ABSTRACT
Background: Two human factors studies evaluated whether a stable liquid formulation of glucagon in a prefilled syringe (G-PFS) could be safely and effectively administered and evaluated the effectiveness of the product label guide and instructions-for-use (IFU).
Research design and methods: In a formative study, 11 participants received orientation with the G-PFS instructional materials and performed a single unaided rescue attempt. In the validation study, 75 adult and adolescent participants received training or familiarized themselves with the G-PFS IFU, Label Guide, and device. All participants returned 1 week later to perform a single unaided rescue attempt of a simulated person with diabetes suffering from an emergency severe hypoglycemic event.
Results: The formative study resulted in a 100% success rate across all rescue dose attempts. The validation study resulted in 74/75 (99%) of participants successfully using the G-PFS to administer the full glucagon rescue dose, and validated that intended users could learn from, comprehend, and recall the G-PFS instructions to successfully use the product.
Conclusion: The G-PFS provides a familiar, easy-to-use alternative to currently marketed lyophilized glucagon kits for treating severe hypoglycemia. The G-PFS IFU and Label Guide enable even untrained users to successfully administer a full rescue dose of stable liquid glucagon.
1. Introduction
Hypoglycemia is a potentially serious complication that occurs with insulin use in persons with both type 1 and type 2 diabetes [Citation1,Citation2]. Between 4% and 10% of deaths in persons with type 1 diabetes are attributed to hypoglycemia [Citation3], and the risk of death is 3.4-fold higher among those with severe hypoglycemia compared to those with no or mild hypoglycemia [Citation4]. Persons with type 1 diabetes experience approximately 2 symptomatic hypoglycemic events per week, and severe events occur at least once a year [Citation2]. Additionally, persons with type 2 diabetes experience several hypoglycemic episodes annually, including 1–2 severe events [Citation1], and the incidence of severe hypoglycemic events in this population has been reported to be 1.4% [Citation5].
Glucagon is the standard of care for treating severe hypoglycemia episodes [Citation6], and should be prescribed for all persons with diabetes (PWDs) who are at risk of clinically significant hypoglycemia in the outpatient setting where access to intravenous dextrose is not immediately available. Glucagon administration is not limited to health care professionals; caregivers, school personnel, or family members of PWDs who are at risk should know where glucagon is readily located, and when and how it should be administered [Citation6]. When successfully administered, marketed glucagon emergency rescue kits (GEKs) are known to reduce mortality and morbidity [Citation7–Citation9]. A critical component in the treatment of severe hypoglycemia is the prompt and successful administration of glucagon. The functional efficacy of glucagon is dependent upon the ability of any individual to successfully provide external assistance in a timely manner: to correctly prepare and reliably administer a full dose of the drug during an acute emergency. The consideration of a rescue product with high functional efficacy is critical for appropriate user groups, including adolescent and adult, and trained and untrained users in these scenarios. Human factors, including usability by the appropriate user in the appropriate use environment, influence the safety and efficacy of a device or drug-device combination product. As part of a product registration package, drug product manufacturers typically conduct a comprehensive risk assessment of the usability of a combination product including human factors formative and validation studies designed to mitigate usability risks [Citation10].
GEKs, however, require reconstitution of the lyophilized glucagon powder immediately prior to use – a disadvantage that necessitates multiple steps to prepare the dose prior to administration. Reconstitution and drug administration require thorough education and often re-education of PWDs, their families, and other caregivers. Despite training, errors or delayed administration of the full dose of glucagon occur within emergency situations [Citation11–Citation13]. Previous human factors studies have demonstrated that only 13% of caregivers and 0% of acquaintances can deliver full doses of injectable glucagon in such settings [Citation14].
Development of a soluble glucagon formulation for rapid administration has been hampered by the physicochemical properties of glucagon. Following reconstitution, native glucagon is a highly unstable peptide that may undergo spontaneous polymerization and formation of amyloid-like fibrils which are cytotoxic and lack the bioavailability of monomeric glucagon, and limit stability [Citation15–Citation17]. Development of a stable liquid ready-to-use glucagon formulation in a pre-measured format enables rapid treatment of severe hypoglycemia in an acute rescue setting. A stable, readily usable glucagon product may help reduce fear of hypoglycemic events [Citation18–Citation21].
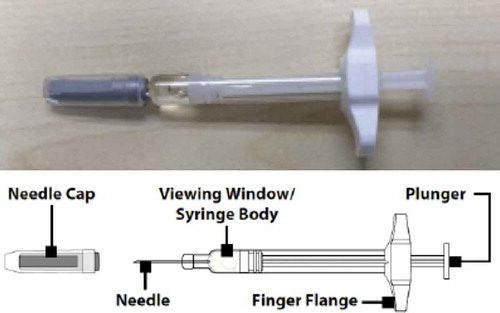
A proprietary, non-aqueous formulation of glucagon (G-PFS) is under development that uses biocompatible, polar aprotic solvents to create a highly soluble and highly stable formulation [Citation22]. The use of a polar aprotic solvent effectively suppresses the fibrillation observed in aqueous solutions of glucagon and enables chemical stability similar to lyophilized powders to facilitate long-term storage [Citation22]. The G-PFS is a concentrated, low volume, stable glucagon formulation that is pre-mixed and pre-loaded into a 1 mL disposable syringe with a 0.1 mL (0.5 mg – Pediatric Use) or 0.2 mL (1 mg – Adult Use) fill volume (). The G-PFS provides 1) a ready-to-use pre-measured liquid glucagon with no reconstitution required to enable rapid dosing; 2) simple preparation and administration to reduce the potential for errors associated with currently available GEKs; and 3) enhanced portability and availability due to room temperature stability.
We report results from two separate human factors studies undertaken to evaluate a stable formulation of liquid glucagon within a prefilled syringe (G-PFS). The objectives of the studies were to determine if G-PFS can be safely and effectively administered by the appropriate user groups and to evaluate the effectiveness of product labeling for providing instructions on the injection procedure.
2. Participants and methods
2.1. Description of G-PFS device
G-PFS is supplied () with a primary container closure system consisting of a 1-mL long, prefilled syringe with a 27-Gauge, 1/2” needle. G-PFS is supplied in 2 premeasured dose strengths of 0.5 mg and 1 mg. G-PFS is a sterile subcutaneous injectable non-aqueous formulation of synthetic human glucagon intended for treatment of severe hypoglycemia. A proprietary non-aqueous formulation platform is utilized to eliminate water from the formulation, thus providing greater stability of the concentrated glucagon peptide at room temperature. All excipients in the G-PFS are USP/NF grade and used in currently approved drug products and/or listed on the FDA Inactive Ingredient Guide (IIG). The G-PFS is portable and does not require refrigeration; it should be stored at room temperature.
2.2. Ethical considerations
This was a non-significant risk study where no therapeutic intervention was performed. Participants provided written informed consent prior to completing any human factors study activities.
2.3. Formative human factors study
The primary objectives of the formative study were to assess whether the intended user population could safely and effectively use the G-PFS, and to evaluate the effectiveness of the labeling (Instructions for Use [IFU] and Label Guide) ().
People in a metropolitan area of the U.S. were screened by telephone for participation in the study. Eligible participants were an actual caregiver of a PWD, as these individuals are most likely to administer glucagon in the real world. Eligible adult caregivers were age 18–79 years and adolescent caregivers were age 12–17 years. Participants were excluded if they participated in a prior study with GEKs, if they did not currently have a role as a caregiver, if they had any medical or genetic condition that could prevent them for using the glucagon administration device, or for a lack of English reading and speaking abilities. Participants provided written informed consent prior to study participation.
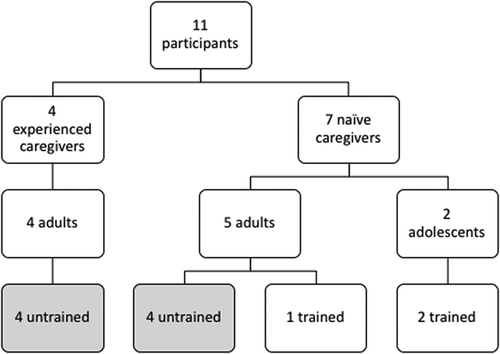
Participants were separated into 2 study groups consisting of individuals with some level of self-reported experience with a GEK (Experienced Caregivers), and users with no self-reported previous knowledge or experience with a GEK (Naïve Caregivers). To assess usability of the G-PFS in adolescents, two of the naïve caregivers were between the ages of 12 and 17 years old.
To assess the effectiveness of the IFU, Experienced and Naïve Caregivers were assigned to 1 of 2 training conditions: trained and untrained (). Eight participants were untrained, and 3 participants were trained. The untrained group included 4 experienced caregivers and 4 naïve caregivers. Untrained participants were given time to review the IFU prior to administering glucagon. All 3 trained participants were naïve to GEKs (1 adult and 2 adolescent participants). Trained participants were naïve caregivers and received a short, but representative, training prior to attempting a rescue injection.
All participants took part in 2 study sessions (). During the first session, trained participants received training and untrained participants were given time to familiarize themselves with the instructional materials. During the second session, all participants performed a single unaided rescue attempt followed by post interaction questions, knowledge probe questions, reading comprehension questions, and a review of the instructions. Study assessments included ():
Evaluate the readability and comprehension of the content in the IFU and Label Guide.
Determine if there were any areas of the labeling which lead to undue confusion, difficulty or errors when attempting to perform the injection procedure using the G-PFS.
Determine if the G-PFS could be safely and effectively used and if performance of tasks lead to patterns of preventable use errors or harm – to the PWD or user; including compromised medical care.
Identify any potential remaining use-related hazards that could be mitigated.
Determine if the G-PFS and associated labeling were ready for validation.
Table 1. Summary of study design and procedures.
Table 2. User tasks evaluated to assess use of the G-PFS.
2.4. Summative human factors validation study
The primary objectives were to assess whether the intended user population could correctly, safely, and effectively use the G-PFS, and to evaluate the effectiveness of the labeling (IFU and Label Guide ()).
Selection criteria included participants who were likely to be present in the event that a PWD suffered a severe hypoglycemic emergency and would have the knowledge that the PWD required external assistance (rescue) with glucagon. This included First Responders (i.e. emergency personnel, health care providers, and school nurses) and Caregivers (i.e. family) of either an adult or minor with diabetes who was prescribed glucagon. This study included Caregivers who were naïve in the use of GEKs or had self-reported experience with GEKs. Participants were excluded for the same criteria discussed in the formative study above. Participants provided written informed consent prior to participation.
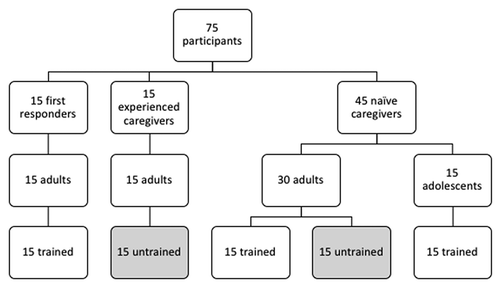
To assess the effectiveness of the IFU, participants were assigned to 1 of 2 training conditions: trained and untrained (). As healthcare professionals, First Responders are required to be knowledgeable and responsible for all procedures they perform, including any device that is used during these procedures. Therefore, all First Responders received training prior to performing an unsupervised glucagon rescue attempt. In addition, half of the Naïve Adult Caregivers also received training. It is possible that minors in the adolescent age range of 12–17 may have to administer a rescue injection to a parent or sibling. It is expected they receive some form of training by either a healthcare professional or the person for whom which would be receiving the care, and thus for this study all Naïve Adolescent Caregivers were trained. In order to test the worst-case-scenario, all Experienced Caregivers and the remaining Naïve Adult Caregivers did not receive training prior to performing their unaided rescue attempt.
All participants took part in two study sessions (). During the first session, trained participants were introduced to the purpose, dosing, and details of the device and procedure, provided time to review the IFU, Label Guide, and device, and were given a verbal walkthrough of the procedure. Untrained participants were given time to familiarize themselves, as they desired, with the device, IFU, and Label Guide. The Label Guide consisted of instructions printed on the device storage pouch.
All participants then returned approximately 1 week later (6 to 8 days) from the training or self-familiarization of the injection procedure, when participants had access to only the Label Guide as the sole source of guidance to perform a single unaided rescue attempt that simulated the rescue of a PWD suffering from an emergency hypoglycemic event. During the rescue attempt, participants were only provided the device in the sealed poly-foil pouch (with Label Guide). Participants were not provided the IFU on the assumption that a PWD may not carry the IFU with them. Representative ambient noise was playing in the background while participants performed the unaided rescue. After performing the rescue attempt, all participants were asked a series of post-interaction questions, critical knowledge probes, and reading comprehension queries with the IFU. Lastly, participants provided feedback on the effectiveness of the IFU and Label Guide. Study assessments included ():
Validate that the intended user populations (Family/Caregivers of PWDs, or First-Responders) could successfully use the G-PFS and associated IFU and Label Guide.
Validate that the G-PFS and associated labeling (IFU and Label Guide) could be safely and effectively used, and that performance of tasks did not lead to any patterns of preventable use errors on critical tasks or result in harm to the PWD or user including compromised medical care.
Validate that use-related hazards for the final/finished product were eliminated or that the residual risks that could not be mitigated were acceptable (the benefit of product approval outweighs the residual risk).
3. Results
3.1. Formative human factors study
This study was conducted with 11 participants representing the intended user population of caregivers to PWDs (). Overall, participants successfully executed the steps of the procedure to administer glucagon via a G-PFS. All 11 participants successfully performed the following tasks: 1) open pouch, 2) remove syringe, 3) remove needle cap, 4) inject into the exposed skin on the abdomen, 5) pinch the skin and hold the pinch during the injection, 7) insert the needle at correct angle, 8) administer the full dose without loss of drug or premature expulsion of drug, 9) roll the PWD (manikin) onto their side, and 10) set the syringe aside until it could be disposed of into an FDA-cleared sharps container.
Table 3. Baseline demographics for comparative and validation studies.
No failures or errors were observed. None of the participants called for help or displayed any overt/undue visible signs of difficulty. No significant differences in performance were observed between user groups (experienced vs. naïve or trained vs. untrained). Additionally, neither of the adolescent participants had any difficulty performing the procedure correctly.
Following the injection, all 11 participants reported that they successfully delivered the injection without any difficulty. One Naïve Adult Untrained participant reported some concern with performing an injection in the future because of concerns about damaging the syringe when removing the cap. This concern did not prevent the participant from performing the injection correctly during the unaided rescue.
All participants successful identified/comprehended critical information in the IFU without difficulty regarding: 1) the allowable injection site, 2) injection into bare skin, 3) storage of the syringe at room temperature, 4) checking the syringe expiration date, 5) checking the solution (liquid drug), and 6) turning the PWD (manikin) on their side after the injection. Overall, all 11 participants indicated that 1) they did not have difficulty understanding the IFU, 2) the IFU was readable in terms of spacing, font type, and font size, and 3) the illustrations were clear and effective.
Despite the above positive feedback about the IFU, 4 participants suggested minor enhancements to the IFU and/or Label Guide. Related to the instructions, participants suggested adding an instruction about air bubble removal to the IFU, and making the instruction related to putting the syringe in a safe place after the injection more prominent and clearer in the IFU. Related to the Label Guide, participants suggested adding the same instruction about putting the syringe in a safe place after injection to the Label Guide and changing the location of the expiration date on the Label Guide to make it easier to identify.
3.2. Summative human factors validation study
The summative study was conducted with 75 participants comprised of 3 user groups: 15 First Responders, 15 Experienced Caregivers, and 45 Naïve Caregivers (30 adults and 15 adolescents). All First Responders (N = 15), half of the Naïve Adult Caregivers (N = 15), and all Naïve Adolescent Caregivers were assigned to the trained condition. All Experienced Caregivers (N = 15) and half of the Naïve Adult Caregivers (N = 15) were assigned to the untrained condition. The age, ethnicity, and education level of the participants varied widely ().
All 75 participants (100%) performed a successful rescue and injected glucagon via the G-PFS into the subcutaneous tissue at the designated injection site (). Seventy-four (99%) participants delivered the full dose. One participant, an untrained Experienced Caregiver, attempted to prime the syringe and remove the air bubble. In doing so, a small fraction of the drug was expelled prior to injecting, although approximately 90% of the dose was administered successfully. The prior experience of this participant as a caregiver who previously had administered a glucagon injection influenced the participant to remove air. The participant stated that the instructions and warning message (‘do not attempt to remove air’) were clear and offered no changes to mitigate this event.
Table 4. Unaided rescue performance in the validation study.
The success of the rescue injections demonstrated that users could perform the following steps: (1) opening and removing the device from the pouch, (2) removing the needle cap from the device, (2) selecting the correct injection site, (3) exposing the skin on the injection site, (4) inserting the needle into the subcutaneous tissue at a 90-degree angle, (5) administering the full dose of the medication by pressing the plunger all the way down, and (6) rolling the manikin onto its side.
Other errors observed that did not impact the PWD receiving the full dose were committed only by untrained caregivers. Two untrained Naïve Adult Caregivers did not pinch the skin at the time of injection. While it is possible this could result in an intramuscular (IM) injection, this scenario is unlikely as the G-PFS uses a ½” needle which is significantly shorter than the 1” to 1½” needles generally used for IM injections [Citation23]. Further, currently marketed GEKs are labeled for both subcutaneous and IM injection. Four untrained Adult Caregivers (3 Naïve, 1 Experienced) attempted to recap the syringe after administering the dose. Though the instructions stated to not recap the needle, participants thought this was safer than having a needle exposed in a public setting or did not recall learning to not recap the needle from their self-familiarization the week earlier. No needle sticks occurred as a result of recapping the syringe. No close calls or use difficulties were observed in removing the cap or administering the full dose. In addition, no participants called for help during their unaided rescue.
Following the rescue, when asked about their experience with the injection procedure, all participants (100%) stated no difficulty with performing any aspect of the process and expressed positive regard for the Label Guide and injection procedure. No significant group differences were observed between trained or untrained participants. Further, no significant differences were observed between First Responders and lay Caregivers or between adults and adolescents.
Regarding IFU knowledge, all participants successfully understood the allowable injection site per the labeling. All participants successfully exposed bare skin on the injection site and understood they should not inject through clothing. All participants successfully interpreted the phrase in the IFU that instructs the user to store the G-PFS at room temperature. No participants stated difficulty understanding these instructions. All participants successfully interpreted the phrase in the IFU that instruct the user to check the expiration date and color of the medication prior to injecting. No participants stated difficulty understanding these instructions. All participants successfully interpreted the phrase in the IFU that instructs the user to turn the PWD on their side after the injection is complete.
Subjective feedback from participants was positive. The instructional review at the end of the study revealed that participants had no difficulty understanding the IFU or Label Guide. All participants stated that the IFU was easy to understand and utilized a readable font size and illustrations that were clear and effective. Minor enhancements were offered to improve the IFU. The most common recommendation was to move the instruction about exposing the bare skin to before the instruction on removing the needle cap. All participants stated that the Label Guide was a good reference to reinforce the intended procedure.
4. Discussion
The results reported here were from formative and summative human factor studies of this novel, stable liquid glucagon formulation administered from a prefilled syringe. In the formative study, all 11 participants successfully administered the full glucagon dose using the G-PFS. The IFU and Label Guide effectively supported users when performing an unaided emergency rescue attempt with the G-PFS. Additionally, all participants successfully identified/comprehended critical information in the IFU without difficulty, and participants had primarily positive feedback. Three participants with glucagon kit experience stated their preference for the G-PFS versus the current GEKs.
In the summative validation study, across all user groups and training conditions, all 75 participants successfully delivered the unaided rescue injection using the G-PFS, with 74 (99%) administering the full dose of glucagon. The only partial failure occurred when one untrained Experienced Caregiver attempted to prime the syringe and remove the air bubble prior to injecting and expelled approximately 10% of the dose. Overall, subjective feedback across the participants was positive, and the instructional review at the end of the study revealed that participants had no difficulty understanding the IFU or Label Guide.
Treating an episode of severe hypoglycemia in PWDs requires the successful and prompt administration of glucagon, which requires the caregiver to correctly prepare and administer a full dose of glucagon within the acute emergency situation. Across multiple human factor studies of simulated severe hypoglycemic emergencies using marketed GEKs, only a small minority (6%-31%) of trained and untrained participants were able to correctly prepare and administer the full dose of lyophilized glucagon [Citation12,Citation15,Citation24]. The complex process of reconstitution and administration of glucagon using currently available GEKs highlights the risk of administering an incorrect or incomplete dose. These findings continue to highlight the need for new glucagon rescue products to enable prompt and reliable delivery of an accurate, full dose of glucagon by trained and untrained caregivers. The optimal scenario during a severe hypoglycemia emergency is a ready-to-use liquid glucagon with a delivery system that is easy to learn and promptly use within a real world setting.
A novel, soluble liquid glucagon formulation that is stable for 2 years at room temperature is in development for severe hypoglycemia rescue [Citation22]. Early clinical studies have demonstrated that the pharmacokinetic profile of soluble liquid glucagon is effective for treating episodes of hypoglycemia [Citation25–Citation27]. An auto-injector, that contains a prefilled syringe with the identical liquid glucagon formulation to G-PFS, has been used in multiple Phase 3 studies. These multiple clinical studies demonstrate that liquid glucagon successfully treats severe, insulin-induced hypoglycemia [Citation28,Citation29], and has comparable efficacy to existing GEKs. Further, human factors studies with the auto-injector have validated that 99% of users can successfully deliver a full dose of drug (i.e. high functional efficacy) within a simulated emergency setting [Citation11].
The availability of a prefilled syringe for delivering glucagon in an emergency situation provides users with another option for treating episodes of severe hypoglycemia compared to lyophilized products, which may be associated with manufacturing and reconstitution problems that could impact their use. A case report describes a cosmetic defect that occurs while filling vials with lyophilized material that could impact the quality of the end product [Citation30]. Lyophilized products also may require extended time for reconstitution because of a high protein concentration [Citation31].
Limitations of the reported human factors studies include the absence of a comparator, the small sample size, and the qualitative rather than quantitative nature of the outcomes. However, human factors studies, rather than providing statistical comparisons, are designed to be qualitative in nature with the objective of identifying errors in the use of a device. As such, in accordance with FDA guidance, human factors studies should generally include 15 subjects per user group, to provide an adequate assessment of the error rate [Citation10]. The design and implementation of these studies were conducted according to this guidance, and the spectrum of appropriate user groups to administer glucagon in an emergency setting were evaluated.
Despite being an important and effective treatment option for severe hypoglycemia, more than a third of adults with type 1 diabetes report that they do not have a current GEK prescription [Citation32]. Glucagon may be underutilized because of perceived difficulties for both preparation (manual drug reconstitution) and administration (dose calibration), by parents and caregivers. Experts recommend that lyophilized glucagon preparation and administration requires ‘hands on’ practice and ‘follow-up assessment of skills’, as 69% of caregivers have demonstrated obvious difficulties [Citation13].
5. Conclusion
The two studies reported here demonstrate that the G-PFS provides an intuitive, usable and efficient method to deliver a full dose of stable liquid glucagon during an emergency hypoglycemic event. User groups studied included both adolescent and adult, and trained and untrained rescuers. Further, the IFU and Label Guide developed for the G-PFS were found to be usable and acceptable for the intended user audiences. Participants were able to remember the instructions and to follow the information as it was presented on the pouch label guide. They were also able to answer questions about important information contained in the IFU. Collectively, these studies validated that intended users, even those without training, could comprehend, recall, and translate the product instructions into successful product interaction during a simulated rescue scenario. G-PFS addresses the current unmet medical need for a simple and ready-to-use liquid glucagon formulation in a pre-measured, prefilled syringe that enables high functional efficacy. It has the potential to provide an easy-to-use alternative to currently marketed lyophilized glucagon kits for the treatment of severe hypoglycemia.
6. Expert Opinion
Hypoglycemia is a potentially serious complication that occurs with insulin use in persons with both type 1 and type 2 diabetes. Glucagon is the standard of care for treating severe hypoglycemia episodes [6] and should be prescribed for all persons with diabetes (PWDs) who are at risk of clinically significant hypoglycemia. ADA Standards of Care advise that glucagon is indicated for the treatment of hypoglycemia in people unable or unwilling to consume carbohydrates by mouth. When successfully administered, marketed lyophilized glucagon emergency rescue kits (GEKs) are known to reduce mortality and morbidity. The functional efficacy of glucagon is dependent upon the ability of any individual to successfully provide external assistance in a timely manner: to correctly prepare and reliably administer a full dose of the drug during an acute emergency. The consideration of a rescue product with high functional efficacy is critical for appropriate user groups, including adolescent and adult, and trained and untrained users in these scenarios. As part of a product registration package, drug product manufacturers typically conduct a comprehensive risk assessment of the usability of a combination product including human factors formative and validation studies designed to mitigate usability risks. The objectives of the two human factors studies reported here were to determine if glucagon from a prefilled syringe (G-PFS) can be safely and effectively administered by the appropriate user groups and to evaluate the effectiveness of product labeling for providing instructions on the injection procedure. These human factors studies were conducted in accordance with Food and Drug Administration guidance, and the spectrum of appropriate user groups to administer glucagon in an emergency setting were evaluated. The results of these studies demonstrated that G-PFS addresses the current unmet medical need for a simple and ready-to-use liquid glucagon formulation in a pre-measured, prefilled syringe that enables high functional efficacy. G-PFS has the potential to provide an easy-to-use alternative to currently marketed lyophilized glucagon kits for the treatment of severe hypoglycemia.
Author contributions
B Newswanger, S Prestrelski, and A Andre contributed to the conception, design, analysis, interpretation, drafting, revision, and final approval.
Declaration of interest
B Newswanger and S Prestrelski are employees of Xeris Pharmaceuticals and hold stock in the company. A Andre is an employee of Interface Associates, which was contracted to perform the studies. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from Expert Opinion on Drug Delivery for their review work, but have no other relevant financial relationships to disclose.
Acknowledgments
The authors would like to acknowledge the editorial assistance of Richard S. Perry, PharmD in the development of this manuscript, which was supported by Xeris Pharmaceuticals, Inc., Chicago, IL.
Additional information
Funding
References
- Amiel SA, Dixon T, Mann R, et al. Hypoglycaemia in type 2 diabetes. Diabet Med. 2008;25:245–254.
- Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–1912.
- Cryer PE. Severe hypoglycemia predicts mortality in diabetes. Diabetes Care. 2012;35:1814–1816.
- McCoy RG, Van Houten HK, Ziegenfuss JY, et al. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012;35:1897–1901.
- McCoy RG, Lipska KJ, Yao X, et al. Intensive treatment and severe hypoglycemia among adults with type 2 diabetes. JAMA Intern Med. 2016;176:969–978.
- American diabetes association: standards of medical care in diabetes – 2019. Diabetes Care. 2019;42(Suppl 1):S1–S193.
- Kahn PA, Wagner NE, Gabbay RA. Underutilization of glucagon in the prehospital setting. Ann Intern Med. 2018;168:603–604.
- Elwen FR, Huskinson A, Clapham L, et al. An observational study of patient characteristics and mortality following hypoglycemia in the community. BMJ Open Diabetes Res Care. 2015;3:e000094.
- Pearson T. Glucagon as a treatment of severe hypoglycemia: safe and efficacious but underutilized. Diabetes Educ. 2008;34:128–134.
- Center for Devices and Radiological Health. Applying human factors and usability engineering to medical devices. Guidance for Industry and Food and Drug Administration Staff. FDA-2011-D-0469. [ Published 2016 Feb 3; Cited 2019 Jul 24]. Accessed from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/applying-human-factors-and-usability-engineering-medical-devices
- Valentine V, Newswanger B, Prestrelski S, et al. Human factors usability and validation studies of a glucagon autoinjector in a simulated severe hypoglycemia rescue situation. Diabetes Technol Ther. Published online June 20 2019. DOI: 10.1089/dia.2019.0148.
- Harris GDA, Sulway W, Wilkinson M. Glucagon administration - underevaluated and underthought. Pract Diabet Int. 2001;18:22–25.
- Kedia N. Treatment of severe diabetic hypoglycemia with glucagon: an underutilized therapeutic approach. Diabetes Metab Syndr Obes. 2011;4:337–346.
- Rylander D Jr. Treating severe hypoglycemia: rapid mixing of lyophilized glucagon and diluent at point of care with the Enject GlucaPen. J Diabetes Sci Technol. 2015;9:34–37.
- Yale JF, Dulude H, Egeth M, et al. Faster use and fewer failures with needle-free nasal glucagon versus injectable glucagon in severe hypoglycemia rescue: A simulation study. Diabetes Technol Ther. 2017;19:423–432.
- Ghodke S, Nielsen SB, Christiansen G, et al. Mapping out the multistage fibrillation of glucagon. Febs J. 2012;279:752–765.
- Pedersen JS. The nature of amyloid-like glucagon fibrils. J Diabetes Sci Technol. 2010;4:1357–1367.
- Johnson SR, Cooper MN, Davis EA, et al. Hypoglycaemia, fear of hypoglycaemia and quality of life in children with Type 1 diabetes and their parents. Diabet Med. 2013;30:1126–1131.
- Wild D, von Maltzahn R, Brohan E, et al. A critical review of the literature on fear of hypoglycemia in diabetes: implications for diabetes management and patient education. Patient Educ Couns. 2007;68:10–15.
- Willis WD, Diago-Cabezudo JI, Madec-Hily A, et al. Medical resource use, disturbance of daily life and burden of hypoglycemia in insulin-treated patients with diabetes: results from an online survey. Expert Rev Pharmacoecon Outcomes Res. 2013;13:123–130.
- Wood JR, Miller KM, Maahs DM, et al. T1D exchange clinic network. Most youth with type 1 diabetes in the T1D exchange clinic registry do not meet American diabetes association or international society for pediatric and adolescent diabetes clinical guidelines. Diabetes Care. 2013;36:2035–2037.
- Newswanger B, Ammons S, Phadnis N, et al. Development of a highly stable, nonaqueous glucagon formulation for delivery via infusion pump systems. J Diabetes Sci Technol. 2015;9:24–33.
- Craven R, Hirnle C. Fundamentals of nursing: Human health and function. 4th ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2003.
- Ching K, Andre A, Crick M, et al. Biodel’s glucagon emergency management autoreconstitution device demonstrates superior usability compared to marketed glucagon kits in human factors study. Abstract presented at Diabetes Technology Meeting; Bethesda, MD. 2015Oct 22–24.
- Castle JR, Youssef JE, Branigan D, et al. Comparative pharmacokinetic/pharmacodynamic study of liquid stable glucagon versus lyophilized glucagon in type 1 diabetes subjects. J Diabetes Sci Technol. 2016;10:1101–1107.
- Haymond MW, DuBose SN, Rickels MR, et al. T1D Exchange mini-dose glucagon study group efficacy and safety of mini-dose glucagon for treatment of nonsevere hypoglycemia in adults with type 1 diabetes. J Clin Endocrinol Metab. 2017;102:2994–3001.
- Haymond MW, Redondo MJ, McKay S. Nonaqueous, mini-dose glucagon for treatment of mild hypoglycemia in adults with type 1 diabetes: a dose-seeking study. Diabetes Care. 2016;39:465–468.
- Christiansen MP, Cummins MJ, Prestrelski SJ, et al. A phase 3 comparison of a novel liquid glucagon autoinjector to glucagon emergency kit for the treatment of severe hypoglycemia. Diabetes. 2018;67(Suppl 1):1239–P.
- Christiansen MP, Cummins MJ, Prestrelski SJ, et al. A phase 3 comparison of a novel liquid glucagon autoinjector to glucagon emergency kit for the symptomatic relief of severe hypoglycemia. Diabetes. 2018;67(Suppl 1):304–OR.
- Mehta SB, Roy S, Yang HC. “Product on Stopper” in a lyophilized drug product: cosmetic defect or a product quality concern? J Pharm Sci. 2018;107:1736–1740.
- Cao W, Krishnan S, Ricci MS, et al. Rational design of lyophilized high concentration protein formulations-mitigating the challenge of slow reconstitution with multidisciplinary strategies. Eur J Pharm Biopharm. 2013;85:287–293.
- Haymond MW, Liu J, Bispham J, et al. Use of glucagon in patients with type 1 diabetes. Clin Diabetes. 2019;37:162–166.