1. Introduction
Nanoparticle (NP)-based drug delivery is a promising approach for delivering drugs to target tissues by overcoming physiological barriers [Citation1,Citation2]. Traditionally formulated drugs (such as tablets or powders for oral administration, solutions or emulsions for intravenous injection) are designed to improve the bioavailability of therapeutic agents to the whole body. In contrast, targeted NP drug delivery focuses on enhancing the bio-distribution of the drug to the affected organ (such as intestine) and reducing the drug’s systemic partition. Targeted NP-delivered drugs have significant advantages over traditional drugs, which include their greater bio-distribution in the specific tissue, better efficacy toward the disease, and fewer side effects to healthy organs [Citation3]. These advantages make targeted NP-based drug delivery particularly beneficial for the treatment of chronic diseases, as they can avoid the accumulated side effects of conventional drugs during long-term treatment [Citation4]. Therefore, targeted NPs represent an ideal drug delivery system for chronic inflammations, such as inflammatory bowel disease (IBD) [Citation5].
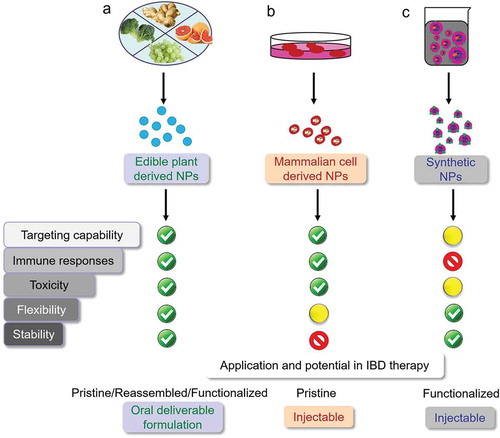
Today, artificially synthesized NPs are the most widely studied targeted platform for delivering therapeutics (such as chemical drugs, siRNAs, proteins, and peptides) to the intestine [Citation6,Citation7]. Although various strategies (including pH-dependent, ROS-responsive, hydrogel-based, and active targeting nano-delivery) have been developed for generating synthetic targeted NPs [Citation8], the high cost of their mass production has limited their applications [Citation9]. Further, for clinical translations, each constituent of the synthesized NPs should be subjected to a comprehensive safety evaluation along with the reduced cost for scaling up synthetic NP production [Citation10]. The use of recently developed targeted NPs derived from naturally occurring NPs (such as edible plant-derived NPs or mammalian cell-derived extracellular vesicles) may overcome these limitations of synthetic NPs (). In fact, natural NPs offer a safer, more sustainable, and better economic drug delivery system. NPs made from natural polymers, including chitosan, sulfated polysaccharides (from marine algae), silk fibroin, and poly amino acids, have been extensively studied and reviewed for their anti-inflammatory potentials [Citation11–Citation15]. In this editorial, we will mainly focus on the new trend of applying novel edible plant- and mammalian cell-derived NP drug delivery systems for IBD treatments.
2. Targeted natural nps from edible plants
Edible plants, including crops, fruits, vegetables, spices, and herbs, are important resources of food and natural medicines for human beings. Natural NPs from edible plants are naturally occurring vesicles with a lipid bilayer membrane and bioactive contents (such as plant secondary metabolites, proteins, and microRNAs). Oral administration of edible plant-derived NPs, such as ginger-derived NPs, have a direct beneficial effect on intestinal inflammation [Citation16]. This approach was based on the hypothesis that the lipid bilayer of plant-derived NPs may protect its inherent bioactive components from degradation by saliva, stomach fluids, active proteolytic enzymes, and intestinal tract fluids [Citation10]. Due to their unique lipid composition, many plant-derived NPs naturally target the intestine [Citation17]. For example, grapefruit-derived nanovesicles (GDNs) were shown to be selectively taken up by intestinal macrophages and reduce dextran sulfate sodium (DSS)-induced colitis in mice [Citation18,Citation19]. The anti-inflammatory effects of GDNs were found to be mediated via the upregulation of heme oxygenase-1 (HO-1) and the downregulation of tumor necrosis factor (TNF)-α and interleukin (IL)-1β in intestinal macrophages. Grape exosome-like nanoparticles (GELNs) were observed to pass through the gut and specifically taken up by intestinal stem cells. Orally administrated GELNs markedly improved the organoid formation of stem cells, promoting the regeneration of intestinal stem cells, and mediating the renewal of intestinal tissues [Citation17]. Using animal models of IBD, our group has shown that ginger-derived nanoparticles (GDNPs) efficiently targeted the colon. They were primarily absorbed by cells of the intestinal lining, which are the active sites of IBD-associated inflammation. In fact, oral administration of GDNPs was also found to increase the survival and proliferation of intestinal epithelial cells (IECs) by reducing the pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) levels, and increasing anti-inflammatory cytokines (IL-10 and IL-22) levels in colitis models. This implies that GDNPs can attenuate damaging factors as well as can promote healing effects [Citation16]. Edible plant-derived NPs that naturally target colonic tissues and have anti-inflammatory properties, therefore represent a novel natural and nontoxic therapy that could easily be scaled up for the treatment of the IBD patients [Citation10].
Beyond the inherent targeting properties of edible plant NPs, self-assembled lipid NPs made from edible plant NP-derived lipids have huge potential for exhibiting a well-defined and robust nano-platform for targeted drug delivery. For example, self-assembled NPs made from GDN lipids were biocompatible, biodegradable, and bio-stable across a wide range of pH values. When the anti-inflammatory drug – methotrexate (MTX), incorporated into the GDN NPs, the encapsulated MTX showed significantly lower toxicity and greater therapeutic efficacy against DSS-induced colitis in mice compared to free MTX [Citation18]. In another impressive study, oral administration of NPs composed of GDNP-derived lipids loaded with siRNA-CD98 effectively targeted the colon tissues and significantly reduced the CD98 expression. This strategy expanded the current perspectives on siRNA-delivery systems by natural NPs and laid the foundation for a safe siRNA-based delivery system to treat the IBD patients [Citation20]. Finally, GELN lipids were found to induce Lgr5+ stem cells. Liposome-like nanoparticles (LLNs) assembled with lipids from GELNs targeted intestinal stem cells, attenuated the production of Lgr5+ stem cells by inhibiting β-catenin–mediated signaling [Citation17]. These findings indicate that re-assembled lipids from plant-derived NPs represent a novel and natural delivery mechanism for preventing and treating IBD affected patients.
3. Exosomes: natural NPs from mammalian cells
Exosomes are extracellular vesicles (EVs) that are secreted by most mammalian cells; they are considered to be natural NPs because their sizes (around 30 to 100 nm) match the criteria of NPs. Their innate ability to deliver naturally incorporated cargo (such as proteins, mRNAs, and microRNAs derived from host cells) to the recipient cells has inspired many innovative drug delivery approaches [Citation21]. Moreover, the homing property of intestinal exosomes has sparked new ideas for the treatment of intestinal diseases, such as IBD [Citation22]. For example, intestinal epithelial cell (IEC)-secreted exosomes were found to be enriched with transforming growth factor beta 1 (TGF-β1) and showed immunosuppressive activity. The transfer of these IEC exosomes into mice with DSS-induced colitis was shown to prevent the development of colitis by inducing regulatory T cells (Tregs) and immunosuppressive dendritic cells (DCs) [Citation23]. In addition, exosomes released by granulocytic myeloid-derived suppressor cells (G-MDSC) were also found to attenuate DSS-induced colitis in mice. G-MDSC treated mice exhibited a decreased proportion of Th1 cells and an increased proportion of Tregs in mesenteric lymph nodes. Moreover, lower serum levels of interferon (IFN)-γ and TNF-α were detected in G-MDSC-treated mice [Citation24]. One another study showed that the exosomes derived from IL-10-treated bone marrow-derived DCs suppressed the trinitrobenzene sulfonic acid (TNBS)-induced colitis [Citation22]. The therapeutic effects of IL-10-induced exosomes were found to be associated with the upregulation of the IL-10 mRNA and the downregulation of the IL-2, IFN-γ, and TNF-α mRNAs in colonic tissue, as well as with the increased number of Tregs in the colonic lamina propria [Citation25]. These studies indicate that exosomes are effective natural nanocarriers for delivering innate anti-inflammatory biological contents for treating IBD.
In addition to suppress inflammation, healing of damaged cells and tissues in the intestine is equally beneficial for the efficient IBD treatment. Several studies have shown that exosomes secreted by mesenchymal stem/stromal cells (MSCs) could serve as therapeutic tools in the repair of tissue injuries in colitis. Exosomes isolated from MSCs of bone marrow, umbilical cord, and adipose tissue are of great interest in such regenerative therapy [Citation25–Citation27]. These MSC-secreted exosomes have the ability of migrating to the injured sites, promoting functional recovery, and modulating immune responses. Thus, MSC exosomes also qualify as a candidate for efficient targeted NP-based delivery system aimed to treat intestinal inflammation.
4. Expert opinion
Targeted nano-therapeutics have been widely studied as an approach for IBD treatment and are expected to be much more effective than traditional drug formulations. Most of the recently developed targeted NPs have been made of synthetic polymers, but these xenobiotics may inevitably arouse the immune response, thus compromise the IBD treatment. Naturally occurring NPs derived from edible plants and mammalian cells are much safer compared to the synthetic polymers, as these endogenous vehicles routinely carry cargoes and communicate between cells under supervision of the immune system.
Among the naturally occurring NPs, edible plant-derived NPs (for being composed of nutrients) are the safest for oral administration. Reassembled NPs made of edible plant NP lipids could be a well-defined system for drug delivery. The surface of plant-derived NPs can further be functionalized to specifically target the disease affected tissue. Ligands, antibodies, and even cell membrane have been used to modify the surface of edible plant-derived NPs to achieve the colon-targeting function and promote drug accumulation in IBD therapy-relevant cells [Citation28,Citation29].
Mammalian cell-derived exosomes have also shown tremendous success against various chronic inflammatory diseases, including IBD, in laboratory preclinical studies [Citation25,Citation26,Citation30,Citation31]. Although surface functionalized NPs from mammalian cells have shown potency in targeted anti-inflammatory applications, like treating acute lung inflammation and brain inflammation, they have not yet been well exploited for the treatment of IBD. Based on literature search, we have found that most of the studies related to mammalian cell-derived exosome treatments have used intravenous or intraperitoneal injections as the drug delivery routes, rather than oral administration (the most favorable route for IBD treatment) [Citation21,Citation22,Citation24–Citation27]. This is reasonable because the lipid composition of the exosomal membrane is different from those of plant-derived NPs. Therefore, the reassembled mammalian cell exosomes are less stable compared to reassembled plant-derived NPs in the harsh environment of the gastrointestinal tract. Additionally, the cost of producing reassembled exosomes from mammalian cells is considerably higher than that of manufacturing targeted plant-derived NPs.
Further investigation of the molecular pathophysiology of IBD will advance the development of intelligent NPs that enable the targeted delivery of drugs to inflamed colonic tissues. An ideal targeted delivery system should orally deliver drugs to key IBD therapy-related cells, especially inflamed intestinal stem cells. We believe that this can be achieved by the surface functionalization of reassembled edible plant-derived NPs. In the near future, orally-deliverable edible-plant derived NPs and injectable mammalian cell-derived EVs with direct therapeutic potential should be subjected to clinical study for the prevention and treatment of IBD.
Declaration of interest
No potential conflict of interest was reported by the authors.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Lautenschlager C, Schmidt C, Fischer D, et al. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv Drug Deliv Rev. 2014 May;71:58–76.
- Zhang S, Ermann J, Succi MD, et al. An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Sci Trans Med. 2015;7(300):10.
- Talekar M, Tran TH, Amiji M. Translational nano-medicines: targeted therapeutic delivery for cancer and inflammatory diseases. Aaps J. 2015 Jul;17(4):813–827.
- Salouti M, Ahangari A. Nanoparticle based drug delivery systems for treatment of infectious diseases. In: Sezer AD, editor. Application of nanotechnology in drug delivery. Intech Open; 2014. p. 155.
- Viscido A, Capannolo A, Latella G, et al. Nanotechnology in the treatment of inflammatory bowel diseases. J Crohns Colitis. 2014 Sep;8(9):903–918.
- Laroui H, Wilson DS, Dalmasso G, et al. Nanomedicine in GI. Am J Physiol Gastrointest Liver Physiol. 2011 Mar;300(3):G371–83.
- Chen Q, Xiao B, Merlin D. Nanotherapeutics for the treatment of inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2017 Jun;11(6):495–497.
- Singh R, Lillard JW Jr. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009 Jun;86(3):215–223.
- Hua S, Marks E, Schneider JJ, et al. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: selective targeting to diseased versus healthy tissue. Nanomedicine. 2015 Jul;11(5):1117–1132.
- Yang C, Zhang M, Merlin D. Advances in plant-derived edible nanoparticle-based lipid nano-drug delivery systems as therapeutic nanomedicines. J Mater Chem B. 2018 Mar 7;6(9):1312–1321.
- Gou S, Huang Y, Sung J, et al. Silk fibroin-based nanotherapeutics: application in the treatment of colonic diseases. Nanomedicine. 2019;14(17):2373–2378.
- Gou S, Huang Y, Wan Y, et al. Multi-bioresponsive silk fibroin-based nanoparticles with on-demand cytoplasmic drug release capacity for CD44-targeted alleviation of ulcerative colitis. Biomaterials. 2019;212:39–54.
- Iglesias N, Galbis E, Diaz-Blanco MJ, et al. Nanostructured chitosan-based biomaterials for sustained and colon-specific resveratrol release. Int J Mol Sci. 2019 Jan 18;20(2):398.
- Lee MC, Huang YC. Soluble eggshell membrane protein-loaded chitosan/fucoidan nanoparticles for treatment of defective intestinal epithelial cells. Int J Biol Macromol. 2019 Jun;15(131):949–958.
- Manlusoc JKT, Hsieh CL, Hsieh CY, et al. Pharmacologic application potentials of sulfated polysaccharide from marine algae. Polymers (Basel). 2019 Jul 8;11(7):1163.
- Zhang M, Viennois E, Prasad M, et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–340.
- Ju S, Mu J, Dokland T, et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther. 2013 Jul;21(7):1345–1357.
- Wang B, Zhuang X, Deng ZB, et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther. 2014 Mar;22(3):522–534.
- Wang Q, Zhuang X, Mu J, et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2013;4:1867.
- Zhang M, Wang X, Han MK, et al. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine. 2017;12(16):17.
- Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016 Jul;6(4):287–296.
- Baghaei K, Tokhanbigli S, Asadzadeh H, et al. Exosomes as a novel cell-free therapeutic approach in gastrointestinal diseases. J Cell Physiol. 2019 Jul;234(7):9910–9926.
- Niel GV, Mallegol J, Bevilacqua C, et al. Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut. 2003;52:8.
- Wang YXH, Lu L, Tian J, et al. Exosomes released by granulocytic myeloid-derived suppressor cells attenuate DSS-induced colitis in mice. Oncotarget. 2016;7(13):13.
- Yang J, Liu XX, Fan H, et al. Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS One. 2015;10(10):e0140551.
- Mao F, Wu Y, Tang X, et al. Exosomes derived from human umbilical cord mesenchymal stem cells relieve inflammatory bowel disease in mice. Biomed Res Int. 2017;2017:5356760.
- Gonzalez MA, Gonzalez-Rey E, Rico L, et al. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009 Mar;136(3):978–989.
- Zhang M, Xiao B, Wang H, et al. Edible Ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol Ther. 2016 Oct;24(10):1783–1796.
- Wang Q, Ren Y, Mu J, et al. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015 Jun 15;75(12):2520–2529.
- Momen-Heravi F, Bala S, Bukong T, et al. Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine. 2014 Oct;10(7):1517–1527.
- Luan X, Sansanaphongpricha K, Myers I, et al. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017 Jun;38(6):754–763.