ABSTRACT
Background
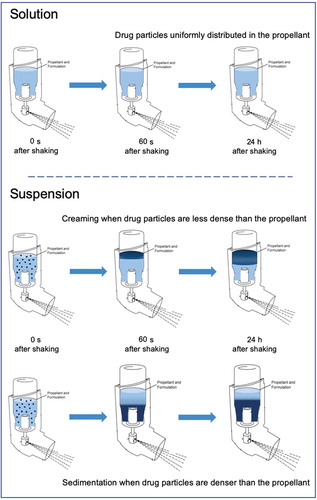
Pressurized metered-dose inhalers (pMDIs) include hydrofluoroalkane (HFA) propellant to generate a drug aerosol upon actuation and drugs can be formulated as solution or suspension. Suspended particles can cream or sediment depending on density differences between drug and propellant and shaking the pMDI is an essential step to ensure a uniform drug dose release.
Research design and methods
The effect of the delay (0, 10, 30, 60 seconds) in pMDI actuation after shaking and the effect of no-shaking during the canister life on the emitted dose (ED) for commercial solution and suspension pMDIs was investigated.
Results
The ED for solutions was unaffected by no-shaking or by the progressive increasing delay in actuation after shaking (between 77% and 97%). For all the suspension products, shaking was demonstrated to be critical to assure the close to nominal drug delivery. In detail, the actuation delay after shaking led to an increase up to 380% or a drop to 32% of ED in relation to the label claim with high variability.
Conclusion
The drug delivered can vary widely for no-shaking and over different shake-fire delays with suspension pMDIs while solution formulations appear to remain stable.
1. Introduction
Orally Inhaled Therapy (OIT) is commonly used for treatment of respiratory diseases such as asthma and Chronic Obstructive Pulmonary Disease (COPD). OIT of bronchodilators and corticosteroids is released by specific inhaler devices. Press-and-breathe pressurized Metered-Dose Inhalers (pMDIs) are a key-family of devices used for delivering OIT. There are several different pMDIs available on the market. The drug into all pMDIs is formulated in liquefied-gas propellants, sealed in an aluminum aerosol container and a predefined dose is released by actuating a metering valve. Although pMDIs usually have a similar external appearance, they may differ largely in characteristics and performances. A main difference relies on drug formulation: many pMDIs are formulated as suspensions, other pMDIs as solutions. As the name suggests, solution-based formulations contain the drug solubilized in a hydrofluoroalkane (HFA) propellant, with or without co-solvents, additives and stabilizers. The pMDIs solutions have the advantage of being homogeneous formulations and for this reason, it is thought not to be necessary to shake them before use. Not all drugs are fully soluble in HFA, thus co-solvents are often added. A primary co-solvent utilized in pMDI formulations is ethanol. It is typically employed to increase drug or excipient solubility and to enhance valve function [Citation1]. However, with the rise of ethanol concentration, the size of atomized droplets becomes larger and droplets evaporate more slowly. Therefore, as the ethanol concentration increases, the droplets have a higher probability of depositing in the upper region and the respirable deposition decreases [Citation2,Citation3]. Moreover, it was demonstrated that the increase of ethanol concentration, retarding the evaporation of droplets, enhances the likelihood of drying particle with a smooth surface [Citation2]. Finally, particle morphology and thermal properties are modified by the addition of non-volatile excipient, such as glycerol, employed to design aerosols with chosen particle size and plume speed generated from HFA-ethanol solution [Citation3,Citation4].
On the other hand, suspension-based formulations contain micronized particles of drug suspended in HFA propellant. A principal consideration for the suspension stability is that the drug must be practically insoluble in the formulation. In these types of products, surfactants are usually added to inhibit particle agglomeration and particle adhesion to device components. Suspended particles are subjected to gravitational stability according to the difference between the density of the drug and the density of the system. Creaming or sedimentation is therefore expected as the drug is less dense or denser than the propellant, respectively (). If a nonuniform suspension gradient is created inside the canister, as a consequence of inexact shaking, the risk of a variability in metered and emitted dose is present.
Figure 1. Gravitational stability of pMDI formulated as solutions or suspensions: solution-based formulations contain the drug solubilized in a hydrofluoroalkane (HFA) propellant with or without co-solvents. HFA suspensions contain suspended drug particles subjected to creaming or sedimentation according to the difference between the density of the drug and the density of the system.
The delivered dose to the lungs could be increased or decreased in comparison with the label claim dose according to the canister life as well. Of course, over time, repeated actuations of lower doses than the label claim will result in a more concentrated drug formulation within the canister; as a consequence, toward the end of the device life, the drug concentration within the canister will be much higher than the label claim, resulting in higher drug deliveries. By contrast, when high-dose deliveries are given in the beginning of the canister life, actuations toward the end of life will contain little, if any, drug. This effect would lead to variable and erratic dosing over time.
It is commonly said that pMDI suspensions should be shaken before using, while this is not relevant for solutions. This difference may be clinically relevant and responsible of ineffective drug mixing and delivery. It has been demonstrated that not shaking pMDI-suspensions may be cause of uncontrolled asthma [Citation5,Citation6]. Not shaking pMDI-suspensions is a common inhaler error in clinical practice [Citation7,Citation8]. Instructions for use of pMDIs generically indicate the need of shaking the pMDI before use, but do not state the maximum acceptable delay between shaking and actuation of the inhaler [Citation9]. Few studies have investigated the consequences of not shaking and firing-shaking delays for most commonly used pMDIs. On this regard, it was in vitro demonstrated that the amount of drug delivered can vary widely over different shake-fire delays with suspension pMDI [Citation10]. Moreover, delays between shaking and actuating a pMDI suspension resulted in an increase of the emitted dose in children with asthma while they were inhaling fluticasone HFA from a valved holding chamber [Citation11].
The hypothesis driving this work was that extent of particle creaming or sedimentation could vary according to the canister manipulation and could be influenced by the excipients added to the formulation. The experiments were conducted in different conditions of misusing in order to evaluate the effects on the gravitational stability and, as a consequence, on the released dose of different pMDIs products.
The aim of this study was to investigate the consequences of not-shaking (at different level of canister content) and shake-fire delays (collection of the dose after firing over a range of delay times) on the emitted dose of some commercial solution and suspension pMDIs. Fourteen products were selected and classified into three categories: i.e. fixed-dose combination (FDC), high dose strength fixed-dose combination (HD_FDC) and monocomponent pMDI. The products were suspensions without excipients, suspensions with excipients, and solutions.
2. Materials
Fourteen HFA commercial branded and generic pMDIs indicated for the asthma and COPD control were tested. The following three categories of products were characterized: fixed-dose combination (FDC), high dose strength fixed-dose combination (HD_FDC) and monocomponent pMDIs. illustrates commercial name, drugs label claim, excipients, density values of the drug and the HFA propellant and therapeutic indication. All the commercial pMDIs were purchased from a local pharmacy except Foster, Trimbow and Clenil, kindly provided by Chiesi Farmaceutici (IT). For analytical purposes, the following reagents were used: acetonitrile HPLC grade (Merk, DE), methanol (VWR International, IT), phosphoric acid 85% (A.C.E.F. S.p.A., IT) and ultrapure water (resistivity = 1–10 MΩ*cm, conductivity = 1–0.1 μS/cm, Purelab Flex, ELGA-Veolia LabWater, UK).
Table 1. Characteristics and therapeutic indications in European Union of fixed-dose combination (FDC_pMDIs), high dose strength fixed-dose combination (HD_FDC_pMDIs) and monocomponent pMDIs drug products.
3. Methods
The drug concentration in samples was determined using a validated HPLC methods using an Agilent 1100 Infinity LC system (Agilent Technologies, US) equipped with a UV–Vis detector, auto-sampler, degassing unit, column oven and operated with an OpenLab CVS Chem Station software (rev.c.01.06 v.A.04.02, Agilent Technologies, US). A specific HPLC method was validated for each single or combination drug analysis. In the supplementary materials HPLC method parameters employed for the API quantification are reported.
3.1. Test procedure
The study was applied to three distinct inhalers for each product in order to consider possible intra-batch variability. Devices were new and the pMDIs were primed by firing three shots to waste before the first use. It should be taken in consideration that for this type of product there is a ‘one-shot delay’ between the shaking and the delivery of the shot corresponding to that shake. In particular a pMDI holds a single shot of drug in the metering chamber and it is this dose that is delivered when the inhaler is actuated. This means that the shot administered for the ‘current’ actuation is a product of the propellant and drug formulation loaded into the valve metering chamber at the end of the previous actuation. Therefore, in all the test, this ‘one-shot delay’ was accounted and each time the dose loaded into the valve was discharged and the new one loaded with the specific shaking maneuver requested by the test.
3.2. Investigation of the emitted dose without shaking during life cycle (Fire no-shaking)
The emitted dose (ED) uniformity was tested using a Unit Spray Collection Apparatus (USCA, Copley Scientific, UK) operating for the duration of time to allow passing 2 L of air as specified in USP42 at flow rates of 28.3 L/min. In all the cases, dose collection was carried out under critical flow control conditions. To determine the emitted dose without shaking at the beginning of life cycle, the inhaler was left standing upright for 24 hours at room temperature, without any movement. Then, three puffs were emitted (priming), the fourth puff and the following ones were analyzed. The collection of the doses was performed at five different levels of the canister content in order to simulate the life cycle of the pMDI. The shot numbers collected were calculated for each product according to the total number of doses loaded in the canister. Between each check point of the life cycle test, the inhaler was left standing upright and the unneeded doses wasted without shaking/moving the position of the inhaler. Afterward, 30 min were waited before the execution of the ED test in order to let the suspension deposit. The test was performed on three inhalers for each product (n = 9 determinations for each canister life point, #3 doses from each inhaler). A high-retention borosilicate glass microfiber filter (Whatman GF/A 25 mm 1820/025) was inserted in the USCA to collect the micro or submicron fraction of aerosol. Active pharmaceutical ingredients (APIs) deposited in the sampling apparatus were quantitatively recovered with 20 mL of solvent mixture the same one used for standard solution preparation and the USCAs were shaken using an automatic shaking apparatus (HS501, IKA-Werke, DE) for 20 min. The APIs were quantified by HPLC.
3.3. Investigation of emitted dose at different times after shaking (Fire delay after shaking)
The emitted dose (ED) was collected after 0, 10, 30, 60 seconds of inhaler shaking in order to investigate the effect of the fire delay on the amount of drug released. pMDIs were used at the life cycle beginning: three puffs were emitted (priming), the fourth puff and the following ones were analyzed. pMDI suspension products were shaken by the operator for 5 seconds (or according to what indicated on the leaflet). The same experiment set was applied to pMDI unshaken solutions where the delay time was calculated between the taking off the cap/connection to mouthpiece and the dose actuation. The sequence of test run was performed randomly on three inhalers for each product (n = 9 determinations for each time delay point, #3 doses from each inhaler). The emitted dose uniformity was tested using a unit spray collection apparatus following the procedure here above described.
3.4. Statistical analysis
Statistical analysis was conducted using the Analysis of Variance (ANOVA test) with a post-hoc test. In this case, the Dunnett’s Test (Dunnett’s Multiple Comparison), comparing means from several experimental groups against a control group was used. This test was used to compare the statistical difference between total emitted dose value (µg) of 0 seconds (control group) and emitted dose values of fire delays after shaking analysis. Statistical significance difference between groups was considered with p-value <0.05.
4. Results
4.1. Fire without shaking
When actuated without shaking, pMDI devices showed three different drug release profiles: no effect on the amount of drug delivered, progressive increase, or a gradual decrease of drug delivered throughout the canister life. , and illustrate the emitted dose behavior of unshaken inhalers of the three categories of products analyzed, i.e. fixed-dose combination (FDC), high dose strength fixed-dose combination (HD_FDC) and monocomponent pMDIs.
Figure 2. Emitted dose (µg) without shaking the canister at different level of canister content for fixed-dose combination (FDC) pMDIs: Foster (BDP-FF) 100 + 6 µg, Symbicort (BUD-FF) 160 + 4.5 µg, Seretide (FP-SX) 125 + 25 µg, Trimbow (BDP-FF-GLY) 100 + 6 + 10 µg, Serzyl (FP-SX) 125 + 25 µg, Flutiform (FP-FF) 125 + 5 µg (n = 9; mean ± st.dev).
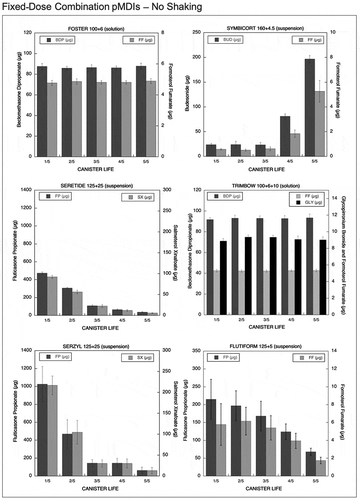
Figure 3. Emitted dose (µg) without shaking the canister at different level of canister content for high dose strength fixed-dose combination (HD_FDC) pMDIs: Foster (BDP-FF) 200 + 6 µg, Seretide (FP-SX) 250 + 25 µg, Serzyl (FP-SX) 250 + 25 µg, Flutiform (FP-FF) 250 + 10 µg (n = 9; mean ± st.dev).
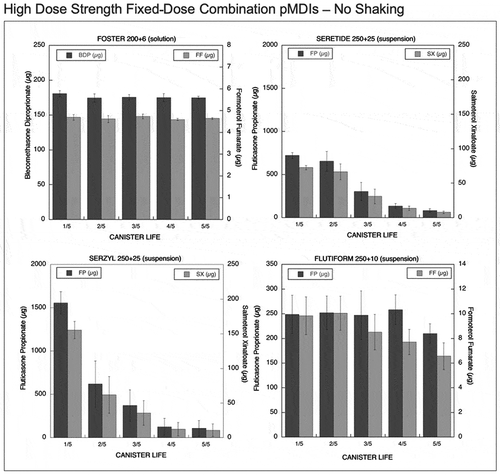
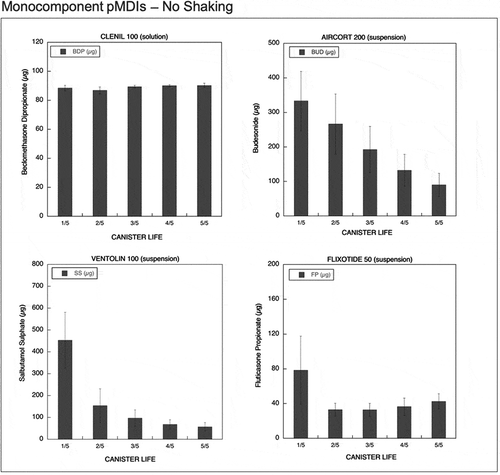
Figure 4. Emitted dose (µg) without shaking the canister at different level of canister content for monocomponent pMDIs: Clenil (100 µg BDP), Aircort (200 µg BUD), Ventolin (100 µg SS) and Flixotide (50 µg FP) (n = 9; mean ± st.dev).
Results for Foster (100/6) and Foster (200/6) (both solution-formulated pMDIs), show that the amount of formoterol fumarate (FF) and beclomethasone dipropionate (BDP) emitted during the entire canister life was unaffected by not shaking these devices before actuating. Similarly, not shaking Trimbow 100/6/10 solution-based pMDI before actuating did not affect the amount of BDP, FF and glycopirronium (GLY) emitted from this inhaler throughout the canister life ( and ).
The suspension-formulated Symbicort is a fixed-dose combination product presenting a label dose of 160 µg of budesonide (BUD) and 4.5 µg of FF. This product showed a low emitted dose of both the drugs at the beginning of the canister life when actuated without shaking (). Only a 13% and 15% of the labeled dose for FF and BUD, corresponding to 0.58 and 24 µg, was released. On the other hand, at the end of the canister life, an increase in the emitted dose of both drugs was observed: 117% and 123% of the labeled dose for FF and BUD, corresponding to 5.3 and 197 µg, respectively.
Seretide is a suspension-formulated pMDI containing fluticasone propionate (FP) and salmeterol xinafoate (SX) in a dose of 125+25 µg for each shot. This product emitted a high dose of the drugs at the start of the canister life: 370% and 378% of the labeled dose for SX and FP, respectively. After that, a decrease in the amount of drug released was seen (24% and 30% of the labeled dose for SX and FP, respectively) as illustrated in . Seretide high dose strength combination (250/25 FP/SX), suspension-formulated pMDI, showed a similar drug release profile: high emitted dose of both SX and FP at the start of the unshaken canister life (290% of the labeled dose for both the drugs), followed by a progressive reduction in emitted doses (around 30% of the expected dose) as illustrated in .
Serzyl pMDIs are formulated as a suspension containing FP and SX in dosage of 125/25 µg for the fixed-dose combination and 250/25 µg for the HD_FDC product. These pMDIs exhibited extremely high emitted doses at the start of the canister life: 868% and 641% of the labeled dose for SX and FP in the strength 125/25 and 620% of the labeled dose for both the drugs in Serzyl 250/25, as shown in and . A decrease in emitted doses to levels equal or below 50% of the labeled dose was seen at the end of the canister life for all drugs.
Flutiform pMDI is formulated as a suspension providing a dose of FP and FF of 125 and 5 µg. When the canister was actuated without shaking, the emitted dose of FF at the start and middle of the canister life was comparable to the labeled dose but presented high variability (20–40% coefficient of variation). On the contrary, the emitted doses of FP were higher in comparison to the labeled dose (172%) but presented a lower variability. At the end of canister life, a relevant decrease in the emitted doses to values well below the labeled dose was observed for both drugs (). The unshaken HD_FDC Flutiform (250/10) pMDI suspension delivered doses for both drugs within acceptable ranges in the early phase of the canister life, and only a slight reduction in the delivered doses were recorded in the last phase of the canister life. The variability of the emitted dose was still relatively high (coefficients of variation in the range 10–20%), even if not as high as in low strenght combination Flutiform ().
A similar difference in the behavior between pMDI solutions and suspensions was observed when unshaken monocompononent pMDIs were actuated (). Aircort, a BUD suspension formulation with 200 µg strength, showed high and extremely variable emitted doses at the start of the canister life (330 µg of BUD corresponding to 166.7% of the labeled dose). A decrease in the emitted doses to levels equal or below 50% of the labeled dose was observed at the end of the canister life. Ventolin is a suspension-formulated pMDI containing salbutamol sulfate (SS) in a dose of 100 µg for shot. This product released a high SS dose compared to the label claim: 453.2% at the beginning of the canister life and 154% at 2/5 of canister content. Afterward, a decrease in the amount of drug released was observed (68.6% of the labeled dose at 4/5 and 57.6% at 5/5). Flixotide pMDI (FP 50 µg) showed a high emitted dose in the early phase of canister life (78 µg corresponding to 157.2% of the labeled dose). Afterward, doses lower than the labeled dose were released during the successive phases of the canister life, i.e. 33, 34, 37, and 43 µg, respectively. On the contrary, not shaking the monocomponent Clenil pMDI, which is formulated as BDP solution 100 µg strength, did not affect the amount of BDP delivered which was constant on the whole canister content.
4.2. Shake-fire delays
, and illustrate the emitted dose behavior when a progressive increasing delay in actuation after shaking was applied. The three categories of products were analyzed as before, i.e. FDC, HD_FDC and monocomponent pMDIs.
Figure 5. Emitted dose (µg) applying increasing time delays after shaking for fixed-dose combination (FDC) pMDIs: Foster (BDP-FF) 100 + 6 µg, Symbicort (BUD-FF) 160 + 4.5 µg, Seretide (FP-SX) 125 + 25 µg, Trimbow (BDP-FF-GLY) 100 + 6 + 10 µg, Serzyl (FP-SX) 125 + 25 µg, Flutiform (FP-FF) 125 + 5 µg (n = 9; mean ± st.dev;*p < 0.05;**p < 0.01;***p < 0.001).
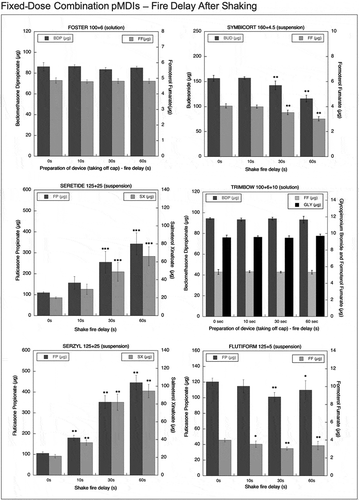
Figure 6. Emitted dose (µg) applying increasing time delays after shaking for high dose strength fixed-dose combination (HD_FDC) pMDIs: Foster (BDP-FF) 200 + 6 µg, Seretide (FP-SX) 250 + 25 µg, Serzyl (FP-SX) 250 + 25 µg, Flutiform (FP-FF) 250 + 10 µg (n = 9; mean ± st.dev;p < 0.05;**p < 0.01; ***p < 0.001).
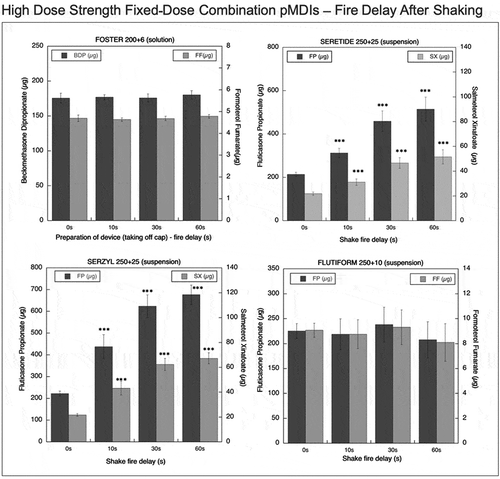
Figure 7. Emitted dose (µg) applying increasing time delays after shaking for monocomponent pMDIs: Clenil (100 µg BDP), Aircort (200 µg BUD), Ventolin (100 µg SS) and Flixotide (50 µg FP) (n = 9; mean ± st.dev; p < 0.05; **p < 0.01; ***p < 0.001).
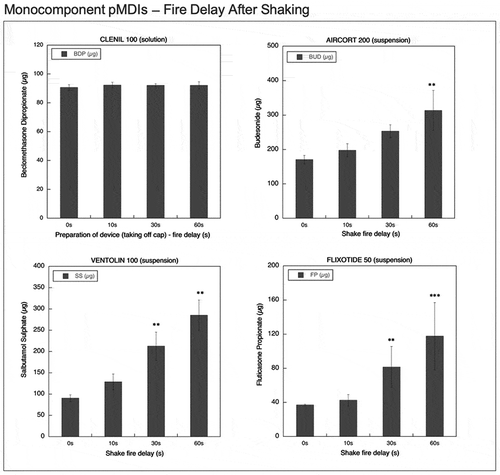
Figure 8. Overall summary data obtained in this study: emitted dose value of each API, released by a fixed-dose, high dose strenght fixed-dose combinations or monocomponent formulation, expressed as percentage in relation to the label claim. The data were collected during canister life cycle without shaking. The number of plotted values for each product were #135, #90 or #45 for triple-, double- or mono-component MDI product, respectively.
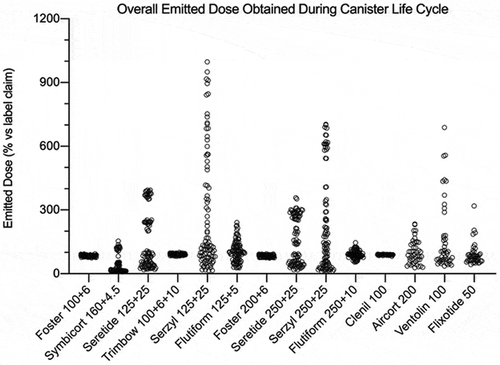
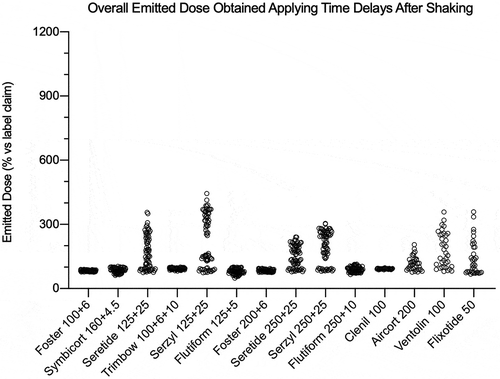
Figure 9. Overall summary data obtained in this study: emitted dose value of each API, released by a fixed-dose, high dose strenght fixed-dose combination or monocomponent formulation, expressed as percentage in relation to the label claim. The data were collected applying time delay after shaking. The number of plotted values for each product were #117, #72, or #36 for triple-, double- or mono-component MDI product, respectively.
The amount of FF and BDP emitted from Foster 100/6 and Foster 200/6 (solution) inhalers was unaffected by fire delays after shaking the device ( and ). A similar behaviour was shown by Trimbow 100/6/10 where the amount of BDP, FF and GLY emitted was unaffected by the progressive increasing delay in fire after shaking ().
The amount of drug delivered by the Symbicort 160/4.5 pMDI decreased progressively. After 60-s of delay, the emitted dose was reduced by 32.7% compared to the labeled dose for FF and by 27.4% for BUD corresponding to 116 and 3 µg, respectively ().
The Seretide 125/25 pMDI exhibited a progressive increase in emitted doses for both drugs. After a 60-s delay, the emitted dose was 265% of the labeled dose for SX and nearly 275% for FP. A similar effect was seen with the Seretide 250/25 where a 60-s delay caused an increase in the amount of drug delivered to 515 and 52 µg for FP and SX, respectively. This growth corresponds to the 206% in comparison with the labeled dose for both the drugs.
The Serzyl 125/25 pMDI showed a progressive increase in the delivered dose, reaching an amount equal to 378% of the labeled dose for SX and 356% for FP at 60-s delay. A similar effect was seen when the Serzyl HD_FDC was actuated with increasing delay times after shaking. With a 60-s delay, the emitted dose was found to be for both drugs equal to 270% of the labeled dose ().
When actuating Flutiform 125/5 pMDI 60-s after shaking, the emitted dose of FF was found to be 31.9% lower than the labeled dose, while in the case of FP this decrease was less evident (12.0%). In both cases with the progressive increase in delay times, there was an increase in the variability of the emitted dose (10-14% coefficient of variation), although minor that the one observed without shaking the device. A similar trend was shown by Flutiform 250/10 where only a relatively mild decrease in the emitted dose was seen at the maximum delay time (−19.0% compared to the labeled dose for FF and −16.9% for FP).
Aircort, Ventolin, and Flixotide pMDIs showed a progressive significant increase of emitted doses with increasing shake-fire delay times (). Statistically significant difference of emitted dose between control group and 30-s delay after shaking (p-value < 0.05) was demonstrated for Flixotide and Ventolin. The difference was significant for all the products (p-value < 0.05) at 60-s delay: the emitted dose was 156.8% of the labeled dose for Aircort, 285.7% for Ventolin, and 235.7% for Flixotide. The amount of BDP emitted by the Clenil was not affected by no-shaking of the inhaler.
5. Discussion
Results from our in vitro experiments underline the importance of shaking suspension-formulated pMDIs before use to deliver a consistent and correct amount of drug per actuation. However, the limitation of the present study lies on the fact that specific controlled conditions of the test were applied to simulate the incorrect use of the product. For this reason, the results of the study do not represent faithfully what could happen in the real-life usage of the device.
Of note, not shaking the suspension pMDIs results in either increased or decreased amounts of drug delivered and, overall, in inhomogeneity of delivered dose. By contrast, actuating unshaken pMDIs does not affect the amount of drug delivered in the case of products formulated as solutions, such as Foster 100/6, Foster 200/6, Trimbow, and Clenil (p-values >0.05 in all the tested conditions). Similarly, actuating pMDIs with increasing shake-fire delays induces inhomogeneity in the amount of drug delivered by all suspension-formulated inhalers but does not affect the emitted dose of solution-formulated pMDIs.
The results obtained can be explained considering that a suspension is a biphasic preparation consisting of solid drug particle suspended in HFA where the gravitational stability is governed by Stokes’s law. Creaming or sedimentation are therefore expected as the drug is less dense or denser than the propellant, respectively, and larger suspended particles will cream or settle faster than smaller particles. Furthermore, as drug particles associate to form large flocculates, they cream or settle with high velocity, leading to a non-uniform suspension gradient and to a variability in metered dose. Therefore, no-shaking the device containing suspensions, the components of the formulation are not adequately mixed, resulting in sedimentation and/or flocculation phenomena. The timing of this separation may depend on the density difference between the drug and the propellant [Citation12]. Moreover, it is described in the introduction that the emitted dose could be increased or decreased according to the level of canister content as well.
In the case of Symbicort (suspension pMDI), the emitted dose of the drug increases at the end of the unshaken canister life, which suggests that the drug concentrates on the upper part of the canister. This result indicated that Symbicort is affected by creaming due to the lower density of the drugs in comparison with the propellant, in this case HFA227. After a 60 second shake-fire delay, the amount of drug delivered decreases by more than a quarter relatively to the expected metered dose, which indicates a relatively fast creaming process.
Seretide 125/25 and Ventolin (suspension pMDIs) seem to be affected by sedimentation, which is caused by a higher density of the drugs in comparison to the propellant. When the Seretide FDC and Ventolin inhalers were not shaken before use, an increase in the amount of drug delivered was observed at the start of the canister life. In addition, the progressive increase of delivered drug with increasing shake-fire delays demonstrates a relatively fast sedimentation of the products after shaking. There was a significant increase in the emitted dose of SX-FP and SS when 30-s or more were waited between the shaking and the dose actuation (p-value < 0.05). Similarly, the incorrect use of Seretide 250/25, Serzyl 125/25, and Serzyl 250/25 induces sedimentation of suspended drug particles which are not adequately mixed together. In all the tests, a significant increase in the emitted dose was observed at 10, 30, and 60 seconds of fire delay (p-value < 0.05). Noteworthy, although the same trend was observed between Seretide and Serzyl formulations ( and ), the latter presented a higher overdosing of both the emitted drugs indicating that the inhomogeneity was more relevant. This result indicates that the intrinsic characteristics of the suspended particles and the addition of ethanol as excipient are crucial for the gravitational stability of the product.
On the other hand, the performance of Aircort was less dependent on the delay time after shaking. There was a significant increase in the emitted budesonide dose only when 60-s were waited between the shaking and the dose actuation (p-value < 0.05). This result can be explained considering the presence of excipients capable to slow down the sedimentation rate. Here, the oleic acid is added likely to prevent particle aggregation and to improve gravitational stability. Surfactants, such as soya lecithin, sorbitan trioleate and oleic acid, which were freely soluble in CFC propellants have very low solubility in HFA propellants. For this reason, ethanol is added as co-solvents to dissolve the sufficient amount of surfactant to formulate stable suspensions.
Flutiform presented two different behaviors for the different strengths in the tests. At low doses (125/5), a progressive decrease in the emitted dose for both drugs was evidenced in the experiment without shaking. Moreover, there was a slight but significant decrease delaying the actuation. At high doses (250/10) these effects was less evident, in particular with emitted dose unmodified up to 4/5 for FP and up to 2/5 for FF of canister life when firing without shaking. Furthermore, no significant effect was detected delaying the actuation up to 60-s for both the drugs. This result was confirmed by ANOVA test and Dunnett’s post-hoc test (p-values > 0.05). This relative stability of Flutiform suggests that is not present an evident sedimentation or creaming of the drug particles as evidenced in previously analyzed suspension products. This peculiar behavior could be explained considering that the formulation contains a third suspended component: sodium cromoglycate, an anti-inflammatory nonsteroidal molecule is included in the product as excipient at a concentration not disclosed. The amount and the nature (size, shape, morphology, etc.) of these solid particles could favor the suspendability of FP anf FF particles in the propellant. Indeed, it is known that gravitational stability can be enhanced by matching the density of the system to the density of the drug by adding excipients or blending propellants to increase or decrease the overall density of the HFA formulation [Citation13].
In the case of Flixotide, the formulation appears clearly affected by dispersed drug sedimentation in both experiments. Delayed actuation after shaking clearly evidences a progressive sedimentation confirmed by the statistically significant increase in the emitted dose (p < 0.05). Similarly, at the beginning of canister life without shaking the average emitted dose is almost the double of the one in regular conditions of actuations. However, this overdosing is not evidenced for late actuation until the end of the canister life. This could be explained by the fact that the product has been reported to be easily redispersed [Citation14]. Considering that the experiment without shaken has been performed discharging the device with a series of continuous actuations, it can be suggested that the progressive manipulation of the product during the test execution, albeit done cautiously, was capable to keep particles in suspension.
and show an overall summary data obtained in this study, where the emitted dose value of each API, released by a combination or monocomponent formulation, was singularly represented in graph. For this reason, the number of plotted values for each product varied from #36 to #135 according to the number of API included in the product and to the type of test conducted.
In detail, summarizes the emitted dose, expressed as percentage in relation to the label claim, for each pMDI during canister life cycle without shaking. All the solution products, single or combination, have resulted in the range 89–95%. On the contrary, for suspension product a characteristic dispersion of the values is evidenced. In particular, the maximum dispersion of data was shown by Seretide and Serzyl where, in the extreme cases, the emitted dose was four or ten times higher than the labeled dose. Symbicort, due to the particle creaming, had the most of the data around 15%. In combination product data representations, both the API included in the formulation had a parallel behavior dependent on the level of the canister content, as detailed by and . Regarding the monocomponent products, Ventolin was the most critical one since the missing of shaking could provide a dose up to 688%. summarizes the overall emitted dose data, expressed as percentage in relation to the label claim, obtained applying time delays after shaking. For all the suspension products the dispersion of the data was narrower compared to data obtained without shaking, as it represents the worst-case scenario for these products use. Seretide and Serzyl showed again the highest emitted dose values for SX and FP reaching peaks around 380% and 450%, respectively. Finally, all the monocomponent suspensions showed an important variability of the data (200–300%) as a consequence of delays after shaking.
Our findings are in agreements with previous in vitro published data. Berlinsky and colleagues showed that a 30-s shake-fire delay increased the amount of drug delivered (+227%) on the second actuation by a fluticasone (Flovent HFA) pMDI. The same group showed that delays between shaking and actuation can have clinical consequences [Citation11]. Hatley et al. demonstrated that a shake-fire delay of 60 seconds vs. no delay increased the mass of drug delivered by the Flovent HFA (+320%), Ventolin Evohaler (+346%), and Airomir Inhaler (+230%) pMDIs; and decreased the mass of drug delivered by the Symbicort budesonide (−75%) and formoterol fumarate (−76%) pMDI [Citation10]. Other studies have found differences from expected dose emissions due to delays, with several suspension pMDIs [Citation15,Citation16].
Results from our study enlarge and update these data also to high dose strength FDC and monocomponent pMDI showing that shake-fire delays also have an important effect on the amount of drug delivered. As expected, shake-fire delays do not influence the amount of drug delivered by pMDIs formulated as solutions like Trimbow, Foster, and Clenil.
The collected data clearly show the variability in the emitted dose as a consequence of device shaking or no-shaking. On the formulation point of view, it is crucial to develop stable suspension with low creaming/sedimentation rate. As previously stated, the stability could be achieved by matching as much as possible the density between the suspended drug particles and propellant. For this reason, excipients are usually added to the HFA to decrease the rate of separation between the drug and the propellant system, to reduce particle agglomeration and to prevent particle adhesion to canister walls and valve components. Alternatively, stable suspensions could be obtained preparing low density particles by engineering techniques. In this respect, PulmoSphere® platform is based on creating lipid porous microspheres by spray drying [Citation17]. Upon shaking, the propellant permeates within the particles providing a uniform suspension for 60-s after shaking compared to those with drug-crystal-only-based formulations which separated rapidly [Citation18]. In this technology, the drug can be incorporated into the porous particles or co-suspended with them as micronized drug crystals [Citation19]. HFA formulations containing PulmoSphere particles and binary or the ternary combination of BUD, GLY, and FF are currently on phase III clinical trial [Citation20–Citation22]. Albeit more stable that the standard suspensions, these products must in any case be shaken before use, in order to release the correct dose. Therefore, from the practical point of view discussed in this article, an increased misuse risk related to no-shaking still remains compared to solution HFA pMDIs.
The negative impact of not shaking pMDI suspensions on pharmacological outcomes has been reported as well in the literature: erroneous administration, in which the canister was shaken only before the first of the five actuations, halved the systemic availability [Citation23]. Another study had shown that if CFC-Ventolin® was not shaken, there was a reduction of the total emitted dose by 25% [Citation24]. It was also observed a reduction in bioavailability of salbutamol inhaling the aerosol after a 20-s delay and this is particularly relevant for a drug used as reliever [Citation25]. This is to be kept in mind considering that the only pMDI, apart from Foster MDI, usable as reliever is a suspension of salbutamol. In fact, the last Global Initiative for Asthma (GINA) strategy strongly suggests the use of inhaled corticosteroids (ICS)-formoterol associations in the contest of maintaining and reliever therapy, explaining the possible risks related to the use of salbutamol inhaler. For the same reasons and based on some clinical trials [Citation26,Citation27], GINA also starts to propose the use of ICS-formoterol combinations as first choice for ‘as needed therapy’. Considering the results of the present experiments, Ventolin misuse in an acute situation, could lead to overdosing or underdosing of the drug with possible negative consequences for the patient. The dose stability of a solution pMDI, containing a combination of ICS-formoterol could support the new strategy proposed by GINA ensuring the correct dose assumption in the acutely symptomatic asthma patients.
Noteworthy, data from the literature show that up to 57% of subjects do not shake their pMDI before actuation. Interestingly, this information is rather generic and not extended also to the second one of two actuations even if two puffs are a common prescription with pMDIs [Citation28,Citation29]. For instance, in an Italian multicenter study (5) including a cohort of 866 experienced pMDIs users when they were asked to show the use of their inhalers to the investigator using a placebo device just as if they would be at home, 20% did not shake the device; in the subset of 456 subjects also observed at the second inhalation, slightly more than 60% did not shake the inhaler (unpublished data of AM). Moreover, manufacturers recommend a 1-min interval between two actuations, this further operation increases the risk of error in the full execution of the dose assumption.
When a spacer or valve holding chamber is prescribed, some tens of seconds can be required after shaking to insert the pMDI into an add-on spacer simply for distraction by external factors [Citation30]. We demonstrated in this work that even few seconds of delay can impact the final emitted dose. Patients and health-care personnel (HCP) should be informed that failure to actuate pMDIs correctly, either by not shaking suspension pMDIs or by actuating the device with delay after shaking, has a significant impact on the amount of drug delivered and, consequently, on treatment. Incorrect inhaler technique in patients with asthma or COPD is unacceptably frequent and has not improved over the past 40 years [Citation31]. Also, a substantial majority of HCP do not use the main inhaler types properly and the correct inhaler technique is decreasing over time [Citation32]. In particular, among HCP the pooled mean error rates in preparation step (take off the cap and shake the inhaler while holding it vertically) were 57% and 63% for pMDI without and with inhalation chamber, respectively [Citation32]. Therefore, inhaler technique skills have not improved in recent years among HCPs and patients, pointing to an urgent need for new approaches to drug delivery.
In this context, beside to a continuous care in patient training, the availability of an inhalation product that doesn’t need to be shaken represents an advantageous choice to reduce the number of possible errors.
6. Conclusion
This work demonstrates the importance of shaking pMDIs formulated as suspensions in order to obtain an efficient emitted dose of the drugs. The high variability and inconsistency of the emitted dose when the inhalers are not shaken properly suggest the importance to respect the leaflet instruction and to train properly the patient on this topic. The delays between shaking and actuation can have clinical consequences as poor maintenance of disease control and specifically, for salbutamol suspension, a risk of poor reliever effect.
On the contrary, the emitted dose of pMDIs formulated as solutions are unaffected by no-shaking or by the progressive increasing delay in actuation after device preparation/cap removal. Hence, this type of formulation could be convenient to reduce the risk of one of the most common error in pMDI misuse.
Furthermore, the effect of shaking on the quality of aerosol plume in terms of respirable dose and aerodynamic particle size distribution will be the purpose of a further publication.
Author contributions
Eride Quarta and Veronica Chierici: investigation and writing-original draft; Luca Cavalieri, Alessio Piraino and Davide Paleari: conception and design; Fabio Sonvico: analysis and interpretation of the data; Andrea S Melani: drafting and revising of the paper; Francesca Buttini: conception design, supervision of the work and manuscript revising and responsible of final approval of the version to be published. All authors have read and agreed to the published version of the manuscript. All authors agree to be accountable for all aspects of the work.
Declaration of interest
L Cavalieri, D Paleari and A Piraino are employees of Chiesi Farmaceutici SpA. F Buttini and AS Melani received several research grants from Chiesi Farmaceutici on project focusing on inhalation products in the last 3 years. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they are affiliated with Aerogen Ltd., Fisher & Paykel, and Hamilton Medical. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
5._Supplementary_Material.docx
Download MS Word (13.1 KB)Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Myrdal PB, Sheth P, Stein SW. Advances in metered dose inhaler technology: formulation development. AAPS Pharm Sci Tech. Springer US. 2014;15(2):434–455. .
- Buttini F, Miozzi M, Balducci AG, et al. Differences in physical chemistry and dissolution rate of solid particle aerosols from solution pressurised inhalers. Int J Pharm. Elsevier. 2014;465:42–51.
- Ganderton D, Lewis D, Davies R, et al. Modulite®: a means of designing the aerosols generated by pressurized metered dose inhalers. Respir Med. W.B. Saunders. 2002;96:S3–S8.
- Lewis DA, Young PM, Buttini F, et al. Towards the bioequivalence of pressurised metered dose inhalers 1: design and characterisation of aerodynamically equivalent beclomethasone dipropionate inhalers with and without glycerol as a non-volatile excipient. Eur J Pharm Biopharm. Elsevier. 2014;86:31–37.
- Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. BioMed Central. 2018;19:1–20.
- Price DB, Román-Rodríguez M, McQueen RB, et al. Inhaler errors in the CRITIKAL study: type, frequency, and association with asthma outcomes. J Allergy Clin Immunol. Elsevier. 2017;5:1071–1079.
- Melani AS, Zanchetta D, Barbato N, et al. Inhalation technique and variables associated with misuse of conventional metered-dose inhalers and newer dry powder inhalers in experienced adults. Ann Allergy Asthma Immunol. 2004;93:439–446.
- Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. W.B. Saunders. 2011;105:930–938.
- Laube BL, Janssens HM, FHC DJ, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. European Respiratory Society. 2011;37:1308–1331.
- Hatley RHM, Parker J, Pritchard JN, et al. Variability in delivered dose from pressurized metered-dose inhaler formulations due to a delay between shake and fire. J Aerosol Med Pulm Drug Deliv. Mary Ann Liebert, Inc. 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA. 2017;30:71–79.
- Berlinski A, Hollen VD, Pritchard JN, et al. Delay between shaking and actuation of a hydrofluoroalkane fluticasone pressurized metered-dose inhaler. Respir Care. 2018;63:289–293.
- Michael Y, Snowden MJ, Chowdhry BZ, et al. Characterisation of the aggregation behaviour in a salmeterol and fluticasone propionate inhalation aerosol system. Int J Pharm. Elsevier. 2001;221: 165–174.
- Sukasame N, Boonme P, ScienceAsia TS. Development of budesonide suspensions for use in an HFA pressurized metered dose inhaler. ScienceAsia. 2011;37:31–37.
- Cogan PS. Appropriate use of pressurized metered-dose inhalers for asthma. US Pharm. 2015;40(7):36–41.
- Liu X, Guo C, Chowdhury B, et al. Valved holding chambers and In Vitro metered dose inhaler performance: effects of flow rate and inhalation delay. J Aerosol Med Pulm Drug Deliv. 2017;30:399–410.
- Prime D, Grant AC, AL SLATER, et al. A critical comparison of the dose delivery characteristics of four alternative inhalation devices delivering salbutamol: pressurized metered dose inhaler, Diskus inhaler, diskhalerinhaler, and Turbuhaler inhaler. J Aerosol Med. 1999;12:75–84.
- Dellamary LA, Tarara TE, Smith DJ, et al. Hollow porous particles in metered dose inhalers. Pharm Res. Kluwer Academic Publishers-Plenum Publishers. 2000;17:168–174.
- Doty A, Schroeder J, Vang K, et al. Drug delivery from an innovative LAMA/LABA co-suspension delivery technology fixed-dose combination MDI: evidence of consistency, robustness, and reliability. AAPS Pharm Sci Tech. Springer US. 2018;19:837–844.
- Vehring R, Lechuga-Ballesteros D, Joshi V, et al. Cosuspensions of microcrystals and engineered microparticles for uniform and efficient delivery of respiratory therapeutics from pressurized metered dose inhalers. Langmuir. 2012;28:15015–15023.
- Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med. Elsevier. 2018;6:747–758.
- Dunn LJ, Kerwin EM, DeAngelis K, et al. Pharmacokinetics of budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler formulated using co-suspension delivery technology after single and chronic dosing in patients with COPD. Pulm Pharmacol Ther. 2020;60:101873.
- (Cited 2020 May 5). https://www.astrazeneca.com/our-therapy-areas/pearl-therapeutics.html
- Thorsson L, Edsbäcker S. Lung deposition of budesonide from a pressurized metered-dose inhaler attached to a spacer. Eur Respir J. European Respiratory Society. 1998;12: 1340–1345.
- Everard ML, Devadason SG, Summers QA, et al. Factors affecting total and “respirable” dose delivered by a salbutamol metered dose inhaler. Thorax. BMJ Publishing Group Ltd. 1995;50: 746–749.
- Clark DJ, Lipworth BJ. Effect of multiple actuations, delayed inhalation and antistatic treatment on the lung bioavailability of salbutamol via a spacer device. Thorax. BMJ Publishing Group Ltd. 1996;51: 981–984.
- Bateman ED, Reddel HK, O’Byrne PM, et al. As-needed Budesonide–Formoterol versus maintenance Budesonide in mild asthma. N Engl J Med. Massachusetts Medical Society. 2018;378:1877–1887.
- O’Byrne PM, FitzGerald JM, Bateman ED, et al. Inhaled combined Budesonide–Formoterol as needed in mild asthma. N Engl J Med. Massachusetts Medical Society. 2018;378:1865–1876.
- van Beerendonk I, Mesters I, Mudde AN, et al. Assessment of the inhalation technique in outpatients with asthma or chronic obstructive pulmonary disease using a metered-dose inhaler or dry powder device. J Asthma. Taylor & Francis. 2009;35: 273–279.
- Ocakli B, Ozmen I, Acarturk Tuncay E, et al. A comparative analysis of errors in inhaler technique among COPD versus asthma patients. Int J Chron Obstruct Pulmon Dis. 2018;13:2941–2947.
- Berg E. In Vitro properties of pressurized metered dose inhalers with and without spacer devices. J Aerosol Med. 1995;8:S–3–S–11.
- Sanchis J, Gich I, Pedersen S. Systematic review of errors in inhaler use: has patient technique improved over time? Chest. Elsevier. 2016;150: 394–406.
- Plaza V, Giner J, Rodrigo GJ, et al. Errors in the use of inhalers by health care professionals: a systematic review. J Allergy Clin Immunol. Elsevier. 2018;6: 987–995.
