle and Ovaleap pen injectors, suggesting that there is a need for additional training and follow-up advice on how to avoid such errors. Type-4 errors did not occur while nurses were using the GONAL-f pen injector and only one was recorded while nurses were using the Bemfola pen injector. In contrast to Type-4 errors, Type-1 errors represented the least severe errors reported in the study, as this type of error can be easily resolved by the patient and is unlikely to impact treatment outcomes. The most common Type-1 error was ‘Opening the pen injector’ which occurred only with the GONAL-f pen injector.
A number of errors recorded for both women with infertility and fertility nurses with the Rekovelle and Ovaleap pen injectors may have resulted from the additional step of inserting the cartridge, and the different locations of the injection button versus the other pen injectors. Despite the Rekovelle and Ovaleap pen injectors being very similar, dose selection was different for the Rekovelle pen injector, as Rekovelle is dosed in μg whereas Ovaleap is dosed in IU. For the Rekovelle pen injector, participants were asked to select a clinically relevant dose of 9.66 µg. The dose selector had marks for 9 and 10 and two dashes in between, representing the thirds, which made the dose harder to select. The majority of errors with the GONAL-f pen injector occurred while preparing the pen injector for use, i.e. removing the cap of the pen injector. This suggests that less steps required to prepare the pen injector and perform an injection might reduce the potential for handling errors. This in turn may increase the ease of use and thereby reduce treatment-related anxiety and discontinuation rates [Citation6,Citation18].
To our knowledge, this is the only simulated-use study that provides such a detailed comparison of the different r-hFSH pen injectors. The study has a number of strengths. Firstly, the study design included the simulated handling of the pen injector which is essential to accurately evaluate the patient’s preference for a medical device. Secondly, the preferences were evaluated for a number of different aspects of the pen injectors both before and after the simulated-use of the device, which demonstrated how actual handling of the pen injector affects the subsequent opinions. Thirdly, the study included a comprehensive assessment of handling errors which provided data on the safety of the different devices, and demonstrated how certain attributes of the device may affect not only the handling of the pen injector, but also result in delay of treatment or even injury. Fourthly, the preference ratings and handling errors were evaluated for both women with infertility intending to undergo ovarian stimulation and fertility nurses. The separate analysis of these two distinct populations provided data essential to assess the different issues that women and nurses might have using pen injectors during infertility treatment. Lastly, the testing order of the pens was randomized to avoid bias. The testing order of the pen was particularly important considering that the processes of preparing the injector for use and performing the injection were similar across devices.
Our study also has a number of limitations. This was a simulated-use study with a single injection into a foam pad, rather than daily subcutaneous injections. The Rekovelle cartridge pen injector was included in our study; a new prefilled Rekovelle pen injector is now used in some countries (Germany and the UK), but was not available at the time of our study. However, although the new prefilled Rekovelle pen has a push knob at the top of the pen to release the dose, the actual dose dial is similar to the older cartridge pen and therefore any handling issues related to dose-setting are unlikely to have changed. It was difficult to fully mask the pen injectors as the cartridge for the Rekovelle and Ovaleap pen injectors distinguishes them from the GONAL-f and Bemfola pen injectors and, as fertility nurses were familiar with the pen injectors and used them before, it is likely they were able to recognize and identify the individual pens. Furthermore, the dose (9.66 μg) that participants were asked to inject with the Rekovelle pen injector was different from the dose that was injected with other pens (62.5 IU), which was another difference that may have led to the unmasking of the pens. As the GONAL-f pen injector was introduced into the market earlier than the other pen injectors, a larger proportion of nurses had previous experience training patients on its use compared with the other pen injectors (87% nurses had previous experience training patients on the use of the GONAL-f pen injector, 43% on the Bemfola pen injector, 25% on the Rekovelle pen injector and 37% on the Ovaleap pen injector). This has the potential for bias as most of the nurses were familiar with the GONAL-f pen injector which may have affected their ratings. The GONAL-f, Bemfola, Rekovelle and Ovaleap pen injectors were all commercially available in the UK, Germany, and Poland at the time of the study; however, previous nurse experience of training women on a specific pen injector depended on individual patient prescription.
The findings reported in this study are consistent with previous studies regarding the evaluation of the GONAL-f pen injector by women with recent or current infertility requiring ART or IVF and fertility nurses [Citation4,Citation5,Citation19]. In a previous study, IVF/ART-experienced and – naïve women with infertility found the redesigned pen injector ‘easy to learn to use’ and ‘easy to use’ [Citation5]. Fertility nurses rated the redesigned pen injector as ‘easy to learn and use’ and ‘easier to teach’ than expected [Citation4]. In addition, IVF/ART-experienced women and fertility nurses in these studies were asked to compare their initial impressions and the use of the GONAL-f pen injector with other self-injection devices that they previously used or taught, based on recall [Citation4,Citation5]. The majority of women said that they would prefer the GONAL-f pen injector over other devices and the majority of nurses would choose to teach the use of the GONAL-f pen injector over other injection devices. Furthermore, a study by Jeannerot et al. (2016) showed acceptable dose accuracy (defined by the International Organization for Standardization 11,608–1:2012/2014 standard) for the GONAL-f pen injector under different conditions and handling processes [Citation19]. To the best of our knowledge, no other pens have had such a comprehensive assessment as the GONAL-f pen injector.
Previous comparative studies of different pen injectors for IVF treatment have reported different results to the findings reported in our study. Two previous studies compared the Bemfola, GONAL-f and Puregon pen injectors and showed that patients preferred the Bemfola pen injector [Citation8,Citation9]. These studies included the handling of different pen injectors and questionnaire-based survey after handling, comparing the ease and convenience of use of the different pen injectors. However, the studies did not evaluate preferences before handling, and neither included harm assessment or evaluation of potential handling errors. Our study included evaluation of the preferences both before and after actual testing of the pen injectors and evaluation of handling errors, which showed that the GONAL-f pen injector was preferred by both women with infertility and fertility nurses.
Another study, looking at the human factor interactions by patients and nurses for the Bemfola pen injector, used a list of pre-defined critical steps according to the IFU to assess the correct handling of the pen injector [Citation20]. Interestingly, no critical errors related to the use of the pen injector were reported in this study and only minor issues were reported related to the priming of the pen injector. By contrast, in our study we observed a number of errors with the Bemfola pen injector related to priming and giving the injection.
An analysis by Zitoun et al. (2019) evaluating the key attributes that patients and fertility nurses consider when selecting an r-hFSH pen injector identified the type of pen injector (multi-use vs single-use, disposable vs reusable) and presence and location of the injection button and dose-setting knob as important considerations [Citation18]. In that study, it was reported that participants preferred a multi-use, reusable device. However, the study did not include actual handling of the pen injectors, but was based on participant recall of previous use and experience. Our study, in which participants actually used the pen injectors, demonstrated that the handling of multi-use pens caused errors due to additional step of inserting the cartridge. In the study by Zitoun et al. (2019), it was also suggested that participants preferred pen injectors with a release button rather than a push knob. Again, in our study, more handling errors were observed with the injectors with the release button on the side of the device, as often the wrong button was pressed to inject the dose. The difference in findings and conclusions in our study versus Zitoun et al. highlights that evaluation of device attributes may not be accurately interpreted without participants actually using and handling the various devices, and rating their preferences and experiences both before and after use.
In this study, we demonstrate that different aspects of the pen injectors may lead to potential handling errors and should be taken into consideration when choosing a pen injector for infertility treatment and when training patients on its use. Preparation of multi-use pens with cartridges requires an additional step; therefore, we recommend that specific training should be provided on how to insert and change the cartridge. For pen injectors with a release button on the side of the device, we also suggest that it should be highlighted in the IFU and during training that pressing the wrong injection button can cause wastage of the product and delay in treatment. Patients using injectors with a cap should be made aware that removing the cap may require a stronger pulling force than initially anticipated. Finally, additional training would be useful for the critical steps, such as priming of the pen injector and giving the injection, and advice given that these steps should be performed with caution. The majority of handling errors observed in our study relate to selecting and injecting the dose, therefore future comparative studies are required to evaluate the dosing accuracy of different pen injectors and provide guidelines on how to avoid such errors.
5. Conclusions
This simulated-use study, involving both women with infertility and fertility nurses, compared the GONAL-f, Bemfola, Rekovelle and Ovaleap pen injectors and showed that, after use, women with infertility rated the GONAL-f pen injector significantly higher than the other devices, and that fertility nurses rated the GONAL-f pen injector higher than the other devices both before and after use. This, among other factors, may be related to an increased number of handling errors (including those that may affect treatment outcomes) observed with the Ovaleap, Bemfola, and Rekovelle pen injectors compared with the GONAL-f pen.
Author contributions
All authors contributed to the interpretation of data and critical review of this manuscript. J Martins and F Beckers contributed to the conception, design, and implementation of the statistical analysis. All authors approved the manuscript for submission to the journal.
Declaration of interest
T D’Hooghe, A Seidler, S Longobardi, and F Beckers are employees of Merck Healthcare KGaA, Darmstadt, Germany. J Martins conducted the statistical analysis for this study, which was funded by Merck KGaA, Darmstadt, Germany. W MacGillivray is an Account Director for Ifop France which conducted the study, which was funded by Merck KGaA, Darmstadt, Germany.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Pen_vs_pen_Manuscript_Suppl_Appendices_0.1.docx
Download MS Word (58 KB)Acknowledgments
Support categorizing the handling errors was provided by Cedric Foucher (Merck KGaA). Writing assistance was provided by Evelina Matekonyte (inScience Communications, Springer Healthcare Ltd), which was funded by Merck KGaA.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Huisman D, Raymakers X, Hoomans EH. Understanding the burden of ovarian stimulation: fertility expert and patient perceptions. Reprod Biomed Online. 2009;19(Suppl 2):5–10. PubMed PMID: 19891842; eng.
- Brandes M, van der Steen JO, Bokdam SB, et al. When and why do subfertile couples discontinue their fertility care? A longitudinal cohort study in a secondary care subfertility population. Hum Reprod. 2009 Dec;24(12):3127–3135. PubMed PMID: 19783833; eng.
- Jeannerot F, Studeli T, Gunther-LaVergne L, et al. Usability engineering study in the European union of a redesigned follitropin alfa pen injector for infertility treatment. Expert Opin Drug Deliv. 2016 Sep;13(9):1221–1229. PubMed PMID: 27329677.
- Schertz J, Worton H. Nurse evaluation of the redesigned fertility pen injector: a questionnaire-based observational survey. Expert Opin Drug Deliv. 2018 May;15(5):435–442. PubMed PMID: 29521156; eng.
- Schertz J, Worton H. Patient evaluation of the redesigned follitropin alfa pen injector. Expert Opin Drug Deliv. 2017 Apr;14(4):473–481. PubMed PMID: 28140682; eng.
- Abbotts C, Salgado-Braga C, Audibert-Gros C. A redesigned follitropin alfa pen injector for infertility: results of a market research study. Patient Prefer Adherence. 2011;5:315–331. PubMed PMID: 21792303; PubMed Central PMCID: PMC3140313. eng.
- Gibreel A, Bhattacharya S. Recombinant follitropin alfa/lutropin alfa in fertility treatment. Biologics. 2010 Feb 4;4:5–17. PubMed PMID: 20161981; PubMed Central PMCID: PMC2819896. eng.
- Quintero LAM, Verdú V, de la Fuente Bitaine L, et al. An evaluation by potential IVF/Donor Oocyte patients of the use and handling of the Bemfola® Pen compared with the Gonal-f® Pen and Puregon Pen®. Revista Iberoamericana De Fertilidad. 2017;34:64–73.
- Imthurn B, McVeigh E, Stiller R, et al. Evaluation of the use and handling of three different pen systems considered for in vitro fertilization treatment. Expert Opin Drug Deliv. 2014 Dec;11(12):1859–1864. . PubMed PMID: 25325925; eng.
- van Overbeeke E, Whichello C, Janssens R, et al. Factors and situations influencing the value of patient preference studies along the medical product lifecycle: a literature review. Drug Discov Today. 2019 Jan;24(1):57–68. PubMed PMID: 30266656; eng.
- Lankreijer K, D’Hooghe TM, Apers S, et al. Hormonal medication in medically assisted reproduction: a systematic review of assessments from patients. Reprod Biomed Online. 2019 Mar;38(3):341–363. PubMed PMID: 30770286; eng.
- Lankreijer K, D’Hooghe T, Sermeus W, et al. Development and validation of the FertiMed questionnaire assessing patients’ experiences with hormonal fertility medication. Hum Reprod. 2016 Aug;31(8):1799–1808. PubMed PMID: 27220980; eng.
- Dancet EA, Nelen WL, Sermeus W, et al. The patients’ perspective on fertility care: a systematic review. Hum Reprod Update. 2010 ( Sep-Oct);16(5):467–487. doi: 10.1093/humupd/dmq004. PubMed PMID: 20223789; eng.
- Dancet EA, D’Hooghe TM, van der Veen F, et al. “Patient-centered fertility treatment”: what is required? Fertil Steril. 2014;101(4):924–926.
- Dancet EA, D’Hooghe TM, Sermeus W, et al. Patients from across Europe have similar views on patient-centred care: an international multilingual qualitative study in infertility care. Hum Reprod. 2012 Jun;27(6):1702–1711. PubMed PMID: 22427309; eng.
- Dancet EA, D’Hooghe TM, Spiessens C, et al. Quality indicators for all dimensions of infertility care quality: consensus between professionals and patients. Hum Reprod. 2013 Jun;28(6):1584–1597. PubMed PMID: 23508250; eng.
- Mahony MC, Patterson P, Hayward B, et al. Human factors engineering and design validation for the redesigned follitropin alfa pen injection device. Expert Opin Drug Deliv. 2015 May;12(5):715–725. PubMed PMID: 25895897; PubMed Central PMCID: PMC4496816. eng.
- Zitoun P, Parikh J, Nijs M, et al. Analysis of patient and nurse preferences for self-administered FSH injection devices in select European markets. Int J Womens Health. 2019;11:11–21. PubMed PMID: 30662286; PubMed Central PMCID: PMC6327888. eng.
- Jeannerot F, Cusin A, Schertz J. Dose accuracy of the redesigned follitropin alfa pen injector for infertility treatment. Expert Opin Drug Deliv. 2016 Dec;13(12):1661–1669. PubMed PMID: 27636195.
- Saunders H, de la Fuente Bitaine L, Eftekhar C, et al. Functionality of a novel follitropin alfa pen injector: results from human factor interactions by patients and nurses. Expert Opin Drug Deliv. 2018 Jun;15(6):549–558. PubMed PMID: 29595399; eng.