1. Therapeutic significance of phyto-therapeutics
Herbs are preferred by many instead of conventional medications to relieve their disease symptoms. As a result, the recent pharmaceutical industry focused on discovering novel therapeutic agents from medicinal plants. About 25% of the natural new molecular entities approved by the FDA are coming from plant origin. For example, the first plant-derived NME is the painkiller morphine which is a benzylisoquinoline alkaloid isolated from Papaver Somniferum and authorized for utilization in 1827 [Citation1]. Its action on the nervous system is majorly due to induced neurotransmitter discharge inhibition from presynaptic neurons [Citation2].
Morphine was followed by the discovery of more active entities as paclitaxel, an alkaloid extracted from the pacific yew plant (Taxus brevifolia) [Citation3]. Paclitaxel stabilizes the microtubule polymer, by binding to β-tubulin, inhibiting the dynamic microtubule disassembly process needed for appropriate mitotic spindle formation and chromosome segregation meanwhile cell division [Citation4]. Presently, paclitaxel is utilized in various types of cancer including ovarian, breast, lung, pancreatic, and human herpesvirus-8-induced Kaposi sarcoma and others. Also, Catharanthus roseus is the herb from which the indole alkaloids vinblastine and vincristine were isolated [Citation5]. They are broad-spectrum anticancer drugs that attach to a site of β-tubulin other than that of the paclitaxel to prevent microtubule polymerization leading to M-phase specific cell cycle arrest.
Unfortunately, some challenges complicate the efficient delivery of phyto-therapeutics including the physicochemical drawbacks like poor solubility and instability. In addition, pharmacokinetic challenges, like poor absorption and low bioavailability, can cause problems in clinical trials. Moreover, the hurdles of translation of the phyto-therapeutics in pharmaceutical industry including batch-to-batch variation and the low potency remain a great barrier [Citation6].
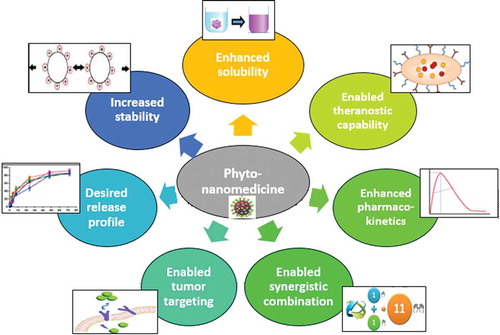
From the few examples mentioned previously, the significance and the challenges facing the herbal medicine in treatment of major diseases is shown. The matter has drawn the attention toward providing it in a safe and effective form. Nowadays, novel delivery systems such as liposomes, phytosomes, nanoparticles, and nanocapsules have been confirmed to be more suitable as delivery systems for the phyto-medicinal constituents compared to the conventional systems. The utilization of those delivery systems enhanced bioavailability, pharmacological activity, extended delivery, and offered physical and chemical stability prolonging the half-life () [Citation7]. Some of the most outstanding examples of herbal nanomedicines are summarized in .
Table 1. Examples of nanomedicine-based phyto-therapeutics for drug delivery applications.
2. Herbal nanomedicines: challenges and benefits
2.1. Improved solubility
Most of the biologically active polyphenolic phytoconstituents suffer from poor water solubility leading to poor systemic absorption, and low bioavailability upon oral administration. This poor solubility also limits the route of administration as they cannot be taken intravenously.
The poor solubility of diosmin (DSN), a potent herbal drug with chemo-preventive action, in water and most organic solvents encouraged researchers to overcome this challenge by nano-encapsulation. For example, DSN was successfully incorporated in a nano matrix composed of the hydrophobic protein gliadin via pH-modulated nanoprecipitation technique for oral therapy of hepatocellular carcinoma [Citation8]. The cationic protein, lactoferrin, was then electrostatically coated onto the surface of gliadin nanospheres to enhance their intestinal internalization and tumor targeting. Gliadin NPs successfully solubilized DSN within its hydrophobic core while being compatible with the aqueous medium based on their nanosize therefore the release profile was significantly improved. Another solubilizing approach includes the hydrophobic complexation of the water-insoluble flavonoid resveratrol (RSV) within phospholipid bilayer enveloping casein micelles encapsulating monascus yellow pigments (MYPs) [Citation9,Citation10]. The developed multi-reservoir nanocarriers proved synergistic aromatase inhibiting the action of both drugs which resulted in enhanced antitumor efficacy as demonstrated by remarkable reduction in tumor volume from 1466.3 mm3 for positive control to 770.55 and 590.3 mm3 for free RSV/MYPs combination and nanocarriers, respectively.
Among the most efficient approaches to increase the solubility of water-insoluble herbal drugs is their conversion into nanosuspension ‘nanocrystals’. The saturation solubility of the poorly soluble herbal drug Silymarin (SLM) nanosuspension after its formulation into lyophilized tablet has been significantly increased to 4616.1 mg compared to only 1324.3 mg for pure SLM. Moreover, the dissolution rate of the tablet expressed as T90 was increased up to 44.2 min [Citation11]. This effect could be correlated to the big increase in surface area of SLM by size reduction as well as the improved wettability imparted by PVA as a hydrophilic polymer used in tablet formulation.
2.2. Sustained release
Rapid clearance and decreased bioavailability represent a great challenge in formulation when dealing with highly water-soluble herbal drugs. Moreover, the fast drug release causes many side effects after administration as a result of the premature release in systemic circulation before reaching its target [Citation12,Citation13].
Berberine (BRB), an isoquinoline alkaloid has relatively high aqueous solubility, demonstrating relatively fast initial release in aqueous media. To prolong its release, two techniques were utilized by Abdelmoneem et al. [Citation14] including hydrophobic ion pairing with sodium deoxycholate (SDC) to facilitate its entrapment into the hydrophobic core of amphiphilic casein micelles. Moreover, chemical crosslinking of the nanoparticles with a crosslinker of much lower cytotoxicity, genipin, was conducted prior to spray-drying into a stable dry micellar powder. The micelles successfully enabled sustained release of BRB and enhanced the efficiency of tumor targeting. While free BRB demonstrated a fast release nearly 100% after only 1 h, the genipin-crosslinked micelles significantly decreased the burst release with 45–55.5% of BRB was released after 24 h.
In another study, hydrophobic ion pairing (HIP) with SLS to BER was done before its entrapment into lipid NPs [Citation15]. After 2 h, HIP complexation could lower greatly the initial burst release of drug from NPs from 69.65% to 43.8%. After 24 h, the accumulated drug release came to 68.4% from BER/SLS-NPs as to 79.45% without HIP complexation. These results proved the significant role of HIP complexation to increase the drug release time. The HIP made the drug more hydrophobic and hence improved its entrapment in the lipid matrix of NPs thereby assigning slow drug release.
2.3. Increased stability
Clinical translation of many of the pharmacologically active herbal drugs is hampered by their poor chemical stability. Therefore, many approaches including nano-encapsulation were attempted to improve their stability and protect those sensitive compounds from premature degradation and hence improve their bioavailability. One example of the sensitive herbals is Epigallocatechin-3-gallate (EGCG) being vulnerable to pH, temperature, and oxygen, thus restricting its applications. Incorporation of EGCG into solid lipid nanoparticles (SLNs) was found to enhance its stability under both gastric and intestinal conditions with a prolonged release of about 21.8% of the drug at the end of the incubation in SIF (pH = 6.8, containing pancreatin) at 37°C [Citation16]. In addition to lipid nanocarriers, the efficient mucoadhesive nature of chitosan nanoparticles encouraged its use for entrapment of EGCG to facilitate its adsorption to the intestinal wall, and hence increase its absorption and bioavailability. Pre-complexation of the drug within the inclusion cavity of sulfobutylether-cyclodextrin prior to encapsulation into chitosan nanoparticles resulted in better retention of the antioxidant activities of EGCG with improved ferric reducing power in high pH (7.0) compared to acidic pH (2–5) [Citation17]. This could be attributed to the flocculation of nanoparticles at higher pH thus inhibiting the drug release conferring more protection [Citation18].
2.4. Improved pharmacokinetics
Nano-encapsulation of drugs offers various pharmacokinetic privileges, such as improved biodistribution, high metabolic stability, increased membrane permeability, enhanced bioavailability, and sustained duration of action. The herbal flavonoid, quercetin was successfully encapsulated into phytosomes formed by its complexation with phosphatidylcholine based on hydrogen bonding as well as hydrophobic interaction [Citation19]. The pharmacokinetic analysis showed significant enhancement in quercetin absorption when formulated as phytosomes compared to free unformulated quercetin at the same dose. The calculated Cmax and AUC of the quercetin phytosomes exhibited 20- and 18-fold higher than free quercetin. In another investigation, the oral bioavailability and pharmacokinetics of resveratrol has been remarkably improved by its encapsulation into zein nanocapsules [Citation20]. The nanocapsules exhibited longer half-life (8.16 h) and lower mean clearance (0.072 ml/h) of resveratrol compared with 1.25 h and 1.02 ml/h for free drug cosolvent, respectively. The improved pharmacokinetics can be explained by its solubilization into the oily core of nanocapsules as well as higher oral stability and resistance to digestion imparted by glutaraldehyde crosslinking of the water-insoluble zein shell.
Encouraged by the potent anticancer and anti–inflammatory activity of the water-insoluble herbal drug celastrol (CST), Freag et al. [Citation21] developed self-assembled phytosomal nanocarriers of CST to improve its solubility and gastrointestinal absorption and hence enhance its oral bioavailability. Oral administration of CST phytosomes into rabbits revealed higher Cmax (460 ng/ml) with relative bioavailability (AUC 0–8) of 410.7% compared with crude CST suspension (Cmax of 90 ng/ml). The phytosomes were thought to enhance the drug bioavailability by increasing its solubility, as well as the improved uptake into the intestinal mucosa caused by phospholipids.
2.5. Enhanced tumor targeting
Active targeting nanoparticles offer several pros including boosting drug selectivity to target cells and selective cytotoxicity to bypass side effects to normal cells, improvement of anticancer activity of the drug and its accumulation in cancer cells, and effectiveness in control of drug release [Citation22].
Dual tumor targeting of quercetin and sorafenib to hepatocellular carcinoma cells is conducted in one study where lactobionic acid (LA) and glycyrrhetinic acid (GA) were chemically coupled to lactoferrin (LF) shell of nanocapsules through carbodiimide coupling reaction [Citation18]. LA and GA decoration were used to target ASGP and GA receptors, respectively; moreover, LF has intrinsic targeting action for liver cancer cells via binding to LDL-related protein receptors. This maximized tumor-targeting capability decreased the interaction with body tissues, hence reduced opsonization and the rapid clearance of LF-nanocapsules. The enhanced tumor-targeting effect was demonstrated as a significant reduction of Ki-67 expression in mice treated with the GA/LF- nanocapsules (20%) compared to (∼93.76%) for diethyl nitrosamide-induced HCC untreated mice models revealing decreased tumor cell proliferation where the number of liver tumor nodules is lowered by >93%. Moreover, the enhanced liver tumor-targeting resulted in decreasing the side effects and increased safety as reflected by significant improvement in the level of liver enzymes ALT and AST [Citation18]. In another investigation, the high systemic and male reproductive toxicities of the herbal drug Triptolide, greatly effective against HCC, was efficiently reduced by its loading into galactosylated-chitosan nanoparticles [Citation23]. This can be displayed as significantly decreased indices of testes and epididymis as well as liver & kidney indexes. This can be accounted for the tumor-targeting effect mediated internalization of nanoparticles through asialoglycoprotein receptor resulting in high liver tumor accumulation in vivo.
2.6. Enabled synergistic combinations
Combination drug therapy in cancer can have additive or synergistic effects, minimize drug resistance, or fight against expected resistance resulting in better safety and efficacy. In our laboratory, we have used a drug combination of the herbal drug wogonin (WOG) and rapamycin (RAP) co-encapsulated into amphiphilic zein-lactoferrin nano-micelles for breast cancer therapy [Citation24]. WOG can synergistically circumvent the feedback survival activation pathway induced by RAP monotherapy by initially arresting mTOR/PI3K/AKT pathways. Coadministration of the dual drug-loaded micelles into Ehrlich’s mammary tumor-bearing mice has maximized the antitumor efficacy demonstrated as synergistic decrease in tumor volume to about 29.27% compared to the control group showing progressive rise in tumor volume (717.43%) after 3 weeks of treatment [Citation24]. For targeting of gastric cancer, the herbal drug ginsenoside was co-incorporated with paclitaxel inside liposomes [Citation25]. In addition to its anticancer effect, ginsenoside can act as a targeting ligand via binding to GLUT of tumor cells. The nano-combination successfully achieved induction of apoptosis via synergistic cell-cycle arrest. In vivo, compared to two PTX nano-formulations, Lipusu® and Abraxane®, the dual therapy liposomes remarkably reduced the tumor size and weight where the tumors had almost disappeared [Citation25].
On another avenue, another mode of synergistic cancer chemo/radiation therapy is promoted by the co-delivery of curcumin and bismuth sulfide nanoparticles (Bi2S3) via folate-targeted albumin nanoparticles (Bi2S3@BSA-FA-CUR). Bi2S3 nanoparticles could both sensitize X-ray proficiency and carry curcumin whereas the folate moiety enhanced the tumor accumulation of albumin nanoparticles. Therefore, intravenous injection of Bi2S3@ BSA-FA-CUR nanoparticles combined with X-ray radiation in tumor-bearing mice showed enhanced antitumor efficacy and significant tumor size reduction compared to single drug or radiation therapy [Citation26].
Among the most promising approaches for cancer therapy, nanoparticle-mediated photo-thermal (PTT) and photodynamic therapy (PDT) attracted much attention. In PTT, near-infrared light irradiation is converted into heat energy by some types of nanoparticles thus killing the tumor cells. The photothermal properties of gold-resveratrol (Au@Res) nanoparticles were exploited to induce cancer-cell apoptosis. The nanoparticles demonstrated powerful killing of A375 cells under 808-nm laser irradiation with outstanding apoptosis increase in comparison with Au@Res nanoparticles without irradiation [Citation27].
On the other hand, PDT needs the presence of light, a photosensitizer and oxygen in the target tissue. The photosensitizing agent reaches its excited state at specific wavelengths, initiating a cascade of events where reactive oxygen species (ROS) are generated mediating cytotoxic effects. Irradiation of MCF-7 breast adenocarcinoma cells and HFF-1 fibroblasts treated with curcumin, a potential photosensitizer, nanoemulsion demonstrated high phototoxic effects, thus decreasing the cell viability. Two doses post-irradiation resulted in 52% and 28% viability of HFF-1 and MCF-7 cells, respectively. An indicator of apoptosis, the level of Caspases 3 and 7 activity, was significantly elevated post-treatment with curcumin nanoemulsion in comparison with the control group treated with blank nanoemulsion [Citation28].
2.7. Enabled theranostic capability
Multifunctional nano-theranostics contain a targeting agent that directs the carrier to the cancer cells, an agent for imaging the tumor tissue, and a therapeutic agent to be selectively delivered to the tumor. Theranostic nanocapsules for breast cancer were developed by including a therapeutic combination of the herbal alkaloid honokiol together with celecoxib and quantum dots (QDs) as fluorescence imaging probe [Citation29]. While the drugs were solubilized within the oily core of nanocapsules, the QDs were chemically coupled to the lactoferrin tumor-targeting shell. The strong fluorescence provided by QDs enabled both in vitro visualization of the nanocapsules internalized within MCF-7 cells and ensured their effective localization into the tumor tissues in vivo. However, low fluorescence intensity was detected in the mice liver and renal tissues [Citation29]. In addition to the fluorescent imaging probes, magnetic nanoparticles provide a remarkable capability for tumor imaging using magnetic resonance imaging (MRI). The herbal drug curcumin (CUR)-loaded polyethyleneimine (PEI)-coated magnetic Fe3O4 nanohybrids could be utilized as theranostic MRI contrast agent [Citation30]. Moreover, the strong inherent fluorescence of CUR enabled the detection of the nanoparticles within SK-N-MC cells. The observed t1/2 of CUR loaded MNPs was 2.5 times more than free CUR proving proper serum bioavailability of the system.
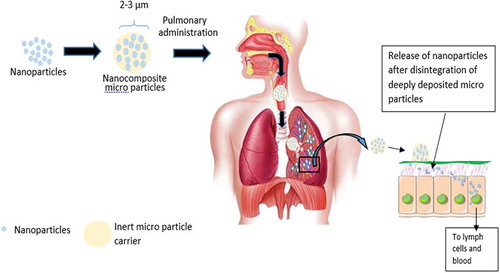
2.8. Enhanced localized drug delivery
Inhalational chemotherapeutics are an encouraging substitute for a localized therapy to lung tumors to systemic therapy. This approach avoids the toxic systemic side effects caused by intravenous administration and delivery of large doses of chemotherapeutics. However, quick exhalation of NPs leads to diminishing their lung deposition [Citation31]. Therefore, microencapsulated nanoparticles (nanocomposites) possess dual advantages of enabling efficient aerosolization, deposition deeply in the lung, and effective tumor cells targeting (). The herbal drug Ellagic acid (EA) was co-formulated with doxorubicin in the form of lactoferrin–chondroitin nanocomplex developed via electrostatic complexation [Citation22]. EA was reported to delay drug resistance via inhibition of P-glycoprotein efflux pumps, allowing DOX to work efficiently. To enhance their lung deposition, the nanocomplex was microencapsulated into mannitol microparticles. The resultant inhalable dry powder nanocomposites of the drug combination showed a significant reduction of the IC50 value by 3.8-fold compared with the freely combined drug solution [Citation22]. In another investigation, the herbal compound naringin was administered to lung through nebulized PLGA nanoparticles together with, celecoxib, the COX-2 inhibitor drug [Citation32]. The radiolabeled drugs exhibited high accumulation in lung tissues 30 min post aerosolization. Noticeable amounts of the drugs were found in metastatic sites of lung cancer such as bones, liver, and brain.
Another mode of localized drug delivery involves the intratumoral administration of herbal nanomedicines. Direct injection of curcumin-loaded PLGA-DSPE-PEG nanoparticles into glioblastoma tissue of rat glioma-2 tumor model showed significant reduction in tumor volume due to the increased drug concentration at the tumor tissue. In comparison, intravenous administration of the curcumin nanoparticles resulted in elevated tumor volume because of its inefficient crossing of the blood–brain barrier [Citation33].
3. Clinical trials/applications of herbal nanomedicines
In a single-arm study, 36 patients received phospholipidated curcumin for treatment of nonalcoholic fatty liver disease in the form of capsules (100 mg curcuminoids) administered thrice daily for 8 weeks [Citation34]. Significant reductions in all lipid indices, along with serum total cholesterol, LDL-C, HDLC, triglycerides, and non-HDL-C were noticed as well as an appreciable decrease in serum uric acid level [Citation34]. In another randomized placebo-controlled study, 40 obese women with a 3-month lifestyle intervention received phytosomes of green tea extract (Greenselect Phytosome®) with the aim of weight maintenance after deliberately weight loss to overcome the weight regain by maintaining energy expenditure [Citation35]. A formula containing 150 mg/dose of Greenselect Phytosome® and 15 mg/dose of pure piperine was administered twice a day, for 3 months. The treated group demonstrated further decrease in weight and fat mass, which retained its stability for 2 and 3 months.
In another controlled study, 160 patients received lecithin curcumin phytosomal complex (Meriva®) [Citation36]. The lecithinized curcumin might lessen the side effects accompanied by chemo‐ and radiotherapy, revealing the beneficial effect of curcumin phytosomes as an assistant agent for cancer therapy. This effect can be assigned to the enhanced anti‐oxidative responses and suppressed inflammatory pathways by curcumin.
4. Expert opinion
Active pharmaceutical agents from herbal origin are gaining a tremendous attention as alternatives and/or adjuvants for conventional chemotherapeutic agents. A promising pharmacological activity has been proved for many of herbal ingredients including antineoplastic, anti–inflammatory, hepatoprotective, cardiotonic, etc. However, many challenges impede the clinical translation of those phyto-therapeutics such as poor solubility, low bioavailability, chemical instability, short half-life, nonselective biodistribution, and systemic toxicity. Therefore, numerous approaches have been explored to overcome the major limitations of phytomedicines. Among the most outstanding strategies, nano-encapsulation of phyto-therapeutics has been proposed for improving their physicochemical properties and increasing their efficacy through various mechanisms. The aqueous solubility and dissolution rate of poorly soluble herbal medicines have been significantly improved upon encapsulation into various types of nanocarriers resulting in enhanced drug bioavailability. The combined use of nanotechnology and controlled drug release strategies such as hydrophobic ion pairing and chemical crosslinking could modulate the rate of drug release from the nanoparticles. Moreover, modification of the surface of nanocarriers with specific tumor-targeting ligands enables targeted delivery of herbal medicines to their specific site of actions and hence increase their efficacy and reduce systemic toxicity. In addition, the use of multi-reservoir (multi-compartmental) nanocarriers enables combined delivery of multiple drugs which facilitates coadministration of herbal and other drugs for synergistic activity. Advanced nanocarrier formulations such as nanocomposite microparticles have been successfully used for effective lung deposition of active herbal drugs to enhance their pulmonary localized delivery.
Based on the abovementioned studies, we can expect the promising future of cancer therapy using herbal nanomedicines. However, some critical issues have to be considered. First, the use of plant extracts seems not practical and less efficient compared to purified isolated herbal ingredients. Presence of mixed components may result in antagonistic effect or unwanted side effects and also affect impact the therapeutic potency of the main ingredient. Second, the anticancer efficacy of the herbal component should be justified in terms of IC50 for cancer cell viability. Herbal drugs with high IC50 values reveal low ant-cancer potency and hence will not represent good candidates. Third, the drug–drug interactions between herbal components and other conventional FDA-approved medicines should be thoroughly investigated to select the proper synergistic combinations.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Patridge E, Gareiss P, Kinch MS, et al. An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov Today. 2016;21(2):204–207.
- Vaughan C, Ingram S, Connor M, et al. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390(6660):611.
- Wani M. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1972;19:2325–2326.
- Snyder JP, Nettles JH, Cornett B, et al. The binding conformation of Taxol in β-tubulin: a model based on electron crystallographic density. Proc Nat Acad Sci. 2001;98(9):5312–5316.
- Johnson IS, Wright HF, Svoboda GH, et al. Antitumor principles derived from Vinca rosea Linn I. Vincaleukoblastine and leurosine. Cancer Res. 1960;20(7):1016–1022.
- Bonifacio BV, da Silva PB, Dos Santos Ramos MA, et al. Nanotechnology-based drug delivery systems and herbal medicines: a review. Int J Nanomedicine. 2014;9:1.
- Elzoghby A. Editorial (Thematic issue: nanocarriers based on natural polymers as platforms for drug and gene delivery applications). Curr Pharm Des. 2016;22(22):3303–3304.
- Abdelmoneem MA, Mahmoud M, Zaky A, et al. Decorating protein nanospheres with lactoferrin enhances oral COX-2 inhibitor/herbal therapy of hepatocellular carcinoma. Nanomedicine. 2018;13(19):2377–2395.
- El-Far SW, Helmy MW, Khattab SN, et al. Phytosomal bilayer-enveloped casein micelles for codelivery of monascus yellow pigments and resveratrol to breast cancer. Nanomedicine. 2018;13:481–499.
- El-Far SW, Helmy MW, Khattab SN, et al. Folate conjugated vs PEGylated phytosomal casein nanocarriers for codelivery of fungal- and herbal-derived anticancer drugs. Nanomedicine. 2018;13:1463–1480.
- Ibrahim AH, Rosqvist E, Smått J-H, et al. Formulation and optimization of lyophilized nanosuspension tablets to improve the physicochemical properties and provide immediate release of silymarin. Int J Pharm. 2019;563:217–227.
- Chen Z, Zhu Q, Qi J, et al. Sustained and controlled release of herbal medicines: the concept of synchronized release. Int J Pharm. 2019;560:116–125.
- Zhou J, Zheng J, Zhang Y, et al. Chitosan hydrogel delivery system containing herbal compound functions as a potential antineuroinflammatory agent. ACS Omega. 2019;4(6):10185–10191.
- Abdelmoneem MA, Mahmoud M, Zaky A, et al. Dual-targeted casein micelles as green nanomedicine for synergistic phytotherapy of hepatocellular carcinoma. J Control Release. 2018;287:78–93.
- Kabary DM, Helmy MW, Elkhodairy KA, et al. Hyaluronate/lactoferrin layer-by-layer-coated lipid nanocarriers for targeted co-delivery of rapamycin and berberine to lung carcinoma. Colloids Surf B Biointerfaces. 2018;169:183–194.
- Shtay R, Keppler JK, Schrader K, et al. Encapsulation of (─)-epigallocatechin-3-gallate (EGCG) in solid lipid nanoparticles for food applications. J Food Eng. 2019;244:91–100.
- Liu F, Majeed H, Antoniou J, et al. pH and temperature stability of (−)-epigallocatechin-3-gallate-β-cyclodextrin inclusion complex-loaded chitosan nanoparticles. Carbohydr Polym. 2016;149:340–347.
- Abdelmoneem M, Elnaggar M, Hammady R, et al. Dual-Targeted lactoferrin shell-oily core nanocapsules for synergistic targeted/herbal therapy of hepatocellular carcinoma. ACS Appl Mater Interfaces. 2019;11:26731–26744.
- Riva A, Ronchi M, Petrangolini G, et al. Improved oral absorption of quercetin from quercetin Phytosome®, a new delivery system based on food grade lecithin. Eur J Drug Metab Pharmacokinet. 2019;44(2):169–177.
- Elzoghby AO, El-Lakany SA, Helmy MW, et al. Shell-crosslinked zein nanocapsules for oral codelivery of exemestane and resveratrol in breast cancer therapy. Nanomedicine. 2017;12(24):2785–2805.
- Freag MS, Saleh WM, Abdallah OY. Self-assembled phospholipid-based phytosomal nanocarriers as promising platforms for improving oral bioavailability of the anticancer celastrol. Int J Pharm. 2018 Jan 15;535(1-2):18–26.
- Abd Elwakil MM, Mabrouk MT, Helmy MW, et al. Inhalable lactoferrin–chondroitin nanocomposites for combined delivery of doxorubicin and ellagic acid to lung carcinoma. Nanomedicine. 2018;13(16):2015–2035.
- Zhang Y-Q, Shen Y, Liao -M-M, et al. Galactosylated chitosan triptolide nanoparticles for overcoming hepatocellular carcinoma: enhanced therapeutic efficacy, low toxicity, and validated network regulatory mechanisms. Nanomedicine. 2019;15(1):86–97.
- Sabra SA, Elzoghby AO, Sheweita SA, et al. Self-assembled amphiphilic zein-lactoferrin micelles for tumor targeted co-delivery of rapamycin and wogonin to breast cancer. Eur J Pharm Biopharm. 2018;128:156–169.
- Hong C, Wang D, Liang J, et al. Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer. Theranostics. 2019;9(15):4437.
- Nosrati H, Charmi J, Salehiabar M, et al. Tumor targeted albumin coated bismuth sulfide nanoparticles (Bi2S3) as radiosensitizers and carriers of curcumin for enhanced chemoradiation therapy. ACS Biomater Sci Eng. 2019;5(9):4416–4424.
- Wang W, Tang Q, Yu T, et al. Surfactant-free preparation of Au@ resveratrol hollow nanoparticles with photothermal performance and antioxidant activity. ACS Appl Mater Interfaces. 2017;9(4):3376–3387.
- Machado FC, de Matos RPA, Primo FL, et al. Effect of curcumin-nanoemulsion associated with photodynamic therapy in breast adenocarcinoma cell line. Bioorg Med Chem. 2019;27(9):1882–1890.
- AbdElhamid AS, Zayed DG, Helmy MW, et al. Lactoferrin-tagged quantum dots-based theranostic nanocapsules for combined COX-2 inhibitor/herbal therapy of breast cancer. Nanomedicine. 2018;13(20):2637–2656.
- Aeineh N, Salehi F, Akrami M, et al. Glutathione conjugated polyethylenimine on the surface of Fe3O4 magnetic nanoparticles as a theranostic agent for targeted and controlled curcumin delivery. J Biomater Sci Polym Ed. 2018;29(10):1109–1125.
- Abdelaziz HM, Gaber M, Abd-Elwakil MM, et al. Inhalable particulate drug delivery systems for lung cancer therapy: nanoparticles, microparticles, nanocomposites and nanoaggregates. J Control Release. 2018;269:374–392.
- Said-Elbahr R, Nasr M, Alhnan MA, et al. Nebulizable colloidal nanoparticles co-encapsulating a COX-2 inhibitor and a herbal compound for treatment of lung cancer. Eur J Pharm Biopharm. 2016;103:1–12.
- Orunoğlu M, Kaffashi A, Pehlivan SB, et al. Effects of curcumin-loaded PLGA nanoparticles on the RG2 rat glioma model. Mater Sci Eng C. 2017;78:32–38.
- Panahi Y, Kianpour P, Mohtashami R, et al. Efficacy of phospholipidated curcumin in nonalcoholic fatty liver disease: a clinical study. J Asian Nat Prod Res. 2019;21(8):798–805.
- Gilardini L, Pasqualinotto L, Di Pierro F, et al. Effects of Greenselect Phytosome® on weight maintenance after weight loss in obese women: a randomized placebo-controlled study. BMC Complement Altern Med. 2016;16(1):233.
- Belcaro G, Hosoi M, Pellegrini L, et al. A controlled study of a lecithinized delivery system of curcumin (Meriva®) to alleviate the adverse effects of cancer treatment. Phytother Res. 2014;28(3):444–450.