1. Introduction
Personalized or precision medicine aims to individualize interventions based on the molecular understanding of the disease, tailor the treatment according to the imaging of the type, stage, and grade of the disease, and optimize treatment operations by real-time monitoring of the therapeutic responses [Citation1]. Cancer development is a complex and multiscale biological process that is correlated with the molecular changes or mutations in some important proteins in cells. Targeted therapy is a type of personalized medicine that precisely blocks specific cancer genes or proteins [Citation2]. But the clinical application of targeted therapy has been limited by the accurate analysis of an individual patient’s tumor, the precise assessment of drug effectiveness, and the inevitable development of drug resistance. Nanoparticle-based drug delivery systems (NDDSs), which can achieve enhanced drug distribution at tumor site via both passive and active targeting, show unique advantages in cancer therapy. A nanoparticle-based theranostic agent that integrates the diagnosis, drug delivery and treatment response monitoring in a single platform, is extremely attractive for personalized cancer therapy. The combination of targeted drugs and nanotheranostics could provide a promising individualized therapeutic paradigm for cancer. In this editorial, we summarize the pros and cons of molecular targeted drugs, describe the use of nanotheranostics for delivery of molecular targeted drugs, highlight the great potential of molecular targeted nanotheranostics for disease- and patient-specific diagnosis and treatment, and dissect the requirements for successful development of molecular targeted nanotheranostics for individualized cancer treatment.
2. Drugs for molecular targeted cancer therapy
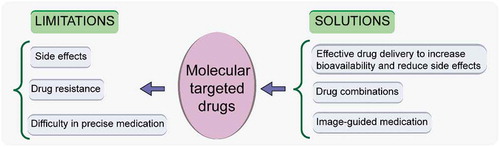
Molecular targeted therapy, a type of treatment that targets specific parts of cancer cells such as signal transduction inhibitors, gene expression modulators, angiogenesis inhibitors, and hormone therapies, has achieved promising results in clinical practice against more than 15 types of cancer. For example, epidermal growth factor receptor (EGFR) mutations are the common oncogenic driver events in advanced non-small cell lung cancer (NSCLC). Conventional chemotherapy cannot effectively treat patients with EGFR-mutant NSCLC, while EGFR-tyrosine kinase inhibitors (TKIs) have significantly improved clinical outcomes. Monoclonal antibodies and small-molecule inhibitors are two main types of molecular targeted drugs. The most studied targeted agents include trastuzumab (Herceptin), lapatinib, trastuzumab-emtansine (T-DM1) for human epidermal receptor 2 (HER2) positive breast cancer, EGFR-TKIs, such as erlotinib and gefitinib for EGFR-mutated NSCLC, and sorafenib targeting vascular endothelial growth factor receptor (VEGFR) for the treatment of hepatocellular carcinoma [Citation2]. To select appropriate targeted drugs, it is necessary to identify specific molecular targets by sequencing various cancer genomes to screen specific genes and proteins. But molecular testing is not standardized and is time-consuming. Accurate detection of the ‘druggable’ targets is still limited by the genetic heterogeneity in cancer. In addition, cancer cells can develop resistance to molecular targeted drugs usually with the second site mutations, and this resistance affects the effectiveness of treatment [Citation3]. Furthermore, targeted cancer therapies can produce substantial side effects such as diarrhea and liver problems. As tumor development undergoes a complex and dynamic process, using real-time imaging to assessing treatment response could be an effective approach to ensure that the right drug is given to the right patient at the right time and, thus, to provide maximum therapeutic benefit. And designing appropriate formulation for effective drug delivery could increase drug concentration in diseased tissue and increase drug bioavailability to improve the effectiveness of treatment, thereby reducing the drug dosage and adverse effects. Using rational drug combinations could be a useful means to overcome resistance to single-agent targeted therapy. The limitations and possible solutions of molecular targeted drugs in cancer therapy are summarized in .
3. Application of nanotechnology in tumor diagnosis and treatment
There are many types of treatment available for cancer, but they often produce severe side effects. Tumors have specific physiological properties with leaky vasculature and abnormal expression of a large number of receptors and growth factors on tumor cells. In passive targeting, macromolecules including nanoparticles are apt to accumulate in tumor tissue due to the enhanced permeability and retention (EPR) effects. Active targeting is the delivery of drugs to target cells using specific ligands through the ligand-receptor binding. NDDSs can deliver therapeutic drugs to tumor tissues and cells through passive and active targeting, thus effectively increasing drug concentration in lesion tissues with improved efficacy, while significantly reducing toxicity on normal tissues. In addition, NDDSs could combine different therapeutic modalities in a single platform to offer promising synergistic effects to reverse drug resistance. There are various NDDSs used for cancer therapy such as lipid-based nanoparticles, polymer- and dendrimer-based nanoparticles, noble metal-based nanoparticles (such as gold and silver), semiconductor nanoparticles, carbon nanotubes, metal oxide nanoparticles, metal-organic frameworks (MOFs), and upconverting nanoparticles (UCNPs).
Besides therapeutic applications, nanomedicines can be employed for diagnostic purposes. Nanoparticles carrying contrast agents such as fluorescent dyes, gadolinium-based probes, and radionuclides have been used for optical imaging, magnetic resonance imaging (MRI), computed topography (CT), single-photon emission computed tomography (SPECT), positron emission tomography (PET), photoacoustic imaging (PA), and ultrasound (US) imaging [Citation4]. These molecular imaging techniques have shown great potential for detection and diagnosis of diseases and monitoring the physiological or pathological processes of organisms at the cellular and molecular level, including gene expression, signal transduction pathways, tumor cell metabolism, proliferation, apoptosis, tumor hypoxia, perfusion, and angiogenesis [Citation5].
Nanoparticles carrying both diagnostic and therapeutic agents in one formulation could be implemented for disease diagnosis and therapy. Nanotheranostics can monitor the drug action site, drug distribution in the body, the characteristics of drug release and the therapeutic efficacy. With additional research, many NDDSs themselves could be used for cancer diagnosis and treatment. Noble metal nanoparticles for optical imaging, semiconductor nanoparticles for fluorescence imaging, UCNPs for X-ray radioluminescence imaging, carbon nanotubes and fullerenes for photoacoustic imaging, and metal oxide nanoparticles (iron oxide, manganese oxide and gadolinium oxide naonoparticles) and MOFs for MRI have been developed for cancer diagnosis and drug response monitoring. Gold, iron oxide nanoparticles, fullerene, and multi-walled carbon nanotubes can be used as photothermal theranostic agents [Citation6]. To improve targeting and drug delivery efficiency, nanotheranostics can be further modified with targeting molecules (antibodies, aptamers, ligands, etc.) on the surface. A large number of targeting-molecule-modified nanotheranostic agents have been developed for cancer treatment [Citation7].
4. Molecular targeted nanotheranostics for precision therapy
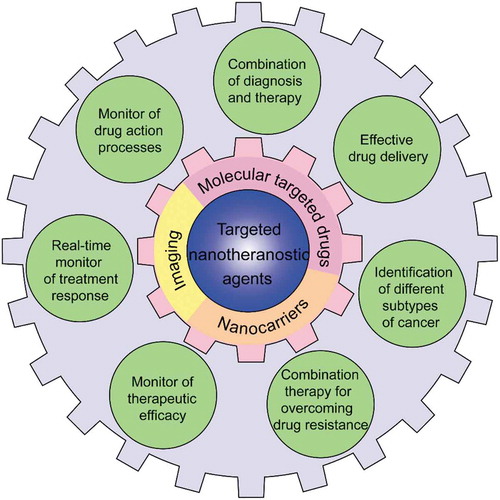
Although molecular targeted drugs have achieved significant efficacy in cancer treatment, targeted therapies are far from satisfactorily precise due to the heterogeneity and drug resistance of cancer. Nanoparticle-based theranostic systems can be applied for delivery of targeted drugs to address these limitations. The targeted nanotheranostic agents are encouraged to be designed not only to diagnose a patient’s druggable gene aberrations to distinguish different molecular subtypes of tumors, but also to monitor drug response during the medication. This valuable information will enable physicians to change treatment strategies (drug doses, patient management protocols, etc.) in a timely manner, thus meeting the needs of each patient. In addition, targeted nanotheranostics can be designed for delivery of drug combinations to overcome drug resistance. Furthermore, effective delivery by NDDSs could improve bioavailability and reduce the side effects of targeted drugs ().
Figure 2. Schematic illustration of the application of targeted nanotheranostic agents to address the limitations of molecular targeted drugs for precision therapy.
Several studies have been carried out using molecular targeted drug-loaded nanotheranostics for accurate cancer diagnosis and treatment. An ultrasmall (<5.5 nm) zwitterionic organic nanocarrier, Harvard dot or H-dot, composed of near infrared (NIR)-fluorescent cyclodextrin-grafted polylysine could selectively deliver imatinib to a mutant stem cell growth factor receptor for effective treatment of unresectable gastrointestinal stromal tumors and for NIR image-guided surgery to distinguish tumors from less than 1 cm of the surrounding tissue. After the tumor surgery, the H-dot could monitor the drug biodistribution and therapeutic efficacy through NIR imaging [Citation8]. Silica nanoparticles functionalized with phthalocyanine (Pc) have been used to entrap small-molecule inhibitors (i.e., dabrafenib and trametinib) to form PcNP@Drug. PcNP@Drug delivered by a microneedle patch could facilitate skin fluorescence imaging to monitor the penetration of nanovehicles into the deep-seated melanoma sites and the synergistic combinational photodynamic and targeted therapy [Citation9].
Nanotheranostic agents could be further modified with targeting moieties to enhance delivery efficiency of molecular-targeted drugs. Polyacrylic acid (PAA)-coated magnetic (iron oxide, Fe3O4) nanoparticles (MNPs) decorated with folic acid and co-encapsulated with two drugs (platinum-based cyanoximate complex Pt(MCO)2 and Hsp90 inhibitor drug ganetespib) and an optical dye Dil, were developed for the treatment of Kirsten rat sarcoma viral oncogene (K-RAS) driven NSCLC. The functional MNPs could target folate receptor-expressing NSCLC, monitor the delivery of drugs by MRI and optical imaging, and obtain the combined effects of ganetespib and Pt(MCO)2 [Citation10]. In another study, ultrasmall fluorescent core-shell silica nanoparticles functionalized with polyethylene glycol (PEG) chains, cyclized Arg-Gly-Asp (cRGD) peptide, PET labels (124I, 89Zr), and dasatinib to form cRGD-dasatinib-nanoparticle drug conjugates (cRGD-Das-NDC) have been investigated for precision drug delivery to brain tumors. The cRGD-Das-NDC could target αv integrins, release drugs in response to protease and pH, and monitor accumulation, diffusion, retention, and pharmacokinetic profiles in brain tumors through fluorescent and PET imaging [Citation11].
Molecular targeted drug-mediated nanotheranostic agents have been investigated to provide more precise treatment for tumors. Superparamagnetic iron oxide (SPIO) wrapped with erlotinib (Er) conjugated gold nanoclusters was developed to precisely treat EGFR over-expressed pancreatic cancer and to determine the epicenter of pancreatic cancer and pancreatic cells in real time using, respectively, the T1/T2 contrast ability of SPIO and the strong red fluorescence of gold [Citation12]. The molecular targeted drug-guided nanotheranostic system has been developed to distinguish molecular druggable mutations and overcome drug resistance. Chitosan was modified with a NIR fluorescent heptamethine cyanine dye, Cy7, and molecular targeted drug, Er, to form CE7Ns, which could distinguish three molecular subtypes of NSCLC cells and effectively inhibit both Er-sensitive and resistant NSCLC tumor [Citation13]. Based on that, a pH/redox dual-responsive nanotheranostic system, EMCI, consisting of indocyanine green (ICG)-loaded mesoporous silica nanoparticles (MSN) lidded with zinc oxide quantum dots (ZnO QDs) and wrapped with Er-modified chitosan was developed for precise molecular subtyping and resistant reversal of EGFR targeted therapy. ECMI could release Er and ICG in response to the pH and redox tumor condition and then activate the fluorescence recovery of ICG to precisely distinguish Er-sensitive NSCLC cells and exert synergistic photodynamic/molecular targeted therapeutic effects to overcome drug resistance [Citation14].
5. Expert opinion
Targeted nanotheranostic systems showed great promise for disease- and patient-specific diagnosis and treatment. They can treat specific diseases on the basis of accurate diagnosis and non-invasively monitor the action process and treatment response of drugs, making the feedback specific and timely, and, thus, conducive to developing personalized treatment programs for patients. However, most of the reports on targeted nanotheranostics were still in the laboratory testing stage. Some critical issues should be addressed for the clinical application of targeted nanotheranostics.
Although there has been a lot of research on nanomaterials in the biological field, only a few have gone through feasible clinical trials. The long-term toxicity and biocompatibility of various nanomaterials have not been clearly studied [Citation15]. The studies on the genotoxicity of nanomaterials are even fewer. Therefore, extensive toxicological and pharmacological studies should be carried out to promote the clinical transformation of nanomaterials. Because the physical and chemical properties of nanomedicine have a great impact on the in vivo biological activities and the action mechanisms, systematic studies on the biosafety of nanomaterials from a multidisciplinary and cross-domain perspective need close collaborative research in chemistry, pharmaceutics, medicine, molecular biology, immunology and other disciplines. In addition, the design of most of the nanotheranostic agents currently reported is in complex systems (such as tedious chemical synthesis routes and multi-step post-modification) with limited translational potential. There is an urgent need to develop nanotheranostic agents with simple preparation procedures and large-scale production to accelerate the clinical transformation. Multi-function materials can be selected to synthesize nanotheranostic agents to reduce composition and simplify preparation steps.
At present, a large number of the developed nanotheranostics are still limited to imaging of drug distribution. They fail to perform real-time monitoring of drug release. Stimuli-responsive drug delivery systems could be employed to develop responsive targeted nanotheranostic systems to release the drug and contrast agent under one or more stimulation to achieve imaging-guided real-time visualization of drug release. The controlled release and activated imaging characteristics of targeted nanotheranostics are beneficial to developing more precise personalized treatment. In addition, targeted nanotheranostic systems can be designed with two imaging or multi-modal imaging modalities, using one signal to visualize the distribution and accumulation of drugs in target sites before the drug is released, and the other to monitor drug release. However, as each molecular imaging method has its advantages and disadvantages, the imaging methods should be carefully selected based on the disease information. Additionally, more advanced and convenient molecular imaging instruments that could combine different imaging modalities need to be developed to make up for the shortcomings of using different instruments.
With the development of molecular biology, the biological diversity of tumors, the sophistication of cellular signaling networks, the complexity of tissue microenvironments, and the various mechanisms of drug resistance are gradually being discovered. Based on this developing knowledge, the nanomaterials should be designed to overcome various biological barriers, and the in vivo processes of nanotheranostics must be studied carefully. Researchers must examine whether entrapping the drugs in nanotheranostics alters their function. Because the clinical development of drug resistance is mostly the result of a combination of multiple factors, targeted nanotheranostic systems can be designed to have a multi-target combination model to inhibit multiple pathways. Combination of molecular targeted therapy with other therapies (including photothermal therapy and immunotherapy) is worthy of investigation. Because every therapeutic/imaging agent has its own strengths and limitations, the optimal combination of therapeutic/imaging agents needs to be optimized to obtain synergistic effects and improved imaging effects. The dose and frequencies of administration for both therapeutic and contrast agents also require optimization in one nanoplatform to obtain the best efficacy with improved patients’ compliance.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Lammers T, Rizzo LY, Storm G, et al. Personalized nanomedicine. Clin Cancer Res. 2012;18(18):4889–4894.
- Ke X, Shen LS. Molecular targeted therapy of cancer: the progress and future prospect. Front Lab Med. 2017;1(2):69–75.
- Izar B, Rotow J, Gainor J, et al. Pharmacokinetics, clinical indications, and resistance mechanisms in molecular targeted therapies in cancer. Pharmacol Rev. 2013;65(4):1351–1395.
- Fass L. Imaging and cancer: a review. Mol Oncol. 2008;2(2):115–152.
- Czernin J, Weber WA, Herschman HR. Molecular imaging in the development of cancer therapeutics. Annu Rev Med. 2006;57:99–118.
- Kievit FM, Zhang M. Cancer nanotheranostics: improving imaging and therapy by targeted delivery across biological barriers. Adv Mater. 2011;23(36):H217–47.
- He J, Li C, Ding L, et al., Tumor targeting strategies of smart fluorescent nanoparticles and their applications in cancer diagnosis and treatment. Adv Mater. 2019;31(40): e1902409.
- Kang H, Stiles WR, Baek Y, et al. Renal clearable theranostic nanoplatforms for gastrointestinal stromal tumors. Adv Mater. 2020;32(6):e1905899.
- Tham HP, Xu K, Lim WQ, et al. Microneedle-assisted topical delivery of photodynamically active mesoporous formulation for combination therapy of deep-seated melanoma. ACS Nano. 2018;12(12):11936–11948.
- Kallu J, Banerjee T, Sulthana S, et al. Nanomedicine-assisted combination therapy of NSCLC: new platinum-based anticancer drug synergizes the therapeutic efficacy of ganetespib. Nanotheranostics. 2019;3(1):120–134.
- Juthani R, Madajewski B, Yoo B, et al. Ultrasmall core-shell silica nanoparticles for precision drug delivery in a high-grade malignant brain tumor model. Clin Cancer Res. 2020;26(1):147–158.
- Nebu J, Anjali Devi JS, Aparna RS, et al. Erlotinib conjugated gold nanocluster enveloped magnetic iron oxide nanoparticles–A targeted probe for imaging pancreatic cancer cells. Sens Actuators B Chem. 2018;257:1035–1043.
- Gao Y, Zhang H, Zhang Y, et al., Erlotinib-guided self-assembled trifunctional click nanotheranostics for distinguishing druggable mutations and synergistic therapy of nonsmall cell lung cancer. Mol Pharm. 2018;15(11): 5146–5161.
- Zhang Y, Zhang L, Lin X, et al. Dual-responsive nanosystem for precise molecular subtyping and resistant reversal of EGFR targeted therapy. Chem Eng J. 2019;372:483–495.
- Peng F, Setyawati MI, Tee JK, et al. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat Nanotechnol. 2019;14(3):279–286.