ABSTRACT
Background
Anti-tumor necrosis factor (anti-TNF) adherence is suboptimal. ava®, a reusable electromechanical self-injection device (e-Device) developed for certolizumab pegol (CZP) administration, aims to overcome some barriers to increase adherence. This study evaluates patient experience of the e-Device and its training materials and determines patient device preference.
Methods
CZP-treated patients were recruited from the Netherlands, Denmark and Sweden. Patients completed a pre-injection Assessment of Self-Injection (ASI) questionnaire investigating self-injection perception. After training, patients administered 3 consecutive self-injections using the e-Device, patient experience of each was assessed using the post-injection ASI. An additional questionnaire evaluated training materials. After Injection 3, patients indicated their preference: the e-Device or their previous device.
Results
59 patients participated; most rated the e-Device highly for satisfaction, self-confidence and ease of use. The (negative) feelings and pain and skin reactions domains had low ratings. Post-injection ASI domain scores were similar following each of the 3 e-Device injections. Training materials were rated highly (video: 8.4/10; step-by-step guide: 8.4/10). 57.1% (32/56) patients preferred the e-Device over their previous self-injection device.
Conclusions
Patients were satisfied with the e-Device and most preferred it over other self-injection devices. By improving patient experience, the e-Device may help increase medication adherence.
1. Introduction
Anti-tumor necrosis factors (anti-TNFs) are effective treatments for moderate to severe chronic inflammatory diseases, including rheumatoid arthritis (RA), axial spondyloarthritis (axSpA), psoriatic arthritis (PsA), plaque psoriasis and Crohn’s disease [Citation1–Citation5]. The majority of anti-TNFs are administered subcutaneously and can be self-injected by patients [Citation6,Citation7]. Self-injection allows more flexibility and independence as patients can administer their treatment in their home without the help of healthcare professionals (HCPs) [Citation7,Citation8]. Regular trips to a hospital can be a burden for patients, both financially (e.g. travel costs, cost of taking time off work) and due to reduced mobility and high levels of fatigue [Citation9]. By providing benefits for the patient, self-injection can also benefit caregivers, the healthcare system and society generally [Citation7].
Patients may encounter challenges when self-injecting anti-TNFs [Citation10,Citation11]. These can include patient needle phobia, a lack of confidence in their own ability to safely and effectively administer injections and remembering the dates of their self-injections [Citation10–Citation12]. As a result, adherence to anti-TNF treatment regimens is often suboptimal, negatively impacting patient outcomes and disease control [Citation13–Citation15]. Many patients with chronic inflammatory diseases (CIDs) are dependent on lifelong treatment to suppress joint damage and to avoid functional impairment. Tailoring self-injection devices to individual patient preference may improve patients’ adoption of the device and, consequently, medication adherence [Citation10,Citation16,Citation17]. Additionally, introducing more advanced technologies in the management of CIDs provides a unique value proposition as this approach may advance patient engagement and empowerment [Citation18].
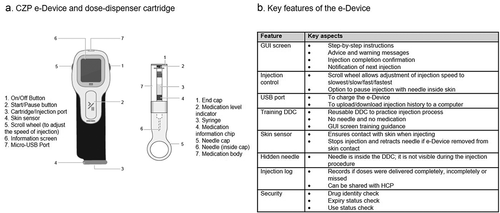
ava® is a new reusable electromechanical self-injection device (e-Device) with disposable dose-dispenser cartridges designed for use with the anti-TNF certolizumab pegol (CZP) [Citation19,Citation20]. It was developed in conjunction with OXO (New York, NY, USA) and with patients, to help personalize their self-injection experience. The e-Device includes a range of features to improve patient experience of self-injection () [Citation21]. This study is the first to report the use of the e-Device in a real-world setting and investigate its usability for patients treated with CZP in clinical practice. Secondly, this study aimed to investigate the patient experience of self-injection with the e-Device independently of their previous experience of self-injection devices. Patient preference for the e-Device compared to their current self-injection device was also determined. Finally, this study also aimed to evaluate the training materials designed for patients using the e-Device.
2. Methods
2.1. Study design
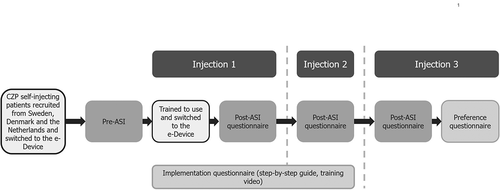
Data were collected before use of the e-Device and immediately after three consecutive injections using the e-Device. Injections were carried out two weeks apart (). Patients initially answered the pre-injection Assessment of Self-Injection (pre-ASI) questionnaire to assess their feelings about self-injection and self-confidence at study baseline. Patients were then trained by an HCP to self-inject using the e-Device and given a step-by-step guide on the usage of the e-Device. In addition, patients were encouraged to watch a provided training video on self-injection with the e-Device. The next three injections in the patients’ treatment regimen were administered using the e-Device at their homes, and are referred to as Injection 1, Injection 2 and Injection 3 (either the maintenance dose of 200 mg CZP every two weeks, or the loading dose of 400 mg CZP every two weeks). Each patient self-injected the CZP dose prescribed by their clinician; the injected drug, volume and excipients were not changed from their previous device. At e-Device training, patients were not permitted to use pain relief (e.g. numbing cream or heat/cold packs) before or after self-injection. After administering each of the three injections, patients completed the post-injection Assessment of Self-Injection (post-ASI), to assess patient experience using the e-Device. In addition, patients answered questions about the training video and step-by-step guide, following their first or second injection. After the third injection, patients were asked their preference between the e-Device or the device(s) they had previously used for self-injection.
2.1.1. Patients
Patients treated with CZP were recruited from the Netherlands, Denmark and Sweden through three rheumatology clinics and gave signed informed consent to be included in the study. Patients were adults (aged between 18 and 85 years) with experience of self-injecting CZP and/or other biologics using either a pre-filled syringe (PFS) or a pre-filled pen (PFP) for RA, axSpA or PsA. Patients were excluded if they suffered from a visual impairment that made it impossible to read or complete the required questionnaires, or if they were not fluent in the language of the questionnaires.
2.1.2. Study evaluations
Patient experience of self-injection, both before and after using the e-Device, were measured using the ASI questionnaire. This questionnaire is a version of the Self-Injection Assessment Questionnaire (SIAQ) modified to assess self-injection using an e-Device. The pre-ASI section comprised six preliminary questions split into two domains, ‘(negative) feelings about self-injection’ and ‘self-confidence’ [Citation11]. Questions asked patients about their feelings regarding needles and injections, and their self-assessment of their ability to correctly, cleanly and safely inject CZP.
After each injection, patients were asked to complete the post-ASI questionnaire comprising of 44 questions evaluating patients’ experience of using the e-Device. The questionnaire was specifically developed to assess patients’ experience of the novel features of the e-Device; the questionnaire did not ask patients to compare the e-Device with their previous self-injection devices. These questions were grouped into six domains for which an overall domain score was calculated [Citation11]. Domain themes included (negative) feelings, self-image, self-confidence using the e-Device, pain and skin reactions, ease of use and overall satisfaction with the e-Device.
Within the first two weeks of the study, patients also completed the implementation questionnaire comprised of 12 questions assessing patient opinions on the training materials provided with the e-Device. Patients rated the training video and step-by-step guide on whether they were easy to understand, detailed enough and interesting (training video) or useful (step-by-step guide). The final question about each training aid asked patients about the overall usefulness of the training materials. The implementation questionnaire also included open-ended questions to collect further information about patients’ opinions on the training materials.
After the third injection, patients completed the preference questionnaire, answering nine questions on their preference for different CZP self-injection devices. This included questions on features specific to the e-Device and on their overall preference. To answer these questions, patients were asked to compare their experience of the e-Device with their previous experience of other self-injection devices. The questions of all questionnaires can be found in the Supplementary Materials.
2.2. Statistical analysis
Patients rated individual questions in the pre- and post-ASI questionnaires on scales of 0–4 or 0–5. To allow comparison between both questions and ASI domains, individual question scores were converted to a 10-point scale. ASI domain scores were calculated using the same method as used for the SIAQ: domains were calculated as the mean of the item scores included in the domain and were only calculated if at least half of the domain items were completed [Citation11].
Patients answered each question of the implementation questionnaire using a rating scale of 0–4, or 1–10 for the overall usefulness rating of the video or step-by-step guide. The mean score was calculated for each question. The number and percentage of patients rating the overall usefulness of the video or step-by-step guide highly (defined as a rating of 8, 9 or 10 out of 10) was also calculated. For the preference questionnaire, the number and percentage of patients for preferring each device was evaluated.
3. Results
3.1. Patient disposition and baseline characteristics
59 patients were eligible, provided written informed consent and entered the study across the three countries (Netherlands: 24, Denmark: 15, and Sweden: 20). Of the 59 included patients, 57 provided data across all three timepoints of the study. Pre- and post-ASI questionnaires were fully completed in Denmark and the Netherlands, however, in Sweden some questions were omitted; therefore, the overall post-ASI domain scores are reported for Denmark and the Netherlands only (n = 39).
Patient characteristics are shown in . The mean age of patients was 54.8 years (standard deviation [SD]: 15.9 years) and 42/59 (71.2%) of patients were female. Overall, most patients were diagnosed with RA (38/59; 64.4%); the remaining patients had received either an axSpA or PsA diagnosis (9/59 [15.3%] and 12/59 [20.3%], respectively).
Table 1. Characteristics of the patients who used the e-Device.
3.2. Self-injection confidence – Results from Pre-ASI (Netherlands and Denmark, n = 39)
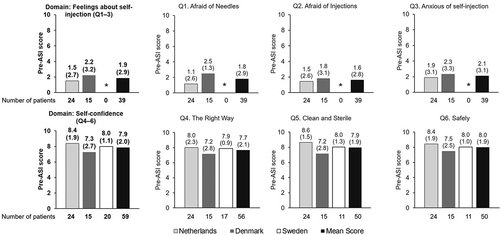
In the pre-ASI, 5/39 (12.8%) patients stated they were ‘very’ or ‘extremely’ afraid of needles, 4/39 (10.3%) were ‘very’ or ‘extremely’ afraid of giving an injection and 4/39 (10.3%) stated they were ‘very’ or ‘extremely’ anxious about self-injecting. However, 33/39 (84.6%) felt ‘very’ or ‘extremely’ confident giving the injection in the right way. 35/39 (89.7%) patients felt they were ‘very’ or ‘extremely’ confident giving injections both in a clean/sterile way and safely. Mean converted scores for all domains, both overall and for individual countries (including Sweden, where applicable), are shown in .
3.3. Satisfaction and experience with the e-Device – Results from the post-ASI (Netherlands and Denmark, n = 39)
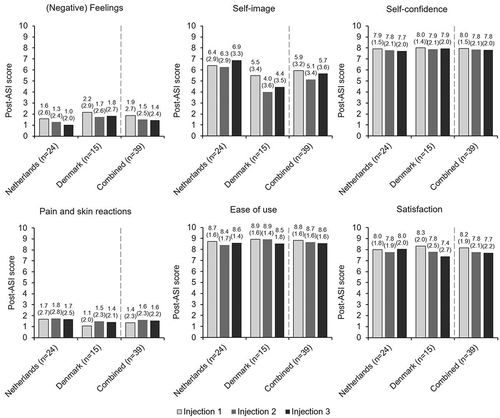
Post-ASI domain scores were comparable following each injection and between countries (). Further analyses of the individual questions in the post-ASI pain and skin reactions domain demonstrate that of the 10 questions asked, patients were most bothered by pain at the injection site, scoring this question highest in the domain, and least bothered by cold or itching, scoring these questions lowest (3.0/10 and 0.2/10, respectively; Supplementary Figure S1). There was very little between-injection variation in question scores in the pain and skin reactions domain. Additionally, there was no difference between the (negative) feelings about self‑injection and self-confidence domain scores in the pre-ASI and post-ASI questionnaires ([negative] feelings about self-injection mean pre-ASI score: 1.6/10 vs. Injection 3 post-ASI score: 1.4/10; self-confidence mean pre-ASI score: 7.9/10 vs. Injection 3 post-ASI score: 7.8/10). Analysis of individual post-ASI questions answered by all three countries can be found in the Supplementary Materials (Supplementary Figures S2–5).
3.4. Patient preference for the e-Device (across all countries)
Overall, most patients preferred the e-Device to their previous CZP self-injection device(s) (32/56 [57.1%]; ). 15/56 (26.8%) patients preferred the injection device they had previously used, and 9/56 (16.1%) patients had no preference for either device. Analysis of individual questions found that patients were most likely to select the e-Device as the easiest to hold (44/57; 77.2%), safe (41/57; 71.9%) and having the most control over the self-injection process (39/57; 68.4%). However, only 13/52 (25.5%) and 20/57 (35.1%) preferred the e-Device for travel and storage, respectively.
Table 2. Patient preference for different self-injection devices.
3.5. Patient opinions of the e-Device training materials (across all countries)
The step-by-step guide and the training video were well received with 39/53 (73.6%) patients and 34/46 (73.9%) patients, respectively, rating the materials as highly useful (giving a score of 8, 9 or 10 out of 10). Scores for individual questions, both by country and overall, are presented in Supplementary Table S1. Patient comments highlighted that users found the training materials helpful (‘I found the step-by-step guide very relevant and educational’) and easily understandable (‘The picture series tells more than clear instructions’).
Examining the results by country, the step-by-step guide was ranked highly for overall usefulness in all countries (average rating in the Netherlands: 8.0/10 [n = 24]; Denmark: 7.9/10 [n = 15]; Sweden: 9.2/10 [n = 20]). The training video was also ranked highly: average ratings were 8.4/10 in the Netherlands (n = 24), 7.9/10 in Denmark (n = 15) and 8.8/10 in Sweden (n = 20).
4. Discussion
Overall, the e-Device was well received: patients found it easy to use and reported high levels of confidence and satisfaction. Furthermore, after three self-injections, most patients preferred the e-Device to their previous self-injection device(s).
Prior to e-Device use, a few patients rated themselves as anxious about needles and injections and, generally, patients were confident about their ability to self-inject, possibly due to their prior self-injection experience. Post-ASI questionnaire results indicate that patients had positive experiences using the e-Device. Satisfaction, self-confidence and ease of use domains were all consistently rated highly, both across countries and over time, corresponding to a positive self-injection experience with the e-Device. The pain and skin reactions domain was generally scored lowest compared to all other domains, and patients also reported low levels of negative feelings regarding e-Device use, again indicating a positive self-injection experience. High patient satisfaction reported with the e-Device indicates it may help address some of the challenges associated with self-injection, such as needle phobia and hand dexterity problems. Previous studies in RA patients have shown a general preference for a large grip as this aids drug administration for individuals with hand dexterity problems [Citation20,Citation23].
Increased patient satisfaction has previously been shown to increase patient adherence[Citation23]. Adherence to anti-TNFs for the treatment of CIDs is known to be low (59% [95% confidence interval: 58–60%]) [Citation13], which in turn can reduce disease control and long-term outcomes [Citation10,Citation14]. High patient satisfaction levels with the e-Device may lead to improved adherence and clinical outcomes. Forgetfulness has also been shown to influence patient adherence [Citation18]. The e-Device notification of the next injection could help reduce non-adherence due to forgetfulness. Similarly, increasing patient control over treatment administration can facilitate patient empowerment [Citation24]. Both device design, such as the ability to vary injection speed or pause an injection with the ava® e-Device, or patient development of habits and ‘rituals’ surrounding self-injection can help increase patient control. Together with increased patient confidence, this in turn can contribute to increased treatment adherence [Citation11,Citation24].
After training and e-Device use, high levels of satisfaction with the step-by-step guide and training video were also reported. Providing step-by-step on-screen instructions has been shown to improve patient confidence in their ability to successfully complete a self-injection [Citation21]. Patient responses to open-ended questions in the Implementation Questionnaire support this idea with multiple individuals commenting on the helpfulness and clarity of the on-screen instructions (‘The picture series tells more than clear instructions’; ‘The information on the device is so good and relevant’).
Fewer patients from the Netherlands responded to the questions referring to the training video in the Implementation Questionnaire compared to patients from Sweden and Denmark. This was due, in part, to fewer patients in the Netherlands watching the video as the video link did not work for some patients. Additionally, patient responses from all three countries suggested the on-screen instructions were so comprehensive that some patients did not feel they needed to watch the training video (‘The animation video is not needed, the step-by-step guide is enough’; ‘I didn’t watch the video because ava® is clear enough’), that the video was too long (‘It takes a long time to watch and its quite long winded’) or that the video was more suitable for patients who had never self-injected before (‘As a first-time user of biologics the video might also be a good idea – but for me it’s too long’).
Overall, the e-Device was the preferred device for most patients. This indicates that the e-Device may meet previously unmet needs of patients using self-injection devices. Patients were most likely to prefer the e-Device when asked about the self-injection process, for example, injection safety, control and ease. This finding may be expected as the e-Device was developed through multiple iterations of patients testing and evaluating its ability to aid successful self-injection [Citation21].
It should be noted that not all patients preferred the e-Device over their previous self-injection device. This suggests differences in the self-injection device features required and/or preferred by individual patients and supports previous research that found different patients place different values on the features of self-injection devices [Citation25]. For example, previous research has suggested patients who prefer using a PFS over a PFP find it easier to control the self-injection process with a syringe [Citation26]. Similarly, patients who are not at ease with technology may choose the CZP PFP in preference to the e-Device [Citation18]. These results highlight the importance of patient choice when selecting a self-injection device.
4.1. Limitations
The number of patients involved in this study was small (n = 59). As a result, it may not be possible to generalize these results to other patient groups or populations. Additionally, patients opted into the study, which may lead to selection bias as patients willing to participate in a study testing new devices may be more open to alternative treatment delivery options. However, both this approach and the number of patients included are common for a pilot study [Citation27]. Furthermore, the patients who participated in this study all had previous experience of self-injection, with the results of the pre-ASI questionnaire suggestive of considerable prior self-injection experience. This may further limit the generalizability of the results to the wider patient population, which includes individuals who are self-injection-naïve.
The e-Device was only used to self-inject three times over this study (although patients could choose to continue to use the e-Device once the study had ended). Furthermore, patients were not recruited based on any reported problems with their previous self-injection device. These factors could have biased the results in favor of patients’ previous self-injection devices, due to more extensive experience and established device-specific self-injection routines that increase feelings of control over drug administration [Citation17]. Indeed, as mentioned previously, research has suggested patients who prefer using a PFS over a PFP find it easier to control the self-injection process with a syringe [Citation26]. In addition, since patients were only followed for four weeks in this study, these results only apply to initial patient preference and satisfaction levels and cannot be generalized to longer timelines.
Finally, patients in Sweden did not answer all questions in the pre- and post-ASI questionnaires; therefore it was not possible to include these patients in the ASI domain analyses. This reduced the number of patients who successfully completed the study. However, for questions that were answered by the Swedish cohort, results are consistent with those from Denmark and the Netherlands (Supplementary Figures S2–5) suggesting additional answers from Sweden would not change overall conclusions.
5. Conclusions
Patients perceived the e-Device as easy to use and handle and were able to successfully administer self-injections. The e-Device was the preferred device for most patients, and the training materials were positively rated. This suggests access to an e-Device may help enhance patient experience, which could improve anti-TNF adherence and patient outcomes. The fact that some patients preferred their previous self-injection device demonstrates the importance of having a portfolio of devices available for patients to choose from, to ensure maximum satisfaction for all patients.
Article highlights
Despite the benefits of self-injecting biologics, patients may encounter challenges when administering their own treatment. By identifying and addressing patients’ needs, involving patients in the device design process has the potential to help overcome these challenges and improve patient experience. Increased patient satisfaction has the potential to increase patient adherence and, as a result, may improve clinical outcomes.
The e-Device is a new electromechanical self-injection device developed in close consultation with patients. Patient feedback on the design was incorporated throughout the design process.
After training and self-injection, patients found the e-Device easy to use and were both self-confident and satisfied when using it. Patients also found the training materials helpful and that they gave enough information to use the device.
This study demonstrated that most patients preferred the e-Device over their previous self-injection device. This suggests that the e-Device may help patients overcome some of the unmet needs and challenges that may preclude successful self-injection.
This study demonstrates that, overall, most patients prefer the e-Device suggesting the device may help improve patient satisfaction in clinical practice. By developing devices, such as this e-Device, with the input of patients, device design may help improve patient experience and clinical outcomes.
Author contributions
Substantial contributions to study conception and design: B Pouls, LE Kristensen, M Petersson, BJ van den Bemt, L Ballerini, R Bruggraber, H Karlen, I Mountian, E van Bracht, S Wiegratz, TS Jorgensen; substantial contributions to analysis and interpretation of the data: B Pouls, LE Kristensen, M Petersson, BJ van den Bemt, L Ballerini, R Bruggraber, H Karlen, I Mountian, E van Bracht, S Wiegratz, TS Jorgensen; drafting the article or revising it critically for important intellectual content: B Pouls, LE Kristensen, M Petersson, BJ van den Bemt, L Ballerini, R Bruggraber, H Karlen, I Mountian, E van Bracht, S Wiegratz, TS Jorgensen;; final approval of the version of the article to be published: B Pouls, LE Kristensen, M Petersson, BJ van den Bemt, L Ballerini, R Bruggraber, H Karlen, I Mountian, E van Bracht, S Wiegratz, TS Jorgensen.
Declaration of Interest
BJ van den Bemt received grant/research support from: UCB Pharma, Pfizer, Abbvie; Speakers bureau: Pfizer, AbbVie, UCB Pharma, Biogen, Sandoz. Delivered consultancy work for UCB Pharma, Novartis and Pfizer.
LE Kristensen received grant/research support from: UCB Pharma, Biogen, Janssen Pharmaceuticals, and Novartis; Speakers bureau: Pfizer, AbbVie, Amgen, UCB Pharma, BMS, Biogen, MSD, Novartis, Eli Lilly, and Janssen Pharmaceuticals.
E van Bracht, R Bruggraber, I Mountian, S Wiegratz, H Karlen, and L Ballerini are employees of UCB Pharma.
TS Joergensen received grant/research support from: AbbVie, Roche, Novartis, UCB Pharma, Biogen, Eli Lilly.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (1 MB)Acknowledgments
The authors thank the patients, the investigators and their teams who took part in this study. The authors also acknowledge Emma Phillips, PhD, and Simon Foulcer, PhD, from Costello Medical, UK, for writing and editorial assistance in preparing this manuscript for publication, based on the authors’ input and direction.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Maruotti N, Cantatore FP. Impact of biological therapy on spondyloarthritis. Eur J Clin Pharmacol. 2014;70:1021–1027.
- Mease PJ. Biologic therapy for psoriatic arthritis. Rheum Dis Clin North Am. 2015;41:723–738.
- Sivamani RK, Goodarzi H, Garcia MS, et al. Biologic therapies in the treatment of psoriasis: a comprehensive evidence-based basic science and clinical review and a practical guide to tuberculosis monitoring. Clin Rev Allergy Immunol. 2013;44:121–140.
- Trivedi I, Hanauer SB. Balancing the risks and benefits of biologic therapy in inflammatory bowel diseases. Expert Opin Drug Saf. 2015;14:1915–1934.
- Wang D, Li Y, Liu Y, et al. The use of biologic therapies in the treatment of rheumatoid arthritis. Curr Pharm Biotechnol. 2014;15:542–548.
- Liu J, Sylwestrzak G, Ruggieri AP, et al. Intravenous versus subcutaneous anti-TNF-Alpha Agents for Crohn’s Disease: a comparison of effectiveness and safety. J Manag Care Spec Pharm. 2015;21:559–566.
- Larsson I, Bergman S, Fridlund B, et al. Patients’ independence of a nurse for the administration of subcutaneous anti-TNF therapy: A phenomenographic study. Int J Qual Stud Health Well-being. 2010;5. DOI:10.3402/qhw.v5i2.5146.
- Mathijssen EG, van Heuckelum M, van Dijk L, et al. A discrete choice experiment on preferences of patients with rheumatoid arthritis regarding disease-modifying antirheumatic drugs: the identification, refinement, and selection of attributes and levels. Patient Prefer Adherence. 2018;12:1537–1555.
- Jacobs P, Bissonnette R, Guenther LC. Socioeconomic burden of immune-mediated inflammatory diseases–focusing on work productivity and disability. J Rheumatol Suppl. 2011;88:55–61.
- Maniadakis N, Toth E, Schiff M, et al. A targeted literature review examining biologic therapy compliance and persistence in chronic inflammatory diseases to identify the associated unmet needs, driving factors, and consequences. Adv Ther. 2018;35:1333–1355.
- Keininger D, Coteur G. Assessment of self-injection experience in patients with rheumatoid arthritis: psychometric validation of the Self-Injection Assessment Questionnaire (SIAQ). Health Qual Life Outcomes. 2011;9:2.
- Cox D, Mohr DC. Managing difficulties with adherence to injectable medications due to blood, injection, and injury phobia and self-injection anxiety. Am J Drug Delivery. 2003;1:215–221.
- Fidder HH, Singendonk MM, van der Have M, et al. Low rates of adherence for tumor necrosis factor-alpha inhibitors in Crohn’s disease and rheumatoid arthritis: results of a systematic review. World J Gastroenterol. 2013;19:4344–4350.
- Pasma A, Schenk CV, Timman R, et al. Non-adherence to disease-modifying antirheumatic drugs is associated with higher disease activity in early arthritis patients in the first year of the disease. Arthritis Res Ther. 2015;17:281.
- Lopez-Gonzalez R, Leon L, Loza E, et al. Adherence to biologic therapies and associated factors in rheumatoid arthritis, spondyloarthritis and psoriatic arthritis: a systematic literature review. Clin Exp Rheumatol. 2015;33:559–569.
- Voshaar MJ, Nota I, van de Laar MA, et al. Patient-centred care in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2015;29:643–663.
- Schiff M, Saunderson S, Mountian I, et al. Chronic disease and self-injection: ethnographic investigations into the patient experience during treatment. Rheumatol Ther. 2017;4:445–463.
- van den Bemt BJF, Gettings L, Domanska B, et al. A portfolio of biologic self-injection devices in rheumatology: how patient involvement in device design can improve treatment experience. Drug Deliv. 2019;26:384–392.
- Lyseng-Williamson KA. Certolizumab pegol administration devices: a profile of their use and usability. Drugs Ther Perspect. 2017;33:515–522.
- Domanska B, VanLunen B, Peterson L, et al. Comparative usability study for a certolizumab pegol autoinjection device in patients with rheumatoid arthritis. Expert Opin Drug Deliv. 2017;14:15–22.
- Domanska B, Stumpp O, Poon S, et al. Using patient feedback to optimize the design of a certolizumab pegol electromechanical self-injection device: insights from human factors studies. Adv Ther. 2018;35(1):100–115.
- European Medicines Agency. Annex 1: summary of product characteristics (CIMZIA). 2019 [cited 2019 Mar 20]. https://www.ema.europa.eu/en/documents/product-information/cimzia-epar-product-information_en.pdf
- Schwartzman S, Morgan GJ Jr. Does route of administration affect the outcome of TNF antagonist therapy? Arthritis Res Ther. 2004;6(Suppl 2):S19–23.
- Nafradi L, Nakamoto K, Schulz PJ. Is patient empowerment the key to promote adherence? A systematic review of the relationship between self-efficacy, health locus of control and medication adherence. PLoS One. 2017;12:e0186458.
- Boeri M, Szegvari B, Hauber B, et al. From drug-delivery device to disease management tool: a study of preferences for enhanced features in next-generation self-injection devices. Patient Prefer Adherence. 2019;13:1093–1110.
- Kivitz A, Cohen S, Dowd JE, et al. Clinical assessment of pain, tolerability, and preference of an autoinjection pen versus a prefilled syringe for patient self-administration of the fully human, monoclonal antibody adalimumab: the TOUCH trial. Clin Ther. 2006;28:1619–1629.
- Whitehead AL, Julious SA, Cooper CL, et al. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res. 2016;25:1057–1073.