RRx-001, a dinitroazetidine-based small molecule in Phase 3 for small cell lung cancer (SCLC) and in a randomized trial for the treatment of COVID-19, is a first-in-class Myc inhibitor that also downregulates the cancer-co-opted antiphagocytic protein, CD47, as well as PD-L1 according to recent unpublished data [Citation1–Citation4]. The molecule has demonstrated anticancer activity in multiple tumor types such as colorectal, glioblastoma, brain metastases, multiple myeloma, neuroendocrine, and lung tumors in the context of minimal toxicity. The main-RRx-001-related adverse event is local venous pain and inflammation at the site of infusion [Citation5–Citation9] () .
Figure 1. RRx-001-mediated release of nitric oxide from the red blood cell during direct IV infusion.
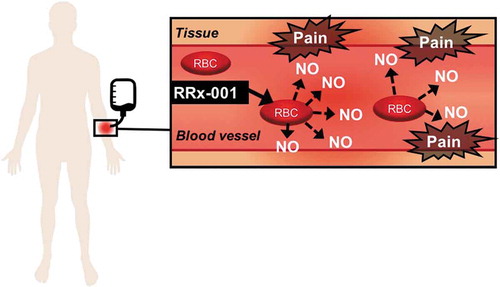
In the Phase 1 first-in-man (FIM) trial, RRx-001 was given by direct intravenous infusion, which led in 85% (21/25) of patients across all dosing cohorts from 20 to 166 mg (10–83 mg/m2) to mostly Grades 1 and 2 localized redness, dilation, tenderness, and edema of the vein, indicative of infusion phlebitis (IP) without infection [Citation10]. Infusion phlebitis occurred no matter the type of vascular access, whether central or peripheral. However, overall, infusion through the antecubital fossa was seemingly better tolerated than infusion through a central venous port or peripherally inserted venous catheter (PICC), due to the additional development of so-called ‘Wasabi or Horseradish Nose,’ that is transient but extreme nasopharyngeal discomfort akin to the burning, painful sensation from bolus ingestion of wasabi or horseradish.
The pathophysiology of RRx-001-mediated IP and ‘Wasabi Nose’ is related to the displacement of nitric oxide (NO) from its binding site on the beta cysteine 93 residue of hemoglobin to which RRx-001 covalently attaches [Citation11].
A venous wall dilator and nociceptive irritant, NO is known to sensitize the so-called wasabi and capsaicin TRPA1 and TRPV1 receptors, which stimulates the release of proinflammatory mediators such as bradykinin, substance P, prostaglandins, histamine, leukotrienes, serotonin, interleukins, and TNF-alpha and elicits the sensation of acute pain [Citation12–Citation14].
Options to mitigate the IP in Phase 1 included prolongation of the infusion for up to 8 hours, which was neither convenient nor feasible on an outpatient basis and marginally effective premedication with corticosteroids. Pretreatment with lidocaine, opioids, benzodiazepines, and hot and cold packs was not appreciably mitigatory.
Given the high phlebitic potential of direct intravenous infusion, and the overall hypercoagulable state of malignancy, more serious complications such as deep vein thrombosis (DVT) and septic thrombophlebitis, i.e., venous thrombosis and bacteremia as well as Lemierre’s syndrome or jugular thrombophlebitis when RRx-001 is given through a central vein are theoretically possible [Citation15,Citation16]. Hence, for safety, the administration of RRx-001 was changed after Phase 1 to an ex vivo admixture with an aliquot of autologous blood and co-infusion over 10–15 minutes. The premise behind this change was that the nitric oxide displaced by RRx-001 would rediffuse across the red blood cell membrane and rebind to hemoglobin without ever interacting with or sensitizing venous TRPA1 and TRPV1 nociceptors, which is, in fact, what occurred. Co-infusion of RRx-001 with 12 mL of autologous blood that is mixed outside of the body in a device called the eLOOP (because blood is routed through it in a circuit; the ‘e’ is for erythrocytes) has resulted in an almost 100% reduction of IP and led to the designation of RRx-001 by the FDA as a drug-device combination.
In conclusion, mixture of RRx-001 with a sample of blood is deemed necessary to eliminate the potential for phlebitis, a common complication during direct IV administration. Phlebitis/thrombophlebitis is generally associated with a benign self-limited clinical course; however, because of its potential to progress, especially in hypercoagulable cancer patients, to deep vein thrombosis and septic thrombophlebitis, for which morbidity and mortality, if not properly treated, are high, direct IV infusion of RRx-001 is not recommended [Citation17]. Moreover, in contrast to the rapidity of RRx-001 blood mix, which takes less than 20 minutes to deliver, direct IV infusion requires a considerably slower rate of infusion, over multiple hours, for acute pain and/or ‘Wasabi Nose’ mitigation, which is not only uncomfortable, intimidating, and inconvenient but also clinically and practically infeasible on an outpatient basis.
Declaration of interest
S Caroen, B Oronsky, C Carter, and T Reid are employees of EpicentRx, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Oronsky B, Scicinski J, Ning S, et al. Rockets, radiosensitizers, and RRx-001: an origin story part I. Discov Med. 2016 Mar;21(115):173–180.
- Oronsky B, Reid TR, Larson C, et al. REPLATINUM Phase III randomized study: RRx-001 + platinum doublet versus platinum doublet in third-line small cell lung cancer. Future Oncol. 2019 Oct;15(30):3427–3433.
- Oronsky B, Reid TR, Oronsky A, et al. Brief report: RRx-001 is a c-Myc inhibitor that targets cancer stem cells. Oncotarget. 2018 May 4;9(34):23439–23442.
- Cabrales P. RRx-001 acts as a dual small molecule checkpoint inhibitor by downregulating CD47 on cancer cells and SIRP-α on monocytes/macrophages. Transl Oncol. 2019 Apr;12(4):626–632.
- Kim MM, Parmar H, Cao Y, et al. Whole brain radiotherapy and RRx-001: two partial responses in radioresistant melanoma brain metastases from a Phase I/II clinical trial: a TITE-CRM Phase I/II clinical trial. Transl Oncol. 2016 Apr;9(2):108–113.
- Das DS, Ray A, Das A, et al. A novel hypoxia-selective epigenetic agent RRx-001 triggers apoptosis and overcomes drug resistance in multiple myeloma cells. Leukemia. 2016 Nov;30(11):2187–2197.
- Oronsky B, Ma PC, Morgensztern D, et al. Nothing But NET: a review of neuroendocrine tumors and carcinomas. Neoplasia. 2017 Dec;19(12):991–1002.
- Oronsky B, Caroen S, Zeman K, et al. A partial response to reintroduced chemotherapy in a resistant small cell lung cancer patient after priming with RRx-001. Clin Med Insights Oncol. 2016 Nov 6;10(105–108). DOI:10.4137/CMO.S40429.
- Oronsky B, Reid TR, Oronsky A, et al. What’s New in SCLC? A review. Neoplasia. 2017 Oct;19(10):842–847.
- Reid T, Oronsky B, Scicinski J, et al. Safety and activity of RRx-001 in patients with advanced cancer: a first-in-human, open-label, dose-escalation phase 1 study. Lancet Oncol. 2015 Sep;16(9):1133–1142.
- Scicinski J, Fisher G, Carter C, et al. The development of RRx-001, a novel nitric-oxide-mediated epigenetically active anticancer agent. Redox Biol. 2015 Aug;5:422.
- Bautista DM, Jordt SE, Nikai T, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124(6):1269–1282.
- Holthusen H, Arndt JO. Nitric oxide evokes pain at nociceptors of the paravascular tissue and veins in humans. J Physiol. 1995 Aug 15;487(1):253–258.
- Caterina MJ, Rosen TA, Tominaga M, et al. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441.
- Schifferdecker B, Merchán J, Ahmar C, et al. Endovascular treatment of septic thrombophlebitis: a case report of a rare complication and a review of the literature. Vasc Med. 2009;14:47–50.
- Allen BW, Bentley TP Lemierre syndrome. (Updated 2018 Oct. 27) In: StatPealrs. StatPearls Publishing, Treasure Island, FL (2018) [cited 2020 Mar 01]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499846/
- Chirinos JA, Garcia J, Alcaide ML, et al. Septic thrombophlebitis: diagnosis and management. Am J Cardiovasc Drugs. 2006;6(1):9–14.
