ABSTRACT
Background: The recent development of high-volume subcutaneous drug delivery using handheld autoinjectors has resulted in longer injection durations. However, the usability of long injections has been neglected. This study aimed to investigate the effects of injection duration on users’ ability to apply injections while holding the device against the skin at the injection site.
Methods: Thirty-two participants among patients, caregivers, and health-care professionals simulated three injections with different injection durations to evaluate usability and user’s force. Linear and quantile regression were then applied to determine the impact of the injection duration on response variables related to user’s force.
Results: A significant negative effect of injection time was found on both the minimum and mean user’s force applied. Initial empirical evidence was also determined on the negative association being more pronounced for users exerting lower force to hold the device against the skin.
Conclusions: The results that are subject to future clinical validation suggest the feasibility of injections lasting up to approximately 30 s using handheld autoinjectors. The participants of the simulated use study successfully applied long-duration injections regardless of disease states, age, or visual and dexterity impairments.
1. Introduction
Subcutaneous administration of drugs is driving changes in global health-care systems [Citation1,Citation2]. In particular, novel device designs enable patient-centered concepts to healthcare and allow users across chronic disease states to routinely self-inject drugs at home, thereby eliminating the need of going to a hospital or infusion center [Citation3–Citation7]. Still, the delivery of around 1 mL of drug has been treated as upper limit using handheld autoinjectors, as the list of recently approved drug products indicates (see ).
Table 1. Injection volume and duration of recently USA FDA-approved drugs using handheld autoinjectors for subcutaneous drug delivery.
The healthcare industry, however, has started to explore the self-administration of single-dose volumes above 1 mL, thus triggering debate around the feasible limits of injectable volume, methods, and rates for subcutaneous drug administration [Citation2,Citation17–Citation19]. The growing interest in subcutaneous injection of large-volume single doses up to 2 mL or more can be attributed to three major causes. First, dosing frequency has been identified as an important factor driving treatment choices across disease states [Citation20–Citation22]. In fact, the complexity of the dosing regimen in terms of frequency, flexibility, and timing has a greater importance on patient perceptions than the efficacy and safety profile of drugs [Citation23]. Second, available studies have advanced our understanding of the pharmacokinetics and tolerability of single high-volume injections, enhancing informed decisions around injection methods and rates [Citation19,Citation24]. Third, technological advances in drug delivery devices and primary packaging formats, such as novel high-volume syringe formats [e.g. Citation25] for use with equally novel and easy-to-use handheld autoinjectors [e.g. Citation4,Citation5,Citation26–Citation28], foster high-volume drug administration. As a consequence, emerging dosing regimens demand the administration of a single bolus dose up to 2 mL [Citation18,Citation19]. In fact, various recently approved biologics and late-stage clinical trials suggest that subcutaneous administration of single high-volume boluses is reaching market introduction [e.g. Citation29–Citation31]. summarizes USA FDA-approved and late-stage development drug products that require the injection of single doses larger than 1 mL.
Table 2. Selected marketed and late-stage development biologics self-administered using 2.25 mL pre-filled syringe (PFS) or handheld 2.25 mL autoinjector (AI). Status by 6 December 2019*.
The self-injection of larger volumes using a single dose, however, has drawbacks, as it translates into longer injection duration. Although patients’ pain sensation during injection appears to be generally low [Citation37] and tends to be independent of the single-dose volume [Citation38], higher perceived pain, subcutaneous pressure, mechanical strain, and injection site leakage have been reported with increasing injection rates [Citation18,Citation19,Citation38,Citation39]. Thus, the benefits of reducing the rate of injection while increasing its duration for single large-volume doses are being pursued. However, the feasibility of longer injection times using handheld drug delivery devices in terms of usability has been mostly neglected. This scarcity of studies is unexpected, because the ability of patients to safely and effectively perform self-injections is emphasized as a prerequisite for successful product validation, regulatory approval, and commercial uptake [Citation26,Citation40].
This study addressed the impact of injection duration on the user’s ability to hold an autoinjector against the skin at the injection site. Usability and user’s force data were collected from simulated self-injections performed by 32 participants including patients, caregivers, and health-care professionals. The user’s force, seen as proxy for the ability to hold the autoinjector against the skin, was collected using in-house foam cushions embedding force sensors. Linear and quantile regression analyses provide initial empirical evidence on the feasibility of longer injection duration in a simulated use setting. Moreover, this study provides insights on the ability of different user groups – including elderly and dexterity-impaired subjects – to withstand such duration. Finally, guidelines for effective self-injection device design are selected to enable safe and effective administration of single high-volume bolus entailing long injection durations.
2. Methods
User’s force and injection duration data were collected from an observational simulated use study based on single-site visits. This study complied with the principles from the User Experience Professionals Association Code of Professional Conduct [Citation41] and the European Pharmaceutical Market Research Association Code of Conduct [Citation42]. Since the participants of the non-interventional study were not exposed to the risk of accidental medication administration, no approval by an ethics committee was required.
2.1. Sample
Thirty-two subjects from the Philadelphia Greater Metropolitan Area, PA, USA, were recruited for this study. Patients (groups G1–G3, n = 19), nonprofessional caregivers (group G4, n = 6) and health-care professionals (group G5, n = 7) were included to reflect the intended usage population of single-use autoinjectors [Citation4,Citation28,Citation43]. From the 24 female and 8 male participants, 20 had injection experience and the remaining 12 did not have experience. The patients were assigned to adolescent (G1, n = 6), adult (G2, n = 6) and elderly (G3, n = 7) groups to evaluate related characteristics (e.g. age, dexterity, visual and manual impairments) that may influence on the ability to maintain sufficient force for safe and effective completion of self-injections. The patients in groups G1–G3 were diagnosed with at least one of the following chronic diseases, which have self-injection treatments available: diabetes, asthma, osteoarthritis, osteoporosis, multiple sclerosis, Parkinson’s disease, psoriasis, psoriatic arthritis, and rheumatoid arthritis. The nonprofessional caregivers (G4) attended patients diagnosed with arthritis, cancer, dementia, diabetes, hypertension, Parkinson’s disease, psoriasis, and stroke. The health-care professionals (G5) were experienced with inpatient (n = 4), outpatient (n = 3), and home-based (n = 2) care, and their experience was related to vials and syringes (n = 6), autoinjectors (n = 2), pre-filled syringes (n = 2), and pen injectors (n = 2). summarizes the participants’ details and occurrence of patients’ chronic disease states. The participants received a small financial compensation after the study.
Table 3. User population and disease states in the simulated use study.
2.2. Study protocol
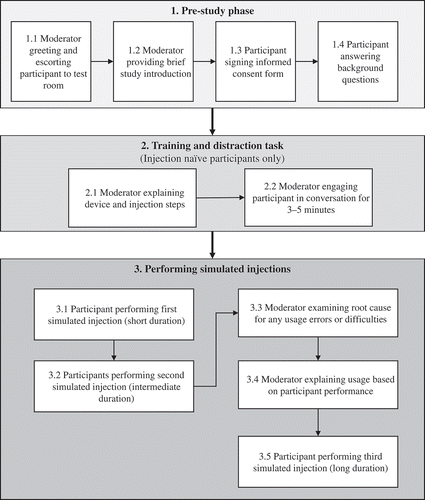
The participants were asked to sequentially simulate injections using handheld autoinjectors at increasing preset injection durations. The study procedure is described in .
As part of the pre-study procedure, a moderator introduced the study purpose and provided general instructions according to a predefined protocol. To verify that the inclusion criteria were met, each participant was asked to complete a questionnaire providing background information on disease states, age, gender, injection experience, handedness, extent of dexterity impairment, vision, neuropathy, and colorblindness (see ). In addition, the participants provided informed consent to use their information for the purpose of this study.
The moderator then presented the autoinjectors and corresponding usage instructions. The usage instructions described the range of the injection duration (7–30 s) and the visual and audible feedbacks occurring at the injection start (audible click), during the injection (colored plunger filling the viewing window), and at the injection end (colored plunger has filled the viewing window; audible click). The injection naïve participants (n = 12) received a verbal walkthrough and a demonstration of the autoinjector usage. This training was followed by a distraction task in which the moderator engaged with each participant in a conversation unrelated to the study purposes for 3 to 5 min. The injection experienced participants (n = 20) received no training. Each participant was then asked to simulate three injections with stepwise increasing preset durations referred to as short (mean: 7.9 s; SD: 1.1 s), intermediate (mean: 17.8 s; SD: 2.3 s), and long (mean: 27.1 s; SD: 2.1 s) injection durations. The instructions for use were available to the participants during the injections, being optional during the first and mandatory during the second injection. The moderator observed and recorded any usage errors or difficulties during the first two injections, and then provided additional training and feedback after the second injection to ensure correct administration for the long injection. No detailed observational usability data were collected during the third injection. Patient groups (G1–G3) simulated self-injections into a sensor-augmented foam cushion attached to their abdomen to resemble a typical self-injection. Nonprofessional caregivers (G4) and health-care professionals (G5) performed the injections into the sensor-augmented foam cushion strapped around the abdomen of a mannequin to resemble injection into another person. Participants held the device in any way they wanted during the injection. There were no observations of patients changing hands during the injection.
2.3. Materials
Each participant sequentially simulated three injections using a standard handheld disposable autoinjector (YpsoMate®, Ypsomed AG, Burgdorf, Switzerland) described earlier [e.g. Citation26,Citation28,Citation44]. The selected autoinjector is well suited for studying the impact of injection duration on user’s force, as its push-on-skin handling concept has been demonstrated to support safe and effective drug self-administration across user groups and disease states [e.g. Citation26,Citation45,Citation46]. To complete the injection, participants needed to sustain a minimum force of 4.0 N to hold the device against the skin during the process. Independent of the user-controlled push-on-skin process to insert the needle into skin, the autoinjector contained a spring-driven mechanism automatically delivering the drug product. The autoinjector samples included in the study contained a 2.25 mL syringe (syriQ®, 27 G needle diameter, Schott, Mainz, Germany) pre-filled with 2.0 mL aqueous solution of polyethylene glycol of varying viscosities to achieve the targeted injection duration of 7–9 s for the short, 15–20 s for the intermediate, and 25–30 s for the long injection at standard temperature and atmosphere. The injection duration referred to the time required to deliver the drug product excluding holding time and safety margins due to system tolerances.
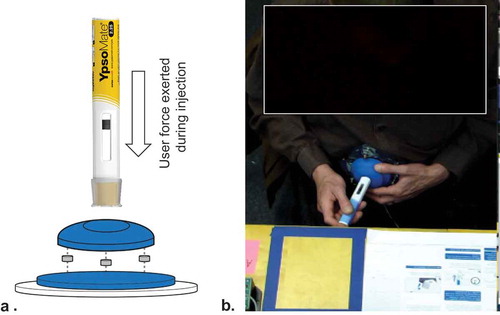
Participants performed simulated injections into custom-built foam cushions embedding force sensors to collect the user’s force data. The force–data were acquired for each simulated injection from three force sensors (LLB-130, FUTEK Advanced Sensor Technology, Irvine, CA, USA) placed between the foam cushion and a round auxiliary plate used to attach the measurement unit to either the patient or mannequin abdomen. The three force sensors were mounted one hundred twenty degrees apart along the edge of the base plate of the injection pad. When a simulated injection was performed, the user’s force was applied to all three sensors. A signal processor (TEDS DB9, FUTEK Advanced Sensor Technology, Irvine, CA, USA) was used to combine the output of all three sensors into a single output value. This approach enabled accurate data collection at any location on the injection pad. The users thus could perform simulated injections on the injection pad wherever they want. After fabrication of the foam cushion base with the force sensors, the sensor readings were verified using a calibrated force meter (DST-110A, IMADA, Toyohashi City, Japan) to ensure measurement accuracy and reliability. illustrates the experimental setup to collect the user’s force data.
Figure 2. Experimental setup for user’s force data collection. Expanded view diagram of sensor-augmented foam cushion to capture user’s force-time data. (a) Three force sensors were placed between the foam cushion and strap to attach the foam cushion on the patients’ or mannequin’s abdomen. Example of patient performing a simulated injection with the sensor-augmented foam cushion attached to abdomen (b) .
2.4. Data analysis
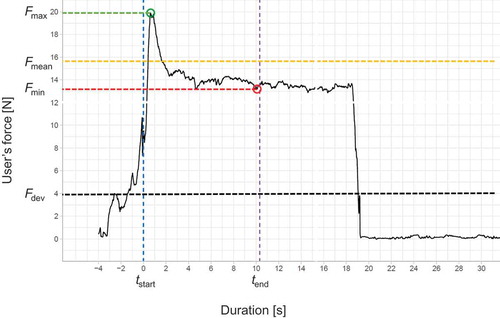
Each user’s force–time curve was recorded using the SENSIT measurement software package (FUTEK Advanced Sensor Technology, Irvine, CA, United States) at a sampling rate of 100 Hz. Raw data were then exported into the R statistical software package for preprocessing, visualization, and identification of relevant variables, namely, injection duration, minimum user’s force (Fmin), mean user’s force (Fmean), and maximum user’s force (Fmax) obtained from the start to completion of the injection. illustrates a user’s force–time curve and defines the user’s force response variables included in the subsequent statistical analyses. also shows the user’s force relative to the user’s force threshold specified by the autoinjector to complete the injection (Fdev = 4 N). The Stata statistical software package version 12 (StataCorp, College Station, TX, USA) was used for descriptive and inferential statistics. For each of the three user’s force variables, Fmin, Fmean, and Fmax, conventional linear regression (pooled OLS) and quantile regression were applied.
Figure 3. Typical user’s force-time curve. The injection duration is the time between injection start tstart and injection end tend. Fmin and Fmax are the minimum and maximum user’s force applied when holding the device against the skin during injection, respectively. Fmean is the mean force exerted during injection to hold the device against the skin. Fdev is the user’s force threshold specified by the handheld autoinjector required to complete the injection.
Three linear regression models (one per force variable) were obtained to study the overall impact of injection duration on user’s force from 86 simulated injections of various durations performed by the 32 participants. Pooled OLS (ordinary least squares) was used because its estimators were preferred over fixed and random effect estimators due to the limited sample size. Compared to fixed or random effect models, pooled OLS provides higher degrees of freedom to improve the accuracy of the estimates. In addition, the Breusch-Pagan test, which determines any participant’s specific effects of injection duration, retrieved no need for random or fixed effect estimators. The three linear regression models (OLS.1–OLS.3) were thus prepared to test the impact of the injection duration on the response variables minimum user’s force Fmin (OLS.1), mean user’s force Fmean (OLS.2), and maximum user’s force Fmax (OLS.3) on a logarithmic scale. The impact of injection duration on user’s force was also assessed using fixed and random effects panel regression (data not shown). The resulting estimators were similar to the reported pooled OLS regression.
Moreover, three quantile regression models (QREG.1–QREG.3) were obtained to complement pooled OLS regression and provide a more detailed description of the effects of injection duration on user’s force variables Fmin, Fmean, and Fmax [Citation47]. Although conventional linear regression (pooled OLS) was appropriate to study the average effect of the injection duration on the user’s force variables, it does not necessarily reflect the full complexity of such relation. In fact, pooled OLS regression provides information only on average effects, neglecting differences across the distribution of user’s force. In contrast, quantile regression allows to identify whether the effects of injection duration on user’s force differs among participants with relatively low and high force levels [Citation48].
The linear regression (OLS.1–OLS.3) and quantile regression (QREG.1–QREG.3) models considered participant age (and its squared effects), gender, injection experience, and dexterity assessed using a standard scale [Citation49] as control variables. The variables were obtained from the questionnaire applied during the pre-study phase. Age and its squared effect were included to control for linear and nonlinear decreasing cognitive performance and grip strength with increasing age [Citation50,Citation51]. Gender controls for differences in related force exertion and dexterity [Citation52,Citation53]. Injection experience controls for learning effects resulting from participants’ prior exposure to other self-injection devices. Dexterity was included to control for participants’ perceived self-rated dexterity impairments caused by chronic inflammatory diseases, such as rheumatoid arthritis [Citation54]. The explanatory variables were stepwise introduced to verify the robustness of the final models.
3. Results
The simulated use study describes the user’s ability to deliver a 2 mL single high-volume bolus with long duration using a handheld autoinjector. Stepwise increasing the average injection duration from 7.9 s (SD: 1.1 s) for the short to 17.8 s (SD: 2.3 s) for the intermediate and 27.1 s (SD: 2.1 s) for the long injection duration did not increase the occurrence of usage errors, usage difficulties, or further deviations from the usage instructions. In contrast, the number of use errors, namely, the removal of the device from the injection site prior to the injection end, decreased from 8 for the short to 2 for the intermediate and 0 for the long injection duration, as users became familiar with the handheld autoinjector. Importantly, all incomplete trials during the first injection were performed by experienced participants, who received no usage training, and all the interruptions occurred within 2 s after the injection onset. Participants’ potential impairments (e.g. age-related conditions, dexterity) did not influence their ability to successfully complete injections. Similarly, usage difficulties and further deviations from the instructions for usage decreased from 31 for the short to 13 for the intermediate injection duration. These events related with users’ difficulties to remove the protective cap (short injection duration: 16; intermediate injection duration: 2), the initiation of the injection process (short injection duration: 1; intermediate injection duration: 0), noncompliance with the holding time (short injection duration: 6; intermediate injection duration: 5), and inappropriate disposal of the device after usage (short injection duration 8; intermediate injection duration: 6).
details the descriptive statistics of the three user’s force variables per group. The highest values for minimum, mean, and maximum user’s force were achieved among health-care professionals (G5). Adolescent patients (G1) exerted the least user’s force to effectively complete self-injections, below both the elder (G3) and adult (G2) patients. A high variation and a decreasing trend in the median values of the user’s force variables was observed when increasing the injection duration. The minimum force (Fmin) decreased 23% on average from the short (mean: 16.59 N; SD: 6.43 N) to the long (mean: 12.76 N, SD: 6.77 N) injection duration. The mean force (Fmean) applied during injection was 18.92 N (SD: 6.75 N) for the short, 17.56 N (SD: 7.20 N) for the intermediate, and 15.44 N (SD: 7.32 N) for the long injection durations. The average maximum force exerted (Fmax) was 25.33 N (SD: 10.04 N) for the short, and 25.69 N (9.25 N) for the mid, and 24.95 N (SD: 12.99 N) for the long injection duration. No user fell below the user’s force threshold specified by the study device (4.0 N) to complete the injection.
Table 4. Minimum (Fmin), mean (Fmean), and maximum (Fmax) user’s force per group for successfully completed injections.
The average effect of injection duration on user’s force was then studied using the three linear regression models (pooled OLS method) by considering the minimum force Fmin (OLS.1), mean force Fmean (OLS.2), and maximum user Fmax (OLS.3) as response variables on a logarithmic scale. shows a graphical representation of the regression models OLS.1–OLS.3.
Figure 4. Average effects of injection duration on user’s force. The red line shows the estimated linear relation (pooled OLS) between injection duration and minimum user’s force Fmin (OLS.1), mean user’s force Fmean (OLS.2), and maximum user’s force Fmax (OLS.3) on a logarithmic scale.
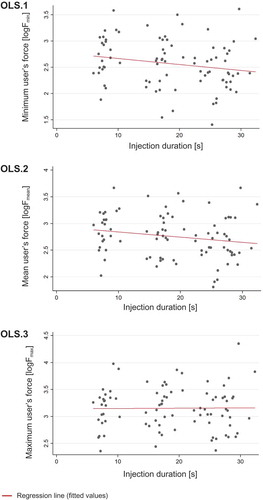
The linear regression model estimates and related standard errors are listed in . The models suggest a significant yet small negative effect of injection duration on the minimum user’s force (coefficient, – 0.013; p < 0.05, OLS.1) and mean user’s force (coefficient, – 0.011; p < 0.01, OLS.2). No significant effect of injection duration on maximum user’s force Fmax was found (coefficient, – 0.000; p > 0.1, OLS.3).
Table 5. Summary statistics for linear regression models (pooled OLS) considering minimum injection force Fmin (OLS.1), mean injection force Fmean (OLS.2), and maximum injection force Fmax (OLS.3) as response variables.
The results also show a significant positive effect of age on minimum user’s force Fmin (coefficient, 0.041; p < 0.05, OLS.1), mean user’s force Fmean (coefficient, 0.042; p < 0.05, OLS.2), and maximum user’s force Fmax (coefficient, 0.051; p < 0.01, OLS.3). Moreover, a significant negative impact was found for the squared age effect on minimum user’s force Fmin (coefficient, –0.001; p < 0.05, OLS.1), mean user’s force Fmean (coefficient, –0.001; p < 0.05, OLS.2), and maximum user’s force Fmax (coefficient, –0.001; p < 0.01, OLS.3). In contrast, dexterity impairment, gender, and injection experience did not show significant impacts on forces Fmin, Fmean, and Fmax. All models showed a similar explanation of the variance in the user’s force, with R2 values ranging from 0.211 (OLS.1) to 0.241 (OLS.3).
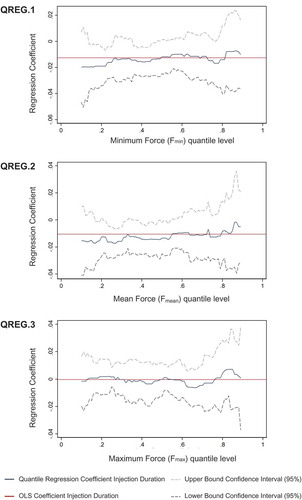
Quantile regression models were then used to describe the varying effects of injection duration across the entire distribution of the user’s force variables. shows the quantile regression results for the three response variables, Fmin, Fmean, and Fmax. The models unveil more complex effects than those using the linear regression. The results of quantile regression model QREG.1 suggest that the negative effect of injection duration depends on the level of minimum user’s force (Fmin). Specifically, the negative effect was more pronounced for the lower levels of minimum user’s force, whereas it diminished and became largely insignificant for the middle and higher quantiles. Similarly, quantile regression for mean user’s force Fmean (QREG.2) highlights that the negative effect of injection duration on this force was the most notable for users exerting the least force to hold the device against the skin, but it diminished for users exerting relatively high mean forces. This suggests that increasing the injection duration has more effect on users exerting relatively low mean forces. Quantile regression model QREG.3 shows no significant effect of injection duration on maximum user’s force Fmax along the entire distribution, thereby confirming the results of linear regression model OLS.3.
Figure 5. Quantile-specific effects of injection duration on user’s force. The graphs show the estimated quantile regression coefficients of injection duration along the vertical axis across the distribution of minimum user’s force Fmin (QREG.1), mean user’s force Fmean (QREG.2), and maximum user’s force Fmax (QREG.3) along the horizontal axis. The two dashed lines show the upper and lower bounds of the 95% confidence interval for the quantile regression coefficients shown as blue lines. The red horizontal line shows the linear regression estimates (pooled OLS) of the mean effect of injection duration on the user’s force variables.
4. Discussion
Despite the growing research interest in subcutaneous administration of higher volumes of drugs using a single large dose [Citation17,Citation19,Citation37,Citation38,Citation55], the understanding of the feasibility of longer injection duration for handheld autoinjectors remains limited. The non-interventional simulated use study reported here provides initial empirical insights on the effect of injection duration on user’s force as proxy for the user’s ability to hold a handheld autoinjector against the skin. The results from this study suggest that users can effectively complete injections of high-volume single doses using handheld autoinjectors with durations beyond 10 s for the actual delivery of the drug product. All participants, regardless of gender, age, dexterity impairment or past injection experience, were able to effectively complete injections with a duration of up to approximately 30 s. The reported empirical analysis contributes to addressing concerns around increasing the injection duration to lower the injection rate and thereby, as past research suggested, reduce perceived pain, subcutaneous pressure, mechanical strain, and injection site leakage [Citation18,Citation19,Citation38,Citation39]. The results show that the administration of 2 mL using a single bolus indeed offers a feasible alternative to the sequential administration of various 1 mL pre-filled syringes or handheld autoinjectors to receive the targeted dose [e.g. Citation56–Citation59].
Usage errors such as incomplete injections mainly occurred for participants with injection experience, who did not receive any device training. Although the injection duration was stepwise increased across subsequent injections, the usage errors and difficulties decreased. The observed decrease may be due to the users’ increasing familiarity with the injection device, the instructions provided before the second injection, and the additional training prior the third injection. Still, the results suggest that usage difficulties were independent of the injection duration. Furthermore, the value of device training and familiarization with the instructions seems important regardless of the user category, as experienced users may be prone to erroneous inferences about the device functionality based on their prior exposure to other types of devices or injection habits.
This study may contribute to quantitatively determining the influence of injection duration on the user’s force exerted throughout the process. Linear regression models (pooled OLS) show a significant yet small negative effect of the injection duration on the minimum and mean user’s force. These results suggest that an extension of the injection duration by 1 s reduces the minimum (Fmin) and mean (Fmean) user’s force by 1.3% and 1.1%, respectively. Characterizing the effects of injection duration on user’s force has important implications for effective device design. The force reduction by the 1-second increase in injection duration may provide a specification of user requirements for the development of future handheld autoinjectors. For instance, consider a user holding a device with 15 N against the skin to trigger injection. Assuming that the device requires sustaining a minimum user’s force of 4 N (the value for the device used in the study) to keep the needle inserted while holding the device against skin, the 1.3% injection duration–minimum user’s force relation suggests the average user would remain capable to effectively complete an injection lasting 56 s. Clearly, the theoretical feasibility shall be distinguished from the patient’s preference and consider the study limitations discussed below. Nevertheless, the results provide important initial empirical support for the feasibility of injection duration beyond the current upper limit.
Interestingly, the analysis did not reveal any significant effect of the injection duration on maximum user’s force Fmax exerted to hold the device against skin at the injection site. In fact, linear regression suggests a non-significant relationship between the injection duration and maximum user’s force, which may be explained by a qualitative assessment of the user’s force–time curves. A pronounced initial user’s force peak occurs when pushing the handheld device against the skin. This initial force is required to ensure needle insertion and trigger injection with the device, and thus this force may be independent of the overall force applied during the injection (see ).
The quantile regression models were then used to further analyze the relation between injection duration and user’s force. Providing a more detailed description of the effects of injection duration across the entire distribution of user’s force, quantile regression complements linear regression (i.e. pooled OLS), which assumes constant regression coefficients per model, and thus only reflects the average effect of the injection duration on the user’s force [Citation48]. Quantile regression unveiled that the negative effect of injection duration on the minimum (Fmin) and mean (Fmean) user’s force was more accentuated for patients exerting the least force throughout the injection. In addition, the negative association between injection duration and user’s force may reduce for participants pushing the autoinjector with high average force against the injection site. Interestingly, further descriptive analyses revealed that participants applying low force primarily comprise adolescent and female elder patients. Hence, these participants were the most sensitive regarding the negative effect of longer injection duration on the exerted force. Thus, besides the evidence on the injection duration negatively impacting the user’s force, quantile regression also details the relation across the distribution of user’s force. In contrast, linear regression may provide misleading information when compared to more complex effects of injection duration on user’s force, as it mainly captures the lower tail of the distribution.
The only control variable that showed a significant overall relationship with the user’s force was age and its nonlinear squared effect. Specifically, the results show that adolescent and elder patients applied a lower mean force than adults. Although prior research has repeatedly shown grip strength to decrease with age [Citation50], the elderly population included in this study did not have any difficulties in applying the force required to effectively complete injections with longer duration. In fact, elder patients consistently exerted higher mean forces than adolescents in this study. The possibly higher confidence of younger patients in their ability to complete an injection may translate into a lower mean user’s force. In contrast, the elder patients’ awareness of impairments potentially impeding their ability to hold the autoinjector against the skin may result in overcompensation, thus increasing the downward pressure on the handheld device during injection. The significant negative impact of the squared age on user’s force, however, shows that the phenomenon is mitigated for the upper tail of the age distribution. The reduction in physical strength eventually takes precedence and yields lower user’s force. On the other hand, injection experience, gender, and dexterity impairment did not exhibit significant effects on the users’ ability to hold the autoinjector against the skin at the injection site.
Another contribution of this study is the methodological advancement for analyzing patients’ behaviors during self-injections. Although prior research on pulmonary drug delivery has routinely used sensor-based approaches to characterize patients’ behaviors [e.g. Citation60–Citation64], such approaches did not stimulate research on subcutaneous self-injections. To the best of the authors’ knowledge, the only exception is the study published by Xiao and colleagues [Citation44] on measurements of needle displacements derived from device motion to assess patients’ completion of injections. However, the motion capture system used to assess the needle position was physically attached to the autoinjector and wired to a measurement unit. Consequently, this technique prevented the use of non-modified injection devices and constrained the movement of the subjects handling the device. The novel methodological approach introduced in the present study using a custom-built sensor-augmented foam cushion does not have the same physical limitations, thus allowing a realistic analysis of the physical performance beyond conventional endpoints of self-injection device usability research in terms of safe and effective use of handheld autoinjectors [e.g. Citation65–Citation68].
Although the results from this study provide important initial insights into the effects of injection duration on the user’s ability to hold a handheld device against the skin, they should be interpreted in the light of some limitations. The results are based on an exploratory non-randomized observational study with limited sample size, and thus they are closely linked to the participants’ characteristics, possibly limiting external validity. Further work should reexamine and extend the understanding of the relations between injection duration and user’s force considering a broader population. The non-clinical simulated use context of this study also calls future research to further investigate the relationship between injection duration, single-dose volume and selected key clinical variables, such as perceived pain, subcutaneous back-pressure, or injection site leakage. Moreover, the effect of injection duration on user’s force may be partially masked by device learning and familiarization effects, including the instructions for use given before the second injection and training before the third injection. Future study designs should address the differential impact of injection duration and device familiarization on user’s force variables by, for instance, randomizing the sequence of injection durations. Furthermore, this study relies on the user’s force as proxy for the ability to hold an autoinjector against the skin at the injection site during injection. Future studies can focus on other variables that may also influence the patient’s ability to handle and accept long injection durations, such as cognitive impairments or the context of device usage. Future research can also explore patient’s ability to complete long injections along with the preference for long injections using handheld devices compared to other emerging devices, such as wearable on-body injectors [e.g. Citation69,Citation70].
5. Conclusion
This simulated use study explored the feasibility of long-duration injection of a single high-volume bolus using a handheld autoinjector. The results from an observational simulated use study with the participation of patients, caregivers, and health-care professionals suggest–subject to validation in clinical practice–the feasibility of injection durations up to approximately 30 s in a simulated use setting. In addition, the occurrence of usage errors and difficulties seems to be independent of the injection duration. Moreover, a significant yet small negative effect of injection duration on the minimum and mean user’s force applied to hold the device against the skin at the injection site is determined. From linear and quantile regression, initial empirical evidence is obtained on the small negative relation of the injection duration with user’s force (i.e. extending the injection duration by 1 s resulted in a reduction of the minimum injection force by 1.3%). This relation was also more pronounced for users who applied lower forces to hold the device against the skin during injection. Finally, the results show that elder female and adolescent patients were the most sensitive regarding the negative effect of longer injection duration on user’s force.
Declaration of interest
A Schneider, J Lange, C Jordi, P Richard and P Müller, are each employees for Ypsomed AG. P Sneeringer, R Nayyar and M Jovanoff are each employees of Design Science. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Kim H, Park H, Lee SJ. Effective method for drug injection into subcutaneous tissue. Sci Rep. 2017 Aug 29;7(1):9613.
- Jones GB, Collins DS, Harrison MW, et al. Subcutaneous drug delivery: an evolving enterprise. Sci Transl Med. 2017 Aug 30;9(405). PubMed PMID: 28855399; eng. DOI:10.1126/scitranslmed.aaf9166.
- Callis Duffin K, Bagel J, Bukhalo M, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017 Jan;31(1):107–113. PubMed PMID: 27500949.
- Hudry C, Lebrun A, Moura B, et al. Evaluation of usability and acceptance of a new autoinjector intended for methotrexate subcutaneous self-administration in the management of rheumatoid arthritis. Rheumatol Ther. 2017 Jun;4(1):183–194. PubMed PMID: 28243967; PubMed Central PMCID: PMC5443726.
- Kivitz A, Baret-Cormel L, van Hoogstraten H, et al. Usability and patient preference phase 3 study of the sarilumab pen in patients with active moderate-to-severe rheumatoid arthritis. Rheumatol Ther. 2017 Dec;5. PubMed PMID: 29209946. DOI:10.1007/s40744-017-0090-2.
- Schiff M, Koo J, Jin E, et al. Usability and acceptability of the abatacept pre-filled autoinjector for the subcutaneous treatment of rheumatoid arthritis. Adv Ther. 2016 Feb;33(2):199–213. PubMed PMID: 26833303; PubMed Central PMCID: PMC4769728.
- Sheikh SZ, Hammer AE, Fox NL, et al. Evaluation of a novel autoinjector for subcutaneous self-administration of belimumab in systemic lupus erythematosus. Int J Clin Pharmacol Ther. 2016 Nov;54(11):914–922. PubMed PMID: 27668697; PubMed Central PMCID: PMC5100660.
- AstraZeneca. Full prescribing information: FASENRA (benralizumab injection, for subcutaneous use; 2019.
- GlaxoSmithKline. Full prescribing information: NUCALA (mepolizumab) for injection, for subcutaneous use; 2019.
- AMAG. Full prescribing information: VYLEESI (bremelanotide injection), for subcutaneous use; 2019.
- Genentech. Full prescribing information: ACTEMRA® (tocilizumab) injection, for intravenous or subcutaneous use; 2019.
- Eli Lilly. Full prescribing information: EMGALITY (galcanezumab-gnlm) injection, for subcutaneous use; 2018.
- Amgen. Full prescribing information: AIMOVIGTM (erenumab-aooe) injection, for subcutaneous use; 2018.
- Sanofi-Aventis. Full prescribing information: KEVZARA (sarilumab) injection, for subcutaneous use; 2019.
- Eli Lilly. Full prescribing information: TALTZ (ixekizumab) injection, for subcutaneous use; 2019.
- GlaxoSmithKline. Full Prescribing Information: BENLYSTA (belimumab) injection, for subcutaneous use. 2019.
- Mathaes R, Koulov A, Joerg S, et al. Subcutaneous injection volume of biopharmaceuticals—pushing the boundaries. J Pharm Sci. 2016 Aug 01;105(8):2255–2259.
- Jain M, Doughty D, Clawson C, et al. Tralokinumab pharmacokinetics and tolerability when administered by different subcutaneous injection methods and rates. Int J Clin Pharmacol Ther. 2017 Jul;55(7):606–620. PubMed PMID: 28590244; PubMed Central PMCID: PMCPMC5480250. eng.
- Dias C, Abosaleem B, Crispino C, et al. Tolerability of high-volume subcutaneous injections of a viscous placebo buffer: a randomized, crossover study in healthy subjects. AAPS PharmSciTech. 2015 Oct;16(5):1101–1107. PubMed PMID: 25693652; PubMed Central PMCID: PMCPMC4674646. eng.
- Qin L, Chen S, Flood E, et al. Glucagon-like peptide-1 receptor agonist treatment attributes important to injection-naïve patients with type 2 diabetes mellitus: a multinational preference study. Diab Ther. 2017 April 01;8(2):321–334.
- Bolge SC, Goren A, Brown D, et al. Openness to and preference for attributes of biologic therapy prior to initiation among patients with rheumatoid arthritis: patient and rheumatologist perspectives and implications for decision making. Patient Prefer Adherence. 2016;10:1079–1090. PubMed PMID: 27390518.
- Nguyen H, Posner J, Kalsekar I, et al. A discrete-choice experiment to quantify patient preferences for frequency of glucagon-like peptide-1 receptor agonist injections in the treatment of type 2 diabetes AU - Hauber, A. Brett. Curr Med Res Opin. 2016 Feb 01;32(2):251–262.
- Stewart KD, Johnston JA, Matza LS, et al. Preference for pharmaceutical formulation and treatment process attributes. Patient Prefer Adherence. 2016;10:1385–1399. PubMed PMID: 27528802.
- Berteau C, Filipe-Santos O, Wang T, et al. Evaluation of the impact of viscosity, injection volume, and injection flow rate on subcutaneous injection tolerance. Med Devices. 2015;8:473–484. PubMed PMID: 26635489.
- Sacha G, Rogers JA, Miller RL. Pre-filled syringes: a review of the history, manufacturing and challenges. Pharm Dev Technol. 2015 Jan 02;20(1):1–11.
- Lange J, Richard P, Bradley N. Usability of a new disposable autoinjector platform device: results of a formative study conducted with a broad user population. Med Devices (Auckl). 2015;8:255–264. PubMed PMID: 26082667; PubMed Central PMCID: PMC4461121.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015 Jun;29(6):1082–1090. PubMed PMID: 25243910.
- Schneider A, Kolrep H, Jordi C, et al. How to prevent medication errors: a multidimensional scaling study to investigate the distinguishability between self-injection platform device variants. Expert Opin Drug Deliv. 2019 Aug 03;16(8):883–894.
- Banerji A, Riedl MA, Bernstein JA, et al. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA. 2018;320(20):2108–2121.
- Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377(22):2113–2122. PubMed PMID: 29171818.
- Thaçi D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016 Jan 02;387(10013):40–52.
- Teva. Full prescribing information: AJOVYTM (fremanezumab-vfrm) injection, for subcutaneous use; 2018.
- Regeneron. Full prescribing information: DUPIXENT® (dupilumab) injection, for subcutaneous use; 2018.
- Valeant. Full prescribing information: SILIQ™ (brodalumab) injection, for subcutaneous use; 2018.
- Novartis. Study of efficacy and safety of secukinumab 2 mL auto-injector (300 mg) in subjects with moderate to severe plaque psoriasis (NCT03589885); 2018 [cited 2019 Feb 02].
- Brightling CE, Chanez P, Leigh R, et al. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. 2015 Sept 01;3(9):692–701.
- Zijlstra E, Jahnke J, Fischer A, et al. Impact of injection speed, volume, and site on pain sensation. J Diabetes Sci Technol. 2018;12(1):163–168. PubMed PMID: 28990437.
- Doughty DV, Clawson CZ, Lambert W, et al. Understanding subcutaneous tissue pressure for engineering injection devices for large-volume protein delivery. J Pharm Sci. 2016 Jul 01;105(7):2105–2113..
- Allmendinger A, Mueller R, Schwarb E, et al. Measuring tissue back-pressure – in vivo injection forces during subcutaneous injection. Pharm Res. 2015 Jul;32(7):2229–2240. PubMed PMID: 25537343; eng.
- Schneider AE, Lange J. Pen devices for self-injection: contrasting measured injection force with users’ perceived ease of injection. Expert Opin Drug Deliv. 2018 Feb 01;15(2):115–125.
- UPA Association. UPA code of professional conduct for usability practitioners. Bloomingdale (IL): Usability Professionals’ Association; 2005.
- EPMR Association. EphMRA 2017 code of Conduct V4.indd. European Pharmaceutical Market Research Association; 2017.
- Tischer B, Mehl A. Patients’ and nurses’ preferences for autoinjectors for rheumatoid arthritis: results of a European survey. Patient Preference Adherence. 2018;12:1413.
- Xiao X, Li W, Clawson C, et al. Evaluation of performance, acceptance, and compliance of an auto-injector in healthy and rheumatoid arthritic subjects measured by a motion capture system. Patient Prefer Adherence. 2018:12:515–526. PubMed PMID: 29674844.
- Barker P, Ferguson GT, Cole J, et al. Single-use autoinjector functionality and reliability for at-home benralizumab administration: GRECO trial results. J Allergy Clin Immunol. 2019;143(2):AB96.
- Bernstein D, Pavord ID, Chapman KR, et al. Usability of mepolizumab single-use prefilled autoinjector for patient self-administration. J Asthma. 2019;1–12. DOI:10.1080/02770903.2019.1630641.
- Koenker R, Hallock KF. Quantile regression. J Econ Perspect. 2001;15(4):143–156..
- Lê Cook B, Manning WG. Thinking beyond the mean: a practical guide for using quantile regression methods for health services research. Shanghai Arch Psychiatry. 2013;25(1):55–59. PubMed PMID: 24948867; eng..
- Sautner J, Andel I, Rintelen B, et al. Development of the M-SACRAH, a modified, shortened version of SACRAH (score for the assessment and quantification of chronic rheumatoid affections of the hands). Rheumatology. 2004;43(11):1409–1413.
- Martin JA, Ramsay J, Hughes C, et al. Age and grip strength predict hand dexterity in adults. PloS One. 2015;10(2):e0117598.
- Cerella J. Information processing rates in the elderly. Psychol Bull. 1985;98(1):67–83.
- Montoye HJ, Lamphiear DE. Recreation. Grip and arm strength in males and females, age 10 to 69. Res Q. 1977;48(1):109–120.
- Haward BM, Griffin MJ. Repeatability of grip strength and dexterity tests and the effects of age and gender. Int Arch Occup Environ Health. 2002;75(1–2):111–119.
- Bellamy N, Sothern RB, Campbell J, et al. Circadian rhythm in pain, stiffness, and manual dexterity in rheumatoid arthritis: relation between discomfort and disability. Ann Rheum Dis. 1991;50(4):243–248.
- Heise T, Nosek L, Dellweg S, et al. Impact of injection speed and volume on perceived pain during subcutaneous injections into the abdomen and thigh: a single-centre, randomized controlled trial. Diabetes Obes Metab. 2014 Oct;16(10):971–976. PubMed PMID: 24720741; eng.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis – results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. PubMed PMID: 25007392.
- Novartis. Full prescribing information: COSENTYX® (secukinumab) injection, for subcutaneous use; 2018.
- Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for crohn’s disease. N Engl J Med. 2016;375(20):1946–1960. PubMed PMID: 27959607..
- Janssen. Full prescribing information: STELARA® (ustekinumab) injection, for subcutaneous or intravenous use revised 10/2017 ed. Horsham, PA: Janssen Pharmaceutical Companies; 2016.
- Azouz W, Chetcuti P, Hosker H, et al. Inhalation characteristics of asthma patients, COPD patients and healthy volunteers with the Spiromax(R) and Turbuhaler(R) devices: a randomised, cross-over study. BMC Pulm Med. 2015 May;1(15):47. PubMed PMID: 25927483; PubMed Central PMCID: PMCPMC4450517. eng..
- D’Arcy S, MacHale E, Seheult J, et al. A method to assess adherence in inhaler use through analysis of acoustic recordings of inhaler events. PloS One. 2014;9(6):e98701. PubMed PMID: 24905012; PubMed Central PMCID: PMCPMC4048229. eng.
- Holmes MS, Seheult JN, Geraghty C, et al. A method of estimating inspiratory flow rate and volume from an inhaler using acoustic measurements. Physiol Meas. 2013;34(8):903.
- Taylor TE, Holmes MS, Sulaiman I, et al. Monitoring inhaler inhalations using an acoustic sensor proximal to inhaler devices. J Aerosol Med Pulm Drug Deliv. 2016 Oct;29(5):439–446. PubMed PMID: 26859629; eng.
- Taylor TE, Zigel Y, Egan C, et al. Objective assessment of patient inhaler user technique using an audio-based classification approach. Sci Rep. 2018 Feb 01;8(1):2164.
- Glenn M, Kate Van B, Alan GZ, et al. Safe and effective use of the once weekly dulaglutide single-dose pen in injection-naïve patients with type 2 diabetes. J Diabetes Sci Technol. 2015;9(5):1071–1079. PubMed PMID: 25901022.
- Limmroth V, Gerbershagen K. Single-use autoinjector for once-weekly intramuscular injection of IFNbeta-1a. Expert Opin Drug Deliv. 2014 Dec;11(12):1969–1978. PubMed PMID: 25255732.
- Seddighzadeh A, Hung S, Selmaj K, et al. Single-use autoinjector for peginterferon-beta1a treatment of relapsing-remitting multiple sclerosis: safety, tolerability and patient evaluation data from the phase IIIb ATTAIN study. Expert Opin Drug Deliv. 2014 Nov;11(11):1713–1720. PubMed PMID: 25073663.
- Pachon JA, Kivitz AJ, Heuer KU, et al. Assessing usability, label comprehension, pen robustness and pharmacokinetics of a self-administered prefilled autoinjector pen of methotrexate in patients with rheumatoid arthritis. SAGE Open Med. 2014;2:2050312114564241. PubMed PMID: 26770759; PubMed Central PMCID: PMC4712753.
- Torjman MC, Machnicki R, Lessin J, et al. Evaluation of an investigational wearable injector in healthy human volunteers. Expert Opin Drug Deliv. 2017 Jan;14(1):7–13. PubMed PMID: 27809609; eng.
- Patel J, Rainess RA, Benfield MJ, et al. Retrospective analysis of clinical outcomes associated with the use of pegfilgrastim on-body injector in patients receiving chemotherapy requiring granulocyte colony-stimulating factor support.0 (0):0018578719867659. Hosp Phar. 2019. DOI: 10.1177/0018578719867659.