ABSTRACT
Objectives
User experience was compared between a new pre-fillable 2.25 mL glass syringe equipped with an ultra-thin-wall (UTW) 8 mm staked needle and a marketed BD Neopak™ syringe equipped with a special-thin-wall (STW) 12.7 mm staked needle.
Methods
Participants simulated subcutaneous injections with both syringes alone (formative Human Factors study) and in combination with a needlestick-prevention device (validation Human Factors study).
Results
Usability results of both studies showed higher success rates for delivering the full dose of 2 mL viscous solution (30 cP) with the 8mmUTW syringe than with the 12.7mmSTW one (63% vs. 42% in the formative study). The use of the 8mmUTW syringe demonstrated also better ease of use and acceptance results and 72% of formative study participants preferred this new syringe over the current one when delivering the viscous solution. Using a shorter needle also showed a benefit in decreasing the injection-related anxiety. Besides, in the case of a non-recommended injection technique, the calculated risk of accidental intramuscular injection is reduced by 2 to 13 times with the 8mmUTW syringe.
Conclusion
Altogether, the results obtained demonstrated an improvement of the user experience with this new syringe compared to the current one in the manual delivery of 2 mL viscous solutions.
1. Introduction
Subcutaneous injection is one of the most common routes of administration used for administration of therapeutic proteins, peptides, and other biologics used in chronic diseases (growth hormone, insulin, heparin, interferons, monoclonal antibodies), mainly due to low oral bioavailability, gastric degradation of oral drugs, local toxicity at the site of absorption, suboptimal pharmacokinetics associated with gastrointestinal absorption or lack of efficient gastrointestinal transport mechanisms for these molecules [Citation1,Citation2]. Moreover, the benefits of home administration by patients themselves or lay caregivers and the increasing interest in new biotherapeutics, such as monoclonal antibody-based therapies, has challenged the limits of volume and viscosity of drug formulations [Citation1,Citation3,Citation4]. Although subcutaneous administration improves patient compliance and quality of life by enabling at-home injections, as compared to intravenous or intramuscular route, the associated anxiety, pain, discomfort, and the frequency of injections can play a significant role in patient non-adherence with chronic subcutaneous treatments [Citation5–8]. In multiple sclerosis patients, anxiety related to injections was often associated with poor adherence to treatment, and the anxiety perceived by some patients with diabetes, for example, resulted in fewer than recommended daily insulin injections performed [Citation9,Citation10]. In order to address these challenges, through reducing the required frequency of subcutaneous injections and increasing injection comfort for novel biotherapeutics, drug delivery systems should be designed to enable the administration of large-volume injections and/or high-viscosity drugs [Citation11,Citation12].
High viscosity and large-volume solutions raise a new technical challenge to keep on allowing manual drug administration for the proportion of users who want to control their injection compared to injections with auto-injectors. These solutions must be designed without affecting the user’s experience in terms of pain, anxiety, ease of use, and acceptance, while several factors can influence the perception of pain, such as injection volume, drug viscosity, or needle insertion [Citation6,Citation13–20].
For subcutaneous insulin therapy, short needles had been introduced to increase the certainty of delivering into the targeted subcutaneous tissue. Outside diabetes, despite the risk of injecting into muscular tissue, the most commonly used needle length for subcutaneous chronic drug delivery is the one-half inch (12.7 mm) [Citation21]. In this context, the accidental administration of insulin into muscular tissue can lead to faster insulin absorption and hypoglycemia episodes [Citation22–25]. Relative to intramuscular, subcutaneous injections also improve the efficacy of Interferon-beta-1a treatment in multiple sclerosis [Citation26,Citation27]. Moreover, several studies demonstrated an improved patient satisfaction and preference for the subcutaneous route of administration [Citation28,Citation29]. Therefore, the use of a 12.7 mm needle length is not necessary and may be associated with unwarranted risk of accidental intramuscular injection, as demonstrated in diabetes care, especially when self-injecting patients do not apply the recommended subcutaneous injection technique. Injection at an angle of 90° or 45° with a skinfold is recommended for subcutaneous administrations with this needle length [Citation30]. In 2010, Frid, A., et al., studied subcutaneous injection practices in 4300 patients using insulin. Despite growing availability of injection technique training, they observed that 43% of these patients released the skinfold too soon and 17% were using an incorrect technique for lifting a skinfold [Citation31,Citation32]. While these errors could result in accidental intramuscular injections, the use of a needle shorter than 12.7 mm may help mitigate this risk. In 2010, Gibney, M., et al., measured the skin thickness and subcutaneous fat depth in the thigh, arm, abdomen, and buttock in adult patients with diabetes [Citation33]. Based on these measurements, they estimated the location of the drug depot (intradermal, intramuscular, or subcutaneous) depending on the needle length. Assuming a needle insertion at 90° without lifting a skinfold, the risk of intramuscular administration is reduced with an 8 mm needle (15%) compared to a 12.7 mm needle (45%). In 2014, Hirsch, L., et al., confirmed that the estimated probability of unintended intramuscular administration is significantly reduced with 8 mm needles compared to 12.7 mm needles [Citation34]. The same trend is also observed in children and adolescents with diabetes, for whom the use of 8 mm needles is estimated to significantly reduce the occurrence of intramuscular injections by about 50% relative to the use of 12.7 mm needles [Citation35]. Accidental intramuscular administration might be responsible for potential side effects such as painful administration, hematoma formation, or risk of anaphylaxis across the range of chronic injectable drugs [Citation36–38]. However, the risk-benefit ratio is likely to be less favorable if patients are exposed to the risk of intradermal administration. In contrast to subcutaneous tissue, the dermis is composed of many specialized immune cells (i.e. dermal dendritic cells), which may induce an undesirable immune response against protein therapeutics intended to treat diseases such as Crohn’s disease or rheumatoid arthritis [Citation39].
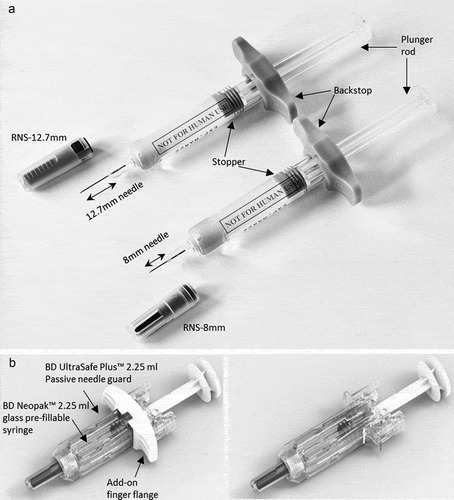
Therefore, to support the delivery of higher drug viscosities and volumes into the targeted subcutaneous tissue, we developed a pre-fillable glass syringe featuring a staked 8 mm needle with an ultra-thin wall (UTW) cannula technology, BD Neopak™ XtraFlow™ 2.25 mL, hereafter referred to ‘8mmUTW’ ()). In comparison to the currently marketed BD Neopak™ 2.25 mL pre-fillable glass syringe equipped with a 12.7 mm special thin wall (STW) needle, hereafter named as 12.7mmSTW, the 8mmUTW offers an optimized flow capacity due to shorter needle length and larger inner diameter of the cannula ()). The UTW technology makes the use of smaller gauge (larger outer diameter) needles unnecessary, which can positively impact the pain perception when enabling the injection of viscous solutions [Citation40,Citation41]. Simulated-use studies with the 8mmUTW syringe alone or combined with an anti-needlestick device have been conducted to assess their safety and efficacy and identify the potential use-related risks. A formative study was undertaken to evaluate the usability, the ease of pushing the plunger rod, and the acceptance of the force required for pushing the plunger rod with the 8mmUTW syringe versus the 12.7mmSTW syringe. The BD UltraSafe Plus™ Passive 2.25 mL Needle Guard, intended to offer needlestick-prevention (X225PLUS), is compatible with the 8mmUTW syringe ()). Among the objectives of the validation study, some were to compare the usability, the ease of use, the acceptance, and the adoption of the X225PLUS–8mmUTW versus the X225PLUS–12.7mmSTW combination product.
Figure 1. (a). BD Neopak™ 2.25 mL glass pre-fillable syringe with special thin-wall 12.7 mm (left) and ultra thin-wall 8 mm (right) needles. (b). BD UltraSafe Plus™ 2.25 mL Passive needle guard combined with BD Neopak™ 2.25 mL glass pre-fillable syringes with special thin-wall 12.7 mm needle with (left panel) and without the Add-on finger flange (right panel)
2. Participants and methods
2.1. Products description
Based on the marketed standard BD Neopak™ 2.25 mL glass syringe equipped with a 12.7 mm 27 G needle with Special Thin Wall (STW), hereafter referred to 12.7mmSTW syringe, a new syringe, BD Neopak™ XtraFlow™ 2.25 mL, equipped with a shorter 8 mm needle and an Ultra-Thin Wall (UTW) technology is currently under development, hereafter referred to 8mmUTW syringe ()). This 8 mm needle offers identical outer diameter (same 27 G) but larger inner diameter and is intended to help the delivery of higher viscosities and/or volumes without degrading the user experience. In addition to the different needle technologies, 2 rigid needle shields (RNS), RNS-8 mm and RNS-12.7 mm, were made of the same elastomer (BD260). The plunger rod, the stopper, and the glass barrel were the same for the 2 tested syringes ()).
As the standard 12.7mmSTW, the 8mmUTW syringe can be assembled with a non-sterile and disposable device with a passive activation of a needle covering safety system named BD UltraSafe Plus™ Passive needle guard 2.25 mL (hereafter abbreviated ‘X225PLUS’), intended to reduce the risk of accidental needle stick injuries after injection. In this study, participants used X225PLUS devices equipped or not with an add-on finger flange providing a larger flange for a better grip when pushing the plunger rod ()).
All the syringes were filled with polyethylene glycol 400 EMPROVE® ESSENTIAL Ph Eur, JP (Merck), diluted to obtain 10 cP or 30 cP solutions. Both solutions behave as Newtonian fluids. The viscosity is not affected by the shear rate of the needle.
2.2. Simulated studies participants
Based on FDA Guidance (2016), ‘Applying Human Factors and Usability Engineering to Medical Devices’ and AAMI/ANSI-HE-75:2009 Part 9.3.2/Table 9.2., a minimum of 5 or 15 participants per user group were recruited for the formative and the validation studies, respectively. Representative end-users over 18 years old were recruited either in the UK or in the US for the formative study and representative end-users over 21 years old were recruited in the US for the validation study:
Naïve patients, diagnosed with at least one chronic disease, did not have any experience in injecting themselves or others with syringes, pens, or autoinjectors in the last 10 years.
Experienced patients diagnosed with at least one chronic disease. In the formative study, these participants had experienced a minimum of 12 self-injections with prefilled syringes for at least 6 months in the past 2 years (only one participant used prefilled syringes with a safety add-on). Experienced participants involved in the validation study had at least 1 year of experience giving self-injections using syringes with active or passive safety systems (a maximum of 10% experienced patients with only self-injecting experience using pen for diabetes treatments and a maximum of 20% of them having only self-injecting experience using an autoinjector).
Hand-impaired patients, diagnosed with Rheumatoid Arthritis or Multiple Sclerosis affecting their fingers and/or hands, characterized by a Cochin score between 10 and 40 at the recruiting stage for the formative study and on the day of testing for the validation study [Citation42,Citation43].
16 Health care workers (HCW) with at least 2 years of working experience and 16 Caregivers (CGV) with at least 6 months of experience and a minimum of 12 injections provided to another individual participated in the validation study, as part of the intended users’ population. Less than 10% and 20% of the caregivers had experience only using reusable pens or autoinjectors, respectively.
All participants signed an informed consent form before participating in the described studies. Both studies got IRB approval for testing conducted in the US.
2.3. Study design of the formative study
The human factors formative study was a multicenter study conducted between August and September 2019 in the US (Boston, MA) and the UK (London). The primary objective was to assess the usability of the 8mmUTW syringe in comparison to the marketed 12.7mmSTW syringe, filled with 2 mL of 10 cP or 30 cP solutions. Secondary objectives were to evaluate the ease of pushing the plunger rod and the acceptance of the force required to push the plunger rod when delivering the solution, as well as the preference between both syringes. This formative testing was performed to confirm that the 8mmUTW syringe is suitable for its intended use without revealing new use-related hazards, use errors, or even without increasing the occurrence of any known risks in comparison to the marketed 12.7mmSTW syringe.
The 39 participants enrolled were not trained on how to use the syringes before the session, but the instructions for use (IFU) of both syringes were available to read during the entire simulated testing. Each participant was asked to perform 4 simulated injections with the 2 types of syringe filled with 2 different viscous solutions, according to an assigned randomization order. The participants tested both syringes by simulating subcutaneous injections into a foam pad attached to a belt that they placed on their body (thigh or abdomen). Acceptance and preference were assessed after each two simulations with a same viscosity, while the root cause interview was performed once the 4 simulations done.
2.3.1. Task assessment
The usability of the syringes was evaluated for the following critical and essential tasks: ‘Remove the needle cover,’ ‘Perform the injection’, ‘Deliver the full dose’ and ‘Disposal of the used syringe.’ As per FDA guidance, critical tasks would or could cause serious harm to the patient or user in case of use error or failure and essential tasks are the ones necessary for successful use of the product and associated with high risk but resulting in severity level below than critical tasks. Correct use was defined as achieving all assessed tasks in compliance with the IFU, including success and success with operational difficulties (SOD). The moderator recorded success with and without operational difficulties and use errors. Usability data are described as categorical data (N), and proportion frequencies (%).
2.3.2. Anxiety assessment
Before starting the simulated injections, naïve patients were asked to evaluate both uncapped 8mmUTW and 12.7mmSTW syringes, provided in front of them, in terms of anxiety if they were going to self-inject a treatment. Subjective patients’ perception and feeling of anxiety was measured using a visual analogue scale (VAS) ranging from ‘no anxiety’ (0%) to ‘worst anxiety’ (100%). Scores were measured in millimeters (120 mm-VAS) and transformed in percentage.
Causes of potential anxiety and feedbacks on the shorter needle perception were recorded for the experienced population after all simulations.
2.3.3. Ease of pushing the plunger rod and acceptance of the force required to push the plunger rod
The ease of pushing the plunger rod and the acceptance of the force required to push it were recorded through a VAS scale after having tested each syringe for a same viscosity (two simulations). This approach allowed the participants to compare the two tested syringes for the same viscosity and marked each syringe on the same VAS scale. Subjective patients’ perception of ease and acceptance was measured using a 120 mm-VAS ranging from ‘very easy/very acceptable’ (0%) to ‘very difficult/very unacceptable’ (100%).
2.3.4. Preference
The moderator asked the participants which syringe they have preferred to use between the 8mmUTW and the 12.7mmSTW and the reason(s) why, after each set of 2 simulations for a same viscosity.
2.4. Study design of the validation study
The human factors validation study was a multi-center study conducted between December 2019 and January 2020 in the US (Newark, NJ; Philadelphia PA; Baltimore, MD and Boston, MA). This study aimed to assess the safe and effective use of the X225PLUS product. Additional objectives were to compare the usability performance of the X225PLUS-12.7mmSTW and X225PLUS-8mmUTW products when filled with 2 mL of a 30 cP viscous solution, as well as the ease and the acceptance of pushing the plunger rod when delivering the full dose was assessed for both product configurations.
No training was provided to the 63 participants enrolled, but all of them received the IFU and were free to read it. Each participant was asked to perform two simulated injections with the X225PLUS-12.7mmSTW and then two simulated injections with the X225PLUS-8mmUTW products. All the participants simulated the subcutaneous injections into a foam pad placed on the thigh or on the abdomen for patients or on a manikin for health care workers and caregivers. The injection technique is limited at a 90° angle with the X225PLUS-8mmUTW product instead of a 45° insertion angle to prevent the risk of intradermal administration. Debriefings on the root cause, ease, and acceptance of pushing the plunger rod as well as willingness to adopt both products were performed after each simulation.
2.4.1. Task assessment
The primary endpoint was the number and percentage of participants that correctly used the X225PLUS–8mmUTW in comparison to the X225PLUS-12.7mmSTW. For both products, 5 critical tasks were evaluated: ‘Remove the needle cover,’ ‘Perform the injection,’ ‘Deliver the full dose,’ ‘Activate the guard,’ and ‘Disposal of the used product.’ For each simulation, the moderator assessed all tasks and recorded the results based according to the IFU of the products, without giving any feedback or indication of performance to the participant. The moderator recorded any use errors, SOD as well as success. For each use error or difficulty encountered by participants, the root cause was investigated. Usability data are described as categorical data (N), and proportion frequencies (%). Both performance-based and subjective data were analyzed to ensure that no new risks were identified.
2.4.2. Ease of pushing the plunger rod and acceptance of the force required to push the plunger rod
Subjective responses were assessed through a questionnaire using a 6-points Likert scale. The percentage of answers with a score ≥5 per question was calculated, representing only strong positive feedbacks.
Likert scale was defined as following: ‘1: very difficult/fully unacceptable’; ‘2: difficult/unacceptable’; ‘3: somewhat difficult/somewhat unacceptable’; ‘4: somewhat easy/somewhat acceptable’; ‘5: easy/acceptable’ and ‘6: very easy/very acceptable.’
2.4.3. Willingness to adopt the product
User’s willingness to adopt each combination if prescribed was assessed using close-ended question. The number and percentage of positive answers were calculated.
2.5. Mathematical model of intramuscular and intradermal risks
A mathematical model was developed with the R statistical software (version 3.6.3) based on published skin thickness data at 4 common injection sites for adults with diabetes (abdomen, arm, buttock, and thigh) [Citation33]. This model estimates intradermal (ID) and intramuscular (IM) injection risks using several needle penetration depths (NPD) for two needle lengths (12.7mmSTW and 8mmUTW) at two injection angles (90° and 45°). These data were stratified by factor (gender, Body Mass Index, injection site). Tissue ID thickness, IM depth, and NPD values were generated using a random number generator function from the respective distributions, each defined by their mean and standard deviation. Logarithm transform was used for IM depth to correct for non-normality of the observed distribution.
Monte Carlo simulations were run for a sample size of 500 for each combination of tissue thickness and
NPD. For each pair of ID depth and NPD and IM depth and NPD, the ID and IM risks were calculated as the proportion of simulated samples where NPD < ID depth and NPD > IM depth, respectively.
Percentages of intramuscular and intradermal risks and ratios were computed between the two syringes of interest (at 45° and 90° injection angles).
3. Results
3.1. Participants demographics
The demographic characteristics of all the participants who took part in the studies are summarized in .
Table 1. Demographic characteristics of the participants
In the formative study 39 participants, Experienced patients (EXP), Naïve patients (NVP) and Hand-impaired patients (IMP) with a Cochin score between 1 and 86 were included in analyzes.
In the validation study, evaluating the use of the 8mmUTW syringe with the needlestick-prevention device X225PLUS, 63 representative end-users were included (Health Care Workers (HCW), Caregivers (CGV), Experienced patients (EXP), and Naïve patients (NVP)). Among the patient participants, 15 were having hand impairment (IMP) characterized by a Cochin Score in the range 12–38.
3.2. Formative study 8mmUTW vs. 12.7mmSTW syringes – Anxiety
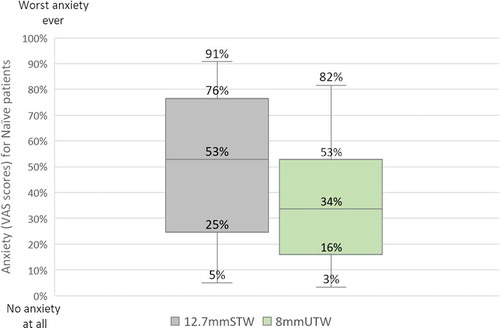
Having the choice between 12.7mmSTW and 8mmUTW syringes, naïve patients indicated to be less anxious by using the syringe with the shorter needle and indicated that they would favor it for self-injection. Indeed, the median VAS score was lower for the 8mmUTW (34%) syringe than for the 12.7mmSTW syringe (53%) (). Among the 23 experienced participants (with or without hand-impairment), 43% reported to be anxious before to self-inject their treatments because they either anticipated the potential pain or side effects, or they were afraid by long needles) and 87% thought a shorter needles could be an advantage (more comfortable, less painful, and intimidating).
Figure 2. Boxplot distributions of the VAS rating of the anxiety perceived by naive self-injecting patients during the formative study. Horizontal lines indicate median values, boxes indicate interquartile range, and whiskers indicate minimum and maximum values. VAS scale ranging from 0% = no anxiety at all to 100% = worst anxiety ever
3.3. Intradermal and intramuscular injection risks
The risks of injecting in a wrong tissue, either intradermal or intramuscular ones, with the 8mmUTW versus the 12.7mmSTW syringes, were estimated based on Gibney M., et al. data () [Citation33]. Regardless of the injection sites, the use of shorter needles (8 mm) compared to conventional ones would not increase the risk of intradermal injections when inserting the needle at 45° or 90° without raising a skinfold. The estimated risk of accidental injection into the muscle according to the 4 considered injection sites (thigh, abdomen, arm, and buttocks), in case of unsuitable injection technique used (injection at 90° without/or with incorrect skinfold) is reduced with the 8mmUTW by 59% to 84%. Considering the injection at 45° without skinfold, the risk of intramuscular delivery is also reduced (from 76% to 92%) for the 8mmUTW syringe versus the 12.7mmSTW syringe ().
Table 2. Estimated risks of intradermal (ID) and intramuscular (IM) injections with the 8mmUTW and the 12.7mmSTW syringes per site of injection and angle of insertion. The percentage of reduction in IM and ID risks and the risk ratio comparing the 8mmUTW and 12.7mmSTW syringes were calculated. N = 500 sample size. Percentages and risk ratio values are rounded up
3.4. Formative study 8mmUTW vs. 12.7mmSTW syringes – usability
The overall usability results obtained by the participants were similar to the 8mmUTW and the 12.7mmSTW syringes, filled with 2 mL of 10 cP or 30 cP solutions ()).
Figure 3. Usability of 8mmUTW and 12.7mmSTW syringes during the formative study. Overall task completion rates (a) and per task assessed (b). Success, use error and success with operational difficulty (SOD) rates are indicated for syringes filled with 2 mL of 10 cP solution. N number of simulated injections; %. (c). Success, use error and success with operational difficulty (SOD) rates are indicated for syringes filled with 2 mL of 30 cP solution. N number of simulated injections; %. * 71% (for 12.7mmSTW) and 83% (for 8mmUTW) of the use errors were caused by syringe priming. (d) Rates of success, use error, and artifact in regard to the injection technique used by patients. N number of simulated injections; %
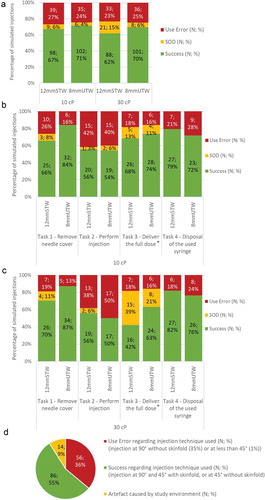
The 8mmUTW syringe improved the removal of the needle cover compared to the 12.7mmSTW syringe () and 3(c). While no success with operational difficulty was observed to remove the RNS-8mm, the success rate was increased by 18% and 17%, at 10 cP and 30 cP, respectively, against the removal of the RNS-12.7mm () and 3(c)). The same trend was observed for all user groups (Figure S1A). The success rate of removing the needle cover was increased with the use of the 8mmUTW syringe relative to the 12.7mmSTW syringe for the experienced and naïve patients, regardless of the viscosity. No improvement was observed for the hand-impaired patients, but the removal of the needle cover was correctly achieved by almost all these participants.
Similar rates of use errors were observed when performing the task ‘Perform injection’ for the two types of syringes at 10 cP and 30 cP () and 3(c)). Overall syringe configurations, impaired and naïve patients experienced 61% and 56% of use errors, respectively, while only 13% were recorded for experienced users (Figure S1B).
Regarding the task ‘Deliver the full dose,’ participants experienced more operational difficulties when using both syringes, 8mmUTW, and 12.7mmSTW, filled with the 30 cP solution compared to those filled with the 10 cP solution. Indeed, more participants were using two hands instead of one to deliver the full dose. Interestingly, while the success rate to deliver the full dose decreased for the high viscosity, the syringe configuration also affects the performance of participants for this task. When participants used the 8mmUTW syringe, they experienced more successes (42% to 63%) and fewer operational difficulties (39% to 21%) on delivering the full dose at 30 cP than when using the 12.7 mm syringe ()). The participants with prior practice in self-injecting experienced more successes to deliver the full dose when using the 8mmUTW syringe relative to the 12.7mmSTW syringe filled with the 30 cP solution (75% vs. 37.5%) (Figure S1C). Regarding both syringes filled with a 30 cP solution, a greater proportion of the hand-impaired patient population correctly achieved the full dose delivery when using the 8mmUTW syringe rather than using the 12.7mmSTW syringe (50% vs. 13%) (Figure S1C). Moreover, hand-impaired patients experienced more successes with operational difficulties with the 12.7mmSTW syringe relative to the 8mmUTW syringe (50% vs. 25%) (Figure S1C). The increase in the number of use errors recorded for impaired and naïve patients was caused by using a wrong injection technique. Injections at a 45° or 90° angle into a skinfold, or at a 45° without a skinfold were considered acceptable. However, 35% of the participants, including experienced ones, simulated the injection at a 90° angle without lifting a skinfold, 1% inserted the needle in the pad with an angle of less than 45° with both 8mmUTW and 12.7mmSTW syringes and 9% used inappropriate injection techniques because of the simulated environment but would correctly inject themselves in standard conditions ()).
Comparable usability results were recorded for the two syringes, 8mmUTW and 12.7mmSTW, and the two tested viscosities, 10 cP and 30 cP, concerning the disposal of the used syringe task () and 3(c)). Overall, the proportion of use errors for naïve patients completing this task was twice higher than for others user groups (Figure S1D). 62% of these use errors were due to participants that attempted to recap the needle after completing the simulated injection. As the moderator intervened to stop the recapping to avoid needle stick injury after the first simulation, less use errors were recorded for the following simulations, regardless of the syringe type.
Overall, the usability of the 8mmUTW syringe is at least equivalent to the marketed 12.7mmSTW syringe, thus there is no difference in risk profile. Regardless of the viscosity, participants experienced less operational difficulties to remove the needle cover with the 8mmUTW syringe. Nonetheless, the use of 8mmUTW syringe filled with a high viscous solution of 30 cP improves the full dose delivery and reduces the operational difficulties encountered by all representative patient groups relative to the 12.7mmSTW syringe, in particular for patients with prior self-injecting experience or those suffering from hand-impairment.
3.5. Formative study 8mmUTW vs. 12.7mmSTW syringes – ease of use, acceptance and preference
As expected, the viscosity directly influenced both ease of pushing and acceptance of the force required to push the plunger rod for all participants.
Across all participants, the ease of pushing the plunger rod and the acceptance of the force required for it were similarly rated for both syringes, the 8mmUTW, and the 12.7mmSTW, filled with 10 cP solution () and 4(b) respectively). These two parameters were rated better at 10 cP than at 30 cP for all participants () and 4(b)). According to fewer operational difficulties experienced by participants to deliver the full dose at 30 cP with the 8mmUTW syringe compared to the 12.7mmSTW syringe ()), participants perceived the pushing of the plunger rod easier with the 8mmUTW syringe than the 12.7mmSTW syringe (overall median VAS score of 43% vs. 61%; )). Consistently, the force required to push the plunger rod was more acceptable for the 8mmUTW syringe versus the 12.7mmSTW syringe (overall median VAS score of 42% vs. 61%; )). Thus, at 30 cP, the 8mmUTW syringe provides an improved ease of pushing the plunger rod and a better acceptance of the force required to push it, especially for naïve and hand-impaired patients. Likewise, 72% of the participants expressed a preference for the 8mmUTW syringe filled at 30cP because of the reduced injection force (57%), the shorter needle (36%) or the easier cap removal (7%). Regardless of the assessed viscosities, the 8mmUTW was preferred by 64% of the participants. At 10 cP the preference toward the 8mmUTW versus the 12.7mmSTW syringe, for the same reasons as mentioned above, was formulated to a lesser extend (56% versus 21%) ().
Figure 4. Boxplot distribution of the VAS scores of the ease of pushing the plunger rod (a) and of the acceptance of the force required to push the plunger rod (b) for all participants of the formative study. Horizontal lines indicate median values, boxes indicate interquartile range, and whiskers indicate minimum and maximum values. VAS scales ranging from 0 = very easy/very acceptable to 100% = very difficult/very unacceptable
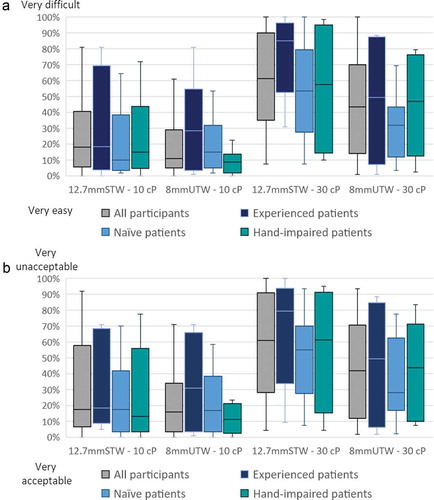
Table 3. Preferred syringe between the 8mmUTW and the 12.7mmSTW during the formative study when both filled with 10 cP and 30 cP solutions across all participants. N number of answers; (%)
3.6. Validation study needlestick-prevention device (X225PLUS) equipped with the 8mmUTW syringe – usability
The usability of the needlestick-prevention device X225PLUS was assessed when equipped with the 8mmUTW syringe or with the 12.7mmSTW syringe, both filled with a high viscous solution (30 cP).
Overall, the usability results were similar between the X225PLUS device equipped with the 8mmUTW syringe or with the 12.7mmSTW syringe ()).
Figure 5. Usability of the safety X225 PLUS device equipped either with the 8mmUTW or the 12.7mmSTW syringes during the validation study. Overall task completion rates (a) and per task assessed (b) for X225 PLUS–8mmUTW and X225 PLUS–12.7mmSTW devices. Rates of success, use error and success with operational difficulty (SOD) are indicated. N number of simulated injections; %. * 20% (for X225-12.7mmSTW) and 28% (for X225PLUS-8mmUTW) of the use errors were caused by syringe priming. Ease of pushing the plunger rod (c) and acceptance of the force required to push the plunger rod (d) for X225 PLUS–8mmUTW and X225 PLUS–12.7mmSTW products. N represents the number of answers rated at 5 (easy/acceptable) and 6 (very easy/very acceptable); (%)
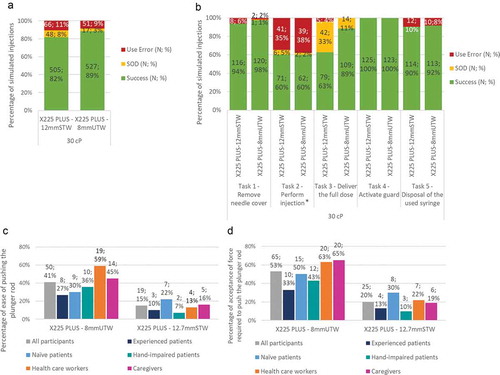
Regardless of the user groups, the number of successes and use errors were not significantly different for participants using the X225PLUS-8mmUTW and the X225PLUS-12.7mmSTW products for the task ‘Remove needle cover’ (98% vs. 94% of successes, respectively).
39% and 41% of use errors were recorded for the task ‘Perform the injection’ with the X225PLUS-8mmUTW and the X225PLUS-12.7mmSTW products, respectively. 39% of the recorded use errors were due to the unappropriated injection technique employed (insertion at 90° without skinfold). 19% of the naïve and 27% of the experienced patients did not perform a skinfold while injecting at a 90° angle.
In comparison to the X225PLUS-12.7mmSTW product, the use of the X225PLUS-8mmUTW product improves the success of the ‘Deliver the full dose’ task ()). No use error was recorded across all user groups with the X225PLUS-8mmUTW product against 4% of use errors with the X225PLUS-12.7mmSTW product. Moreover, the operational difficulties encountered to deliver the full dose was reduced by 22% when participants were using the X225PLUS-8mmUTW product instead of the X225PLUS-12.7mmSTW product. Besides some operational difficulties, users were able to deliver 30 cP solution and activate the needlestick-prevention product. Indeed, the use of the 8mmUTW does not impede the activation of the needle guard, as 100% of the needle guards were correctly activated with both X225PLUS-8mmUTW and X225PLUS-12.7mmSTW products ()).
No significant difference was observed for the task ‘Disposal of the used syringe,’ for which the participants experienced 92% of successes with the X225PLUS-8mmUTW product and 90% with the X225PLUS-12.7mmSTW product ()).
3.7. Validation study needlestick-prevention device (X225PLUS) equipped with the 8mmUTW syringe – ease of use and acceptance
The participants found easier to push the plunger rod with the X225PLUS-8mmUTW product versus the X225PLUS-12.7mmSTW product (41% vs. 15% of easy and very easy rating answers) (health care workers (59% vs. 13%) ()). Similarly, all participants better accepted the force required to push the plunger rod with the X225PLUS-8mmUTW product than with the X225PLUS-12.7mmSTW product (53% vs. 20% of acceptable and very acceptable rating answers) ()).
Therefore, the use of the X225PLUS–8mmUTW product improves the successful delivery of the full dose of high viscous solution (30 cP), which is supported by better perceptions of the ease of pushing the plunger rod and the acceptance of the force required to push the plunger rod. Additionally, the increased proportion of the participants willing to adopt the X225PLUS–8mmUTW product (86%) relative to the X225PLUS-12.7mmSTW product (61%) confirmed the perceived ease of use and acceptance ().
Table 4. Willingness to adopt the X225 PLUS–8mmUTW and the X225 PLUS–12.7mmSTW products across all participants during the validation study. N number of answers(%)
4. Discussion
The two human factors studies, presented in this manuscript, aimed to evaluate the usability of a new pre-fillable glass syringe equipped with a short 8mm and ultra thin-wall needle (BD Neopak™ XtraFlow™ 2.25 mL, 8mmUTW) in comparison to a marketed pre-fillable syringe (BD Neopak™ 2.25 mL, 12.7mmSTW) alone or combined with a needlestick-prevention device (BD UltraSafe Plus™ 2.25 mL Passive needle guard, X225PLUS) in order to improve the subcutaneous administration of high volume (2 mL) and high viscous solutions (30 cP). These human factors studies were conducted per Food and Drug Administration (FDA) guidance and the International Electrotechnical Commission (IEC) 62366–1, and the spectrum of intended user groups were evaluated.
The full delivery of the 30cP viscous solution is improved with the 8mmUTW syringe versus the 12.7mmSTW syringe, resulting in fewer operational difficulties and more successes for all participants. The same trend was observed for the 8mmUTW assembled with the X225PLUS needlestick-prevention device, more successes were recorded to deliver the full dose in comparison to the X225PLUS–12.7mmSTW product. However, the reduced rate of use errors recorded with the X225PLUS–8mmUTW was most probably attributed to the gained experience during previous simulations done with X225PLUS–12.7mmSTW products. Indeed, after 5 simulations with the X225PLUS-12.7mmSTW product, to assess the usability of this product, the users were able to learn how to use this needlestick-prevention device before simulating the injections with the X225PLUS-8mmUTW product. Although this bias must be considered in the interpretation, the user experience is improved, with an easier delivery and a more acceptable force required to push on the plunger rod with the X225PLUS-8mmUTW than the X225PLUS-12.7mm product. A significant difference between the rate of use errors during the validation (0% to 4% of use errors) and the formative (16% to 18% of use errors) studies was observed. During the formative study, the use errors caused by a priming prior the simulations representing 83% and 71% of the use errors for the 8mmUTW and the 12.7mmSTW were recorded during the assessment of the task ‘Deliver the full dose’ as the users failed to deliver the full dose due to the expelled volume expelled before the simulation creating a potential risk of underdosing. These use errors were recorded during the evaluation of the task ‘Perform injection’ in the validation study as occurring before the needle insertion. Despite the difference in terms of task assessment between the two studies, these use errors were not related to the performance of the tested products but were due to an inappropriate use by the participants.
The task ‘Perform injection’ was considered slightly differently between the two studies. It was considered as essential in the formative study and as critical in the validation study. Usually, the instructions in terms of injection technique are précised into the final combination product and therefore are under pharmaceutical companies’ responsibility. However, during the validation study, the injection technique for the X225PLUS-8mmUTW product was restricted (insertion at a 90° angle within a skinfold) and communicated through the IFU to the participants due to the encumbrance of the X225PLUS product related to the shorter needle length, 8 mm, which may increase the risk of intradermal administration if inserted at a 45° angle. Therefore, to assess this mitigation measure the used injection techniques were recorded and the task ‘Perform injection’ was defined as critical during the validation study. Nevertheless, this task was assessed in terms of usability and root cause evaluation during both studies, regardless of its classification.
In terms of safety, regardless of the syringe used, the formative study pointed a hazardous behavior. Indeed, some users attempted to recap the needle once the simulations performed. Although the moderator stopped patients to prevent the recapping of the needle and thus needlestick injury, among them 62.5% were experienced patients in using prefilled syringes (with or without hand impairment) and 37.5% were naïve. Participants located in Boston (US) explained their action because they did not have access to a sharp container at home unlike most of the participants located in London (UK). Thus, the availability of a sharp containers might be critical to change users’ habit toward needle recapping and prevent against needlestick injuries.
Besides the safety and efficacy of drug delivery devices, patients’ perception should also be considered when selecting a drug delivery solution to improve injection experience and potentially their treatment adherence [Citation44–46]. The shorter needle combined with an optimized inner diameter (UTW technology) positively impacts the patients’ perception of the force required to deliver the full dose when using a shorter UTW needle during simulated subcutaneous injections especially for high viscosity solution (30 cP) in both studies. The ability to hold a device may be strongly affected in case of hand and/or fingers deformities and pain observed for some patients suffering from rheumatoid arthritis [Citation45]. Our studies revealed that hand-impaired patients also perceived the 8mmUTW syringe alone and combined with the X225PLUS product as easier to push and better accepted the force required to push on the plunger rod than with the 12.7mmSTW syringe. During the validation study, two participants did not perform any simulation with the X225PLUS-8mmUTW product, as they felt some discomfort after the simulations done with the X225PLUS-12.7mmSTW product. The users’ feedbacks on the ease of pushing the plunger rod and the acceptance of the force required to push the plunger rod may support the interest in using the X225PLUS-8mmUTW product to protect users from fatigue due to the injection of high volumes and/or high viscous solutions. Overall, participants expressed a preference in terms of ease of use and acceptance for the 8mmUTW syringe relative to the 12.7mmSTW syringe due to the perceived reduction of the force required to deliver the solution.
Moreover, in the formative study, the use of an incorrect injection technique observed supports the benefit of using 8 mm needles versus 12.7 mm needles, in terms of chance to reach the correct tissue. Indeed, 35% of participants, including experienced ones, simulated the injection at 90° angle without lifted a skinfold. Based on the described mathematical model the unintended intramuscular injections by reducing the needle length from 12.7 mm to 8 mm when using a non-recommended injection technique, insertion at a 90° angle without raising a skinfold was estimated to be reduced by 59% and 77% in the thigh or abdomen, respectively.
In the formative study, the proportion of participants employing an incorrect injection technique might be overestimated for naïve patients as they were not were aware of the subcutaneous injection technique in contrast with experienced patients, including those with hand-impairment. Moreover, although it is important to note that our data were obtained from a simulated study and with a small sample size and might not reflect real injections, the analysis of a survey addressed to adults with diabetes, using 8 mm or longer needles, showed that only 64% of patients lifted a skinfold, among them 75% did it properly, and less than 50% of them released correctly the skinfold [Citation47]. Therefore, preliminary results obtained in the formative study, as well as published data, highlight that patients’ population often does not know or use the appropriate injection techniques to deliver their treatment in the targeted subcutaneous tissue. In real conditions, these use errors would have increased the risk of injecting in the intramuscular tissue, more with the 12.7mmSTW syringe than with the 8mmUTW syringe, potentially affecting the drug absorption, especially, in case of using an inappropriate injection technique at 90° without raising a skinfold [Citation7,Citation33–35]. In addition to reducing accidental intramuscular administration, the use of the 8mmUTW syringe does not increase the risk of targeting the dermis instead of the subcutaneous tissue. A previous study estimated that the injection at a 90° angle without lifting a skinfold with short needles (4 mm to 8 mm) did not result in accidental intradermal injection, based on skin thickness measurements of the abdomen and the upper arm in adults with diabetes [Citation48]. The data presented in this article confirm that the 8 mm needle is not more likely to induce dermis administration than the 12.7 mm needle when injecting at a 90° as well as at a 45° angle without raising a skinfold regardless of the injection sites (abdomen, arm, thigh, and buttocks).
Limitations of these studies are inherent to the nature of simulated use studies, meaning that participants may perform differently their injections when observed during a simulation study than in their actual use. All subjective feedbacks were recorded according to participants’ point of view including feelings, perceptions, and concerns at the time of the simulated testing. Therefore, clinical studies of the full combination product are required to confirm the results obtained in those human factors studies and go further by evaluating pain and depot localization of the drugs.
5. Conclusion
The new 8mmUTW syringe combined or not to a needlestick-prevention device (X225PLUS) improves the manual delivery of high volumes (2 mL) and viscous solutions (up to 30 cP) for the targeted end-users, especially for patients with prior experience in self-injecting chronic treatments, as well as for patients suffering from hand impairment. The 8mmUTW syringe presents a better usability profile for manual subcutaneous injections in comparison to the 12.7mmSTW syringe by increasing the rate of success and decreasing the occurrence of operational difficulties. Many users perceived the 8mmUTW syringe alone or assembled with the X225PLUS device as easier to push on the plunger rod to deliver the solution and they better accepted the force required to do so in comparison to the 12.7mmSTW syringe. Consistently, most of the users expressed a preference toward the 8mmUTW syringe and were willing to adopt it in combination with the X225PLUS device against the 12.7mmSTW syringe. Our formative study highlighted the use of inappropriate injection technique at a 90° angle without raising a skinfold in 35% of the simulated injections, even for self-injecting experienced patients. In doing so, the needle length reduction could increase the chances of targeting the right tissue (subcutaneous) by 2 to 6 times, thus ensuring an optimal efficacy of chronic injectable therapies while avoiding potential adverse effects for the patients. The 8mmUTW syringe provides an acceptable and easy to use alternative to the current 12.7 mm needle syringes for the delivery of viscous solutions. Additionally, the shorter needle length tends to reduce the injection-related anxiety felt by patients. Altogether, these results demonstrate that the user experience is improved with the BD Neopak™ XtraFlow™ syringe (8mmUTW) which might contribute to enhance treatment compliance in chronic care.
Author contributions
A Pager, K Guerrero, T Tzvetkova-Chevolleau contributed to the conception, design and analysis of the human factors testing. D Morel developed the mathematical model data analysis methods. A Combedazou wrote the paper with the inputs from all authors. All authors reviewed the manuscript. C Frolet and S Glezer supervised the project.
Declaration of interest
All authors are employees of BD Medical Pharmaceutical Systems. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from Expert Opinion on Drug Delivery for their review work, but have no other relevant financial relationships to disclose.
Legend_SupplFile1_User-experience-8mmUTW.docx
Download MS Word (12 KB)Supplementary_FigureS1_User-experience-8mmUTW.tif
Download TIFF Image (587.6 KB)Supplementary material
Supplemental data for this article can be accessed here.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Viola M, Sequeira J, Seiça R, et al. Subcutaneous delivery of monoclonal antibodies: how do we get there? J Control Release. 2018;286:301–314.
- Usach I, Martinez R, Festini T, et al. Subcutaneous injection of drugs: literature review of factors influencing pain sensation at the injection site. Adv Ther. 2019;36(11):2986–2996.
- Collins DS, Kourtis LC, Thyagarajapuram NR, et al. Optimizing the bioavailability of subcutaneously administered biotherapeutics through mechanochemical drivers. Pharm Res. 2017;34(10):2000–2011.
- Zharkov A, Barton B, Heinzmann D, et al., Development pathways for subcutaneous formulations of biologics versus biosimilar development. Expert Review of Precision Medicine and Drug Development, 2019: p. 1–8.
- Bolge SC, Goren A, Tandon N. Reasons for discontinuation of subcutaneous biologic therapy in the treatment of rheumatoid arthritis: a patient perspective. Patient Prefer Adherence. 2015;9:121–131.
- Zijlstra E, Jahnke J, Fischer A, et al. Impact of injection speed, volume, and site on pain sensation. J Diabetes Sci Technol. 2018;12(1):163–168.
- Jin JF, Zhu -L-L, Chen M, et al. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer Adherence. 2015;9:923–942.
- Schweighofer CD, Wendtner CM. First-line treatment of chronic lymphocytic leukemia: role of alemtuzumab. Onco Targets Ther. 2010;3:53–67.
- Turner AP, Williams RM, Sloan AP, et al., Injection anxiety remains a long-term barrier to medication adherence in multiple sclerosis. Rehabil Psychol. 2009;54(1): 116–121.
- Zambanini A, Newson RB, Maisey M, et al. Injection related anxiety in insulin-treated diabetes. Diabetes Res Clin Pract. 1999;46(3):239–246.
- Garidel P, Kuhn AB, Schäfer LV, et al. High-concentration protein formulations: how high is high? Eur J Pharm Biopharm. 2017;119:353–360.
- McLennan DN, Porter CJ, Charman SA. Subcutaneous drug delivery and the role of the lymphatics. Drug Discov Today Technol. 2005;2(1):89–96.
- Bittner B, Richter W, Schmidt J. Subcutaneous administration of biotherapeutics: an overview of current challenges and opportunities. BioDrugs. 2018;32(5):425–440.
- Wago KJ, Skarsvåg TI, Lundbom JS, et al. The importance of needle gauge for pain during injection of lidocaine. J Plast Surg Hand Surg. 2016;50(2):115–118.
- Jorgensen JT, Rømsing J, Rasmussen M, et al. Pain assessment of subcutaneous injections. Ann Pharmacother. 1996;30(7–8):729–732.
- Mathaes R, Koulov A, Joerg S, et al. Subcutaneous injection volume of biopharmaceuticals-pushing the boundaries. J Pharm Sci. 2016;105(8):2255–2259.
- Berteau C, Filipe-Santos O, Wang T, et al. Evaluation of the impact of viscosity, injection volume, and injection flow rate on subcutaneous injection tolerance. Med Devices (Auckl). 2015;8:473–484.
- Alam M, Geisler A, Sadhwani D, et al. Effect of needle size on pain perception in patients treated with botulinum toxin type a injections: a randomized clinical trial. JAMA Dermatol. 2015;151(11):1194–1199.
- Heise T, Nosek L, Dellweg S, et al. Impact of injection speed and volume on perceived pain during subcutaneous injections into the abdomen and thigh: a single-centre, randomized controlled trial. Diabetes Obes Metab. 2014;16(10):971–976.
- Juul KA, Bengtsson H, Eyving B, et al. Influence of hypodermic needle dimensions on subcutaneous injection delivery--a pig study of injection deposition evaluated by CT scanning, histology, and backflow. Skin Res Technol. 2012;18(4):447–455.
- Watt RP, Khatri H, Dibble ARG. Injectability as a function of viscosity and dosing materials for subcutaneous administration. Int J Pharm. 2019;554:376–386.
- Karges B, Boehm BO, Karges W. Early hypoglycaemia after accidental intramuscular injection of insulin glargine. Diabet Med. 2005;22(10):1444–1445.
- Vaag A, Handberg A, Lauritzen M, et al. Variation in absorption of NPH insulin due to intramuscular injection. Diabetes Care. 1990;13(1):74–76.
- Vaag A, Pedersen KD, Lauritzen M, et al. Intramuscular versus subcutaneous injection of unmodified insulin: consequences for blood glucose control in patients with type 1 diabetes mellitus. Diabet Med. 1990;7(4):335–342.
- Frid A, Ostman J, Linde B. Hypoglycemia risk during exercise after intramuscular injection of insulin in thigh in IDDM. Diabetes Care. 1990;13(5):473–477.
- Guo S, Bozkaya D, Ward A, et al. Treating relapsing multiple sclerosis with subcutaneous versus intramuscular interferon-beta-1a: modelling the clinical and economic implications. Pharmacoeconomics. 2009;27(1):39–53.
- Calabrese M, Bernardi V, Atzori M, et al. Effect of disease-modifying drugs on cortical lesions and atrophy in relapsing-remitting multiple sclerosis. Mult Scler. 2012;18(4):418–424.
- Arthur V, Jubb R, Homer D. A study of parenteral use of methotrexate in rheumatic conditions. J Clin Nurs. 2002;11(2):256–263.
- Klein CG, Cicinnati V, Schmidt H, et al. Compliance and tolerability of subcutaneous hepatitis B immunoglobulin self-administration in liver transplant patients: a prospective, observational, multicenter study. Ann Transplant. 2013;18:677–684.
- Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91(9):1231–1255.
- Frid A, Hirsch L, Gaspar R, et al. New injection recommendations for patients with diabetes. Diabetes Metab. 2010;36(Suppl 2):S3–18.
- Strauss K, Gols HD, Hannet I, et al. A pan-European epidemiologic study of insulin injection technique in patients with diabetes. Pract Diabetes Int. 2002;19(3):71–76.
- Gibney MA, Arce CH, Byron KJ, et al., Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations. Curr Med Res Opin. 2010;26(6): 1519–1530.
- Hirsch L, Byron K, Gibney M. Intramuscular risk at insulin injection sites–measurement of the distance from skin to muscle and rationale for shorter-length needles for subcutaneous insulin therapy. Diabetes Technol Ther. 2014;16(12):867–873.
- Tubiana-Rufi N, Belarbi N, Du Pasquier-Fediaevsky L, et al., Short needles (8 mm) reduce the risk of intramuscular injections in children with type 1 diabetes. Diabetes Care. 1999;22(10): 1621–1625.
- Tsapatsaris NP. Low-dose heparin. A cause of hematoma of rectus abdominis. Arch Intern Med. 1991;151(3):597–599.
- Thomas BL. Heparin-induced hematomas. Arch Intern Med. 1992;152(1):204.
- Kim L, Nevis I, Potts R, et al. Patients on subcutaneous allergen immunotherapy are at risk of intramuscular injections. Allergy Asthma Clin Immunol. 2014;10(1):22.
- Baker MP, Reynolds HM, Lumicisi B, et al. Immunogenicity of protein therapeutics: the key causes, consequences and challenges. Self Nonself. 2010;1(4):314–322.
- Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23(1–2):37–43.
- Kreugel G, Beijer H, Kerstens MN, et al. Influence of needle size for subcutaneous insulin administration on metabolic control and patient acceptance. Eur Diabetes Nurs. 2007;4(2):51–55.
- Hudry C, Lebrun A, Moura B, et al. Evaluation of usability and acceptance of a new autoinjector intended for methotrexate subcutaneous self-administration in the management of rheumatoid arthritis. Rheumatol Ther. 2017;4(1):183–194.
- Duruoz MT, Poiraudeau S, Fermanian J, et al. Development and validation of a rheumatoid hand functional disability scale that assesses functional handicap. J Rheumatol. 1996;23(7):1167–1172.
- Koren MJ, Lundqvist P, Bolognese M, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2531–2540.
- Keininger D, Coteur G. Assessment of self-injection experience in patients with rheumatoid arthritis: psychometric validation of the Self-Injection Assessment Questionnaire (SIAQ). Health Qual Life Outcomes. 2011;9(1):2.
- Schiff M, Saunderson S, Mountian I, et al. Chronic disease and self-injection: ethnographic investigations into the patient experience during treatment. Rheumatol Ther. 2017;4(2):445–463.
- Frid AH, Hirsch LJ, Menchior AR, et al., Worldwide injection technique questionnaire study: population parameters and injection practices. Mayo Clin Proc. 2016;91(9): 1212–1223.
- Sim KH, Hwang MS, Kim SY, et al. The appropriateness of the length of insulin needles based on determination of skin and subcutaneous fat thickness in the abdomen and upper arm in patients with type 2 diabetes. Diabetes Metab J. 2014;38(2):120–133.
