ABSTRACT
Introduction
As research in suprachoroidal drug delivery advances, and therapeutic candidates, ranging from small molecule suspensions to gene therapy, progress through clinical trials, an understanding of suprachoroidal space (SCS) biomechanics assumes increasing importance.Areas covered:Numerous anatomic features play an important role in therapeutic access to the SCS. Methods of access include a catheter, a standard hypodermic needle, and a microinjector with microneedle. Physical and fluidic properties of injectates into the SCS, such as volume, viscosity, particle size, osmotic pressure, and ionic charge of formulation can impact the spread and extent of opening of the SCS. Pharmacokinetic data of several small molecule suspensions yielded favorable ocular distribution and pharmacokinetic profiles. Phase 2 and 3 clinical trials have been completed with a suprachoroidally injected corticosteroid; results and information on procedural details with the microinjector are discussed.
Expert opinion
Suprachoroidal drug delivery has been demonstrated to be a reliable and consistent drug delivery method for targeted treatment of retinal and choroidal disorders to potentially maximize efficacy, while compartmentalizing therapies away from the unaffected tissues to potentially enhance safety. These delivery attributes, along with fluid transport properties and formula customization for pharmacological agents, may allow for more tailored treatment of diseases affecting chorio-retinal tissues.
1. Introduction
Ocular drug delivery, for local treatment of common retinal and choroidal disorders, has evolved considerably over the years. Periocular injection, used for decades to deliver corticosteroids, is associated with highly variable drug diffusion across the sclera into the eye [Citation1]. After the 2004 introduction of intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy, intravitreal injection rapidly superseded periocular injection, with over 6 million intravitreal anti-VEGF injections performed yearly in the United States by 2016 [Citation2]. However, intravitreal injection leads to broad diffusion through the eye, including the anterior chamber and lens, with potential for off-target effects. Recently, to achieve more targeted delivery directly to the retina, subretinal injection has become standard for the administration of retinal gene therapy. This procedure requires an operating room based-pars plana vitrectomy with retinotomy and limited retinal detachment, as therapy is injected into the subretinal space. These invasive techniques may lead to complications and additional risks to the patient, and the results are highly variable in controlling the spread of gene therapy under the retina.
To address some of these limitations, in-office drug delivery of gene therapy, biologics and small molecule suspensions via the suprachoroidal space (SCS) is currently under investigation. The injected therapy spreads circumferentially and posteriorly to target the affected chorio-retinal tissue layers, which may maximize efficacy [Citation3–7]. Other potential advantages of suprachoroidal injection include compartmentalization away from nonaffected tissues, providing the potential for enhanced safety, and improved bioavailability, as the choroid and adjacent tissues are exposed to the therapeutic agents in close proximity and high concentration. In addition, several small-molecule suspensions have shown prolonged pharmacokinetic (PK) profiles, supporting the potential for a sustained presence in key ocular tissues [Citation5,Citation8,Citation9].
As new therapies delivered via SCS injection undergo evaluation in clinical trials, there is a need for a comprehensive biomechanical understanding of this delivery route. This review summarizes biomechanical factors in the context of suprachoroidal drug delivery. The first section focuses on existing literature on SCS anatomy and the means to access the space. The second section reviews nonclinical and clinical outcomes of suprachoroidal injection, with emphasis on microneedle-based technology. The final section examines existing evidence of biomechanical impacts after suprachoroidal injection and the feasibility for repeat dosing.
2. Considerations prior to suprachoroidal injections
Potential spaces in human anatomy refer to the area between directly apposed organs or tissue layers. Examples include the pleural space and pericardial cavity, both of which can fill with fluid in a variety of disease states, creating pleural and pericardial effusion, respectively. At the same time, potential spaces represent possible targets for pharmacologic agent delivery, yielding favorable delivery characteristics. One example is the epidural space, often used to deliver anesthetics or corticosteroids via a loss of resistance technique. Similarly, the SCS, a potential space between the sclera and the choroid, has become a promising drug delivery route to the posterior segment of the eye [Citation5,Citation8–12], with the possibility of addressing the most common causes of irreversible vision loss in the industrialized world such as age-related macular degeneration, diabetic macular edema, and other causes of retinal diseases. The SCS has been accessed in a variety of procedures with a variety of devices, including cannulation and injections involving a loss of resistance technique [Citation6,Citation13–16].
2.1. Anatomy of the SCS
As with other potential spaces in anatomy, the sclera and the choroid are normally directly apposed in vivo due to the intraocular pressure (IOP) within the eye. However, with fluid injection and/or mechanical cannulation, these layers can atraumatically separate, creating a true space between them, the SCS. Though the sclera and choroid are adjacent tissues, their functions and mechanical properties are vastly different. The sclera, a dense collagen matrix, serves as the protective outer layer of the eye and is much stiffer than the choroid, a more flexible vascular layer. Specifically, the compression modulus of the sclera is approximately seven times that of the choroid [Citation17], accounting for much greater choroid deformation over that of the sclera at a given pressure, creating the SCS. Histologically, it has been observed that ‘fibrous cords crisscrossed the interface between sclera and choroid’ [Citation18]. Flattened melanocytes and fibrocytes have been observed to be interwoven within a sheet of fine cords that runs parallel to the inner layer of the sclera. Taken together, the difference of the mechanical properties and the lack of strong physical bonds allows for expansion to create the SCS, whether through injection of fluid or mechanical cannulation.
Scleral thickness at the pars plana, where suprachoroidal injection often occurs, is a key anatomical variable for this type of ocular injection as the sclera must be precisely penetrated or cannulated without piercing through choroidal tissues. Scleral thickness in human subjects has been measured with various imaging modalities, such as ultrasound biomicroscopy (UBM), MicroMRI, histology, and optical coherence tomography (OCT). Cross-sectional histological studies of the sclera situated anterior and posterior to the plans plana have measured thicknesses ranging from 500 ± 100 µm at the limbus to 420 ± 130 µm at the ora serrata [Citation19]. Though fixative methods used with enucleated eyes can impact quantitative measurement of scleral thickness, it has been observed that scleral thickness varies along the anterior-posterior axis, decreasing from the scleral-corneal junction to the equator and then increasing again to the posterior pole [Citation20]. Using UBM measurement techniques, Oliveira et al. found the scleral thickness at 3.0 mm post limbus to be 506.5 ± 65 µm in 140 eyes [Citation21]. Data from the literature involving MicorMRI and OCT-based technologies suggest that scleral thickness is relatively consistent at the pars plana and slightly thinner in the superotemporal quadrant of the eye versus the inferonasal quadrant [Citation19,Citation22].
In addition to scleral thickness, the extracellular matrix connections between the sclera and the choroid/ciliary body should be considered in order to understand the boundaries of fluid flow within the SCS. At the scleral spur, the sclera adheres tightly to the ciliary body and thus the suprachoroidal and supraciliary spaces are occluded anteriorly, as seen in . Consequently, fluid introduced into the pars plana region will expand the SCS and flow posteriorly, thereby minimizing anterior chamber exposure [Citation23], as seen in . The flow of fluid in the SCS is further enhance by the natural pressure differential in the eye which decreases posteriorly. Emi et al. measured the hydrostatic pressures in the SCS through direct cannulation, observing pressures 0.8 ± 0.2 mm Hg below the IOP anteriorly and 3.7 ± 0.4 mm Hg below the IOP posteriorly [Citation24]. This pressure differential, the driving force for uveoscleral outflow, may enhance the posterior spread of drugs injected into the SCS, and thereby facilitate the treatment of macular disorders in addition to peripheral chorioretinal disorders.
Figure 1. Cross-sectional anatomy illustration of the human eye. The suprachoroidal space (SCS) is denoted in yellow and is occluded anteriorly by the scleral spur, as seen in the magnified region of the illustration. Image updated from https://www.scientificanimations.com, CC BY-SA 4.0, via Wikimedia Commons
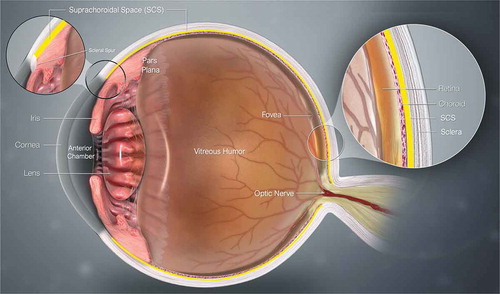
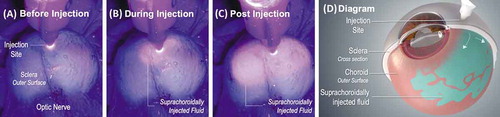
Figure 2. Suprachoroidally injected fluorescing dye under UV light in an ex vivo porcine eye before (A), during (B), and immediately post (C) injection shows posterior and circumferential spread of the injectate within the suprachoroidal space (SCS), as seen by the fluorescing particles emitting light through the scleral tissue. (D) is a diagram of a partial cross-section of an eye with suprachoroidally injected fluid spreading posteriorly and circumferentially between the sclera and choroid around the globe
Lastly, the vasculature in the choroid may also play an important role in the clearance of fluids and molecules from the SCS [Citation25]. Interactions between the choroid and the properties of the injectate, including particle charge and/or size, can significantly influence drug transport. The fenestrated choriocapillaris is estimated to have pore sizes between 6 and 12 nm with a negatively charged glycocalyx matrix [Citation26]. Furthermore, the choriocapillaris also has been shown to impede suprachoroidal fluid patterns [Citation27]. Chiang et al. found that the long posterior ciliary artery (LPCA), attached to the sclera at multiple points, serves as aphysical barrier for fluid and particle flow in the SCS as it can create localized restrictions in the opening of the SCS. Additional venous pathways within the SCS have also been documented with corrosion casting or fluorescent imaging of the space [Citation28,Citation29]. The presence of these pathways may impact both fluid distribution and clearance.
2.2. Methods of accessing the SCS
A variety of methods have been investigated to access the SCS in an effort to deliver therapies to the back of the eye, including the use of a catheter, a standard hypodermic needle, or a microneedle. For catheterization techniques, a catheter is manually inserted through a scleral incision and advanced posteriorly [Citation15]. These procedures require an operating room environment and are more invasive than other ocular injection methods. Advantages of a catheter-based approach include the potential to more precisely target specific posterior regions of the SCS, such as the macula, and the potential for direct visualization of the delivery location. The SCS has also been accessed using a standard hypodermic needle [Citation30,Citation31]. This technique, while employing the use of generic, commercially available materials, requires significant surgical precision, and carries with it the risk for inadvertent injection into the vitreous, the subretinal space, or the subconjunctival region. Microneedle technology, the method primarily explored herein, has potential to access the SCS more reliably than standard hypodermic needles and less invasively than cannulation, as the procedure does not require an incision or surgical imaging in an operating room environment. Microneedles are made to the length of the approximate thickness of the sclera, and are used with a custom injector specifically designed for suprachoroidal drug delivery [Citation3–7]. While there are some challenges with this type of suprachoroidal injection procedure, including the lack of direct intraoperative visualization and the potential need for customized injectate formulations that are compatible with the device [Citation26,Citation32], to date, the procedure has been performed over 1,000 times in clinical trial patients with an acceptable safety profile [Citation33].
Suprachoroidal injection using proprietary microneedle-based technology, the SCS Microinjector® (Clearside Biomedical, Alpharetta, GA) consists of three key steps, unique from other ocular injections. First, the needle of the device is inserted into the sclera at a perpendicular angle to the injection location, at a distance approximately 4.5 mm posterior to the limbus at the pars plana. No surgical incision is required. Second, gentle force is applied to the device so that the hub of the needle contacts and subsequently depresses the globe of the eye, creating a ‘sealing gasket’ effect between the hub of the needle and the conjunctiva. This ‘sealing gasket’ effect ensures that the needle opening reaches the SCS, while minimizing reflux during the injection. The third and final step is to inject into the SCS over 5–10 seconds while maintaining compression of the conjunctiva and perpendicular positioning to the injection site. Because the sclera is comprised of a dense collagen matrix, the injection will not proceed if the opening of the needle is still embedded exclusively within the scleral tissue. When the needle advances inward past the sclera, a loss of resistance is felt with the advancement of the plunger of the device, initiating injection into the SCS.
3. Suprachoroidal injections with microneedles
3.1. Nonclinical characterization
The biomechanics of suprachoroidal injection are detailed in this section, including injection pressures and force measurements, the effects of fluid mechanics properties, such as viscosity, suspension particle size, volume, and osmotic properties, on the degree and nature of fluid spread in the SCS. summarizes these variables and impact on drug delivery to the SCS.
Table 1. Formula and procedure optimization properties, and the relationship they have on key aspects of suprachoroidal delivery, including sustained pharmacokinetic effect, targeting of the posterior pole, and circumferential distribution
3.1.1. Injection forces
Suprachoroidal injection using the SCS Microinjector does not require any external mechanical assistance beyond a physician’s manual forces for the injection of a suspension like CLS-TA, an investigational suspension of triamcinolone acetonide for suprachoroidal injection. An average force of 2.07 N was required to perform injections in an in vivo porcine model using CLS-TA, and an average glide force of 0.73 N has been observed when performing similar injection of water into open air [Citation34,Citation35].
3.1.2. Volume and injections into multiple quadrants
The volume of injectate has been demonstrated to impact the distribution of fluid spread throughout the SCS. In an ex vivo experiment with rabbit eyes, increasing volume increased the overall circumferential coverage of the SCS [Citation27]. Suprachoroidal injection of 75 µL or more of fluorescein covered more than 50% of the entire choroidal surface in rabbit eyes. Interestingly, increasing the fluid volume injected from 25 to 150 µL correlated to a linear increase in fluid coverage across the SCS, rather than an increase in the SCS thickness in the immediate proximity of the location of injection [Citation36]. The peak value of SCS thickness as measured by ultrasound biomicroscopy (UBM) was not significantly affected by injection volume [Citation36]. These results suggest that increasing injection volume is likely to increase overall spread rather than merely increasing the expansion within the same area for a single injection. This observation was further explored in other animal models, and results suggest that the presence of lamellae attaching the choroid to the sclera could limit expansion of the SCS, leading to the spread of the injectate circumferentially and posteriorly around the globe. Another corroborating rabbit study showed that injections of 100 µL distributed to approximately 75% of the posterior globe [Citation37].
Related to volume, injections into multiple quadrants have also been demonstrated to increase the overall choroidal coverage [Citation38]. In a rabbit study, two suprachoroidal injections of sodium fluorescein (50 µL for each injection) were performed in the superotemporal and inferonasal quadrants. The authors found that the entire choroid was covered with two antipodal injections providing greater coverage than with a single injection. This suggests that injections into multiple quadrants may be a strategy to achieve more widespread distribution.
3.1.3. Viscosity and polymeric solution formulations
In addition to volume, the viscosity of the formulation injected into the SCS can impact the spread and extent of opening of the SCS. Chiang et al. injected formulations with various viscosities into the SCS, ranging from Hank’s Balanced Salt Solution (HBSS) (approximate viscosity of 1 cPs), DisCoVisc (viscosity approximation of 75,000 ± 35,000 cPs at a shear rate of 1/s at 25°C) [Citation39], to up to 5% carboxymethyl cellulose (CMC, 700 KDa, with approximate viscosity 200,000 cPs) [Citation36]. Higher viscosities led to increases in SCS thickness. The thicknesses of the SCS opening at the injection site were 0.43 ± 0.06 mm and 2.1 ± 0.1 mm for HBSS and 5% CMC, respectively. Furthermore, the swelling characteristics of CMC draws additional fluid into the formulation after injection, causing the SCS to swell and increase in thickness. Over time, fluids are reabsorbed, collapsing the SCS and returning it to its original thickness. The rate of SCS closure is also dependent on the viscosity of the fluid with slower closure times noted for more viscous formulations. The rate of SCS closure, modeled as a second-order exponential equation, is on the order of 19 minutes for HBSS and up to 9 days for CMC-based formulations. A similar trend was observed when evaluating the clearance of fluorescein mixed with different viscosity solutions. Fluorescent signal was no longer detected after 0.33 ± 0.05 days with the HBSS-based solution and 2.7 ± 0.7 days with the 5% CMC solution [Citation36].
Viscosity also impacts the degree of spread of the injectate around the globe via the SCS. Kim et al. evaluated suprachoroidal fluid spread for formulations with varying shear-thinning characteristics [Citation40]. Unlike Newtonian fluids, where viscosity remains constant for all shear rates, shear-thinning fluids are less viscous at high shear rates. In this case, shear-thinning fluids can be injected through the needle cannula with less viscous resistance and retain other viscous fluid characteristics in the low shear environment of the SCS. In this experiment, three types of fluids were evaluated – HBSS, fluids with moderate shear-thinning characteristics (DisCoVisc and 2.2 wt% 950 kDa hyaluronic acid) and fluids with significant shear-thinning characteristics (1.7 wt% 700 kDa CMC and 3 wt% 90 kDa methylcellulose). Fluid spread was visualized with fluorescent particles mixed into each formulation. As expected, fluid spread immediately post-injection for HBSS was significantly larger than the other two types of formulations. However, the spread increased significantly at 14 days after injection for fluids with moderate shear-thinning characteristics, compared to baseline as well as HBSS, while it remained relatively unchanged for the strongly shear-thinning fluids. These results indicate that the degree of spread of injectates around the globe in the SCS can be customized and tailored based on viscosity [Citation40].
3.1.4. Particle suspensions
In addition to fluid characteristics, some drug formulations contain particles in suspension that can dissolve over time. As noted previously, the fenestrated choriocapillaris has estimated pore sizes between 6 and 12 nm [Citation26], which may limit egress of larger particles through this route. The effects of particle size in the suspension injected into the SCS have been evaluated in multiple publications [Citation27,Citation29,Citation40,Citation41]. Particles between 20 and 1,000 nm were injected into the SCS at a predefined infusion pressure. In this experiment, the infusion was managed by a microcontroller and that the pressure may have been higher than infusion pressures seen using a manual piston resulting in particles being driven in a different distribution pattern compared to manual injections. It was observed that the 20 nm particles were found both in the sclera and the SCS while the larger particles were retained only in the SCS [Citation29]. Under manual piston syringe pressure, a different particle distribution was found, indicating that the nominal pore size of the extracellular matrix of the sclera may be no larger than 20 nm. Kim et al. manually injected particles from 20 nm to 10 µm in size and saw no difference in the area of SCS coverage or overall fluorescence up to 3 months [Citation40]. All particles were found within the SCS. Chiang et al. found similar results indicating that ‘particle size did not significantly affect particle spread circumferentially’ [Citation27].
Compared to injections of particles in suspension, injections of solutions result in much greater circumferential fluid distribution across the SCS with more rapid clearance from the SCS [Citation41,Citation42]. This was demonstrated by the injection of microparticles of poly(lacto-co-glyolic acid) (PLGA) loaded acriflavine (ACF), a molecule being studied as a potential inhibitor for choroidal neovascularization (CNV). It is important to note that this small molecule, ACF, in its solution form clears rapidly from the eye. Sustained treatment is necessary to achieve the desired clinical effect in treating CNV, and therefore PLGA loading was evaluated. Suprachoroidal delivery of these microparticles via transscleral microneedle has shown durability and compartmentalization in rat and rabbit models. When particle size was approximately 7 µm, increased durability was seen throughout the duration of a 16-week study in Brown Norway Rats [Citation42]. Chiang et al. compared the distribution of fluorescein solution and fluorescent polymeric particles in HBSS in a rabbit model. Fluorescein spread much more broadly around the SCS compared to polymeric particles and cleared at a much faster rate, no longer detectable at 1 day. Polymeric particles were observed for as long as 21 days (last time point assessed). Further evaluation of the clearance kinetics and particle size was conducted by Chiang et al. with fluorescent molecules of different molecular weights in HBSS were injected suprachoroidally [Citation43]. Clearance kinetics were evaluated by both fundoscopy and fluid collections from different parts of the eye. The authors found that increasing molecular weight (MW) resulted in longer clearance time: While fluorescein (320 Da in MW or hydrodynamic radius of 0.5 nm) [Citation44] were cleared within 12 hours, 2 MDa fluorescent dextran (hydrodynamic radius estimate of 27 nm) [Citation45] was observed for up to 4 days. The clearance routes include diffusion or convection into the sclera and choroid, transscleral leakage, and choroidal blood flow. Taken together, this indicates that particle size and molecular weight are additional variables which can be tailored and optimized to control fluid spread and clearance, allowing for greater potential flexibility for formulation design.
3.1.5. Osmotic characteristics and ionic charges of formulation
Osmotic characteristics of the formulation, such as the ability for the formulation to absorb fluid and swell, has also been demonstrated to have an effect on fluid spread in the SCS. Chiang et al. observed that the SCS thickness continued to increase after the injection of CMC formulations for a few hours [Citation36]. It was believed that the increase in thickness was due to the swelling of the CMC hydrogel material. This swelling characteristic was utilized to push formulations posteriorly [Citation46]. Jung et al. injected suprachoroidally a low concentration hyaluronic acid (HA) formulation, containing fluorescent particles, followed by a high concentration of HA, acting as a pushing hydrogel. Over 6 hours, the fluorescent particles were observed to move posteriorly due to the swelling and then stabilization of the pushing hydrogel. This phenomenon was even more pronounced with a high-salt concentration formulation. In addition to osmotic pressure, ionic charge of the drug formulation may also play a role in drug distribution with the application of iontophoresis [Citation47]. Jung et al. suprachoroidally delivered negatively charged nanoparticles. By applying a cathodal iontophoresis current to the eye along the anterior-posterior axis, the authors were able to significantly increase the nanoparticle distribution to the back of the eye. Suprachoroidal delivery of non-viral DNA with the application of electric current has also been evaluated in a rat model with increased transfection efficiency as DNA is naturally negatively charged [Citation48].
3.1.6. Compartmentalization and duration of injectates in the SCS
Delivery of pharmacological agents to the SCS yields a unique compartmentalization of those agents; ocular tissues non-adjacent to the SCS appear to be minimally exposed to those injected agents. In animal models, this was confirmed via suprachoroidal delivery of red-fluorescent sulforhodamine B to ex vivo porcine eyes. Eyes were then frozen and cut into cross-sections. Visualization under brightfield microscopy between the sclera and choroid, in the SCS, confirmed a successful suprachoroidal injection as well as compartmentalization of the injectate between the sclera and choroid [Citation29].
Compartmentalization was further validated in an in vivo rabbit model comparing fluorescent signals along the visual axis following a suprachoroidal or intravitreal injection of a fluorescent injectate [Citation23]. Fluorescent signal was concentrated in the back of the eye in the suprachoroidally-injected eyes, compared to a uniform fluorescent intensity along the entire visual axis in the intravitreally injected eyes. Quantitatively, the ratio of fluorescent signal between the choroid/retina and the lens was measured as an indicator of compartmentalization. This ratio was approximately 1 for intravitreal injections and between 10 and 100 for suprachoroidal injections.
Lastly, the compartmentalization and durability of various small molecule suspensions have been evaluated. A proprietary suspension of triamcinolone acetonide injected suprachoroidally into a rabbit model was not found in the aqueous humor over the 91 days studied; negligible concentrations were found in the anterior chamber overall [Citation49]. Conversely, the highest concentration of triamcinolone acetonide was found in the sclera/choroid/retinal pigment epithelium (RPE). It was shown that there was approximately 12 times more triamcinolone acetonide delivered to the sclera/choroid/RPE when injected suprachoroidally as compared with intravitreally delivered.
Other small molecule suspensions have shown similar ocular distribution profiles for suprachoroidal injections. A proprietary formulation of a tyrosine kinase inhibitor, axitinib, injected suprachoroidally was found to have the highest concentrations in the sclera/choroid/RPE for the 67 days studied in a rabbit model. Concentration of axitinib found in the vitreous humor was negligible 1 week post-injection. Similarly, a small molecule complement inhibitor, A01017, demonstrated highest concentrations in the sclera/choroid/RPE for duration up to 90 days tested in a rabbit model [Citation50,Citation51].
3.2. Clinical evaluations
In addition to extensive preclinical evaluations to investigate the effects of various biomechanical factors on compounds delivered via suprachoroidal injection, CLS-TA was delivered suprachoroidally in multiple clinical studies via the SCS Microinjector; results have largely confirmed the findings in the preclinical studies [Citation3,Citation6,Citation7,Citation52,Citation53]. lists clinical trials completed to date that involve suprachoroidal delivery with CLS-TA. In the 24-week Phase 3 PEACTHREE trial for uveitic macular edema (NCT02595398), targeted delivery of CLS-TA resulted in clinically meaningful efficacy, while maintaining a favorable safety profile [Citation7]. A sustained presence of this small molecule suspension was validated by MAGNOLIA (NCT02952001), a 24-week observational extension study to PEACHTREE, in which approximately 50% of patients completed the trial without requiring rescue therapy [Citation53].
Table 2. Clinical trials involving suprachoroidal injections of an investigational formulation of triamcinolone acetonide (CLS-TA)
3.2.1. Safety, efficacy, durability of CLS-TA
3.2.1.1. Efficacy
To date, CLS-TA is the only pharmacologic agent delivered suprachoroidally evaluated in a randomized, controlled Phase 3 trial. The efficacy and safety of suprachoroidally injected CLS-TA was tested in this Phase 3 PEACHTREE trial. In the study, patients in the experimental arm received a suprachoroidal injection of CLS-TA at Day 0 and Week 12, versus the control arm which received sham procedures at those timepoints. At Week 24, 46.9% of patients receiving CLS-TA had gained 15 letters or more in visual acuity (representing a clinically meaningful improvement of 3 lines or more on an ETDRS eye chart or a doubling of the visual angle) compared to 15.6% of patients in the control arm. Central subfield retinal thickness (CST), assessed via spectral domain OCT (SD-OCT), was reduced by an average of 152.6 µm compared to 17.9 µm in the control arm [Citation7]. Since suprachoroidal delivery of CLS-TA was not compared to another type of local corticosteroid uveitis therapy in PEACTHREE, comparisons cannot be made relative to efficacy and safety of other types of local corticosteroid treatments. However, a post hoc analysis compared visual function results in unrescued CLS-TA to rescued control patients. A total of 86.4% of CLS-TA patients did not require rescue therapy, compared to the 71.9% of patients in the control group who received rescue therapy. The type of rescue therapy was at the discretion of the Investigator; all rescued control patients received corticosteroids, with intravitreal or periocular injections being prescribed to 80.4% of them. In the unrescued CLS-TA group, mean gain in BCVA was 15.7 letters, versus a 10.9 letter improvement in rescued control patients who completed the study (P =.080). Reduction in CST showed a similar trend [Citation7]. Given the post hoc nature of the analysis, further study is warranted.
3.2.1.2. Safety
At the time of writing, over 1,000 suprachoroidal injections of CLS-TA with the SCS Microinjector have been administered to patients across multiple clinical trials, as summarized in . As discussed earlier, preclinical models have shown that corticosteroid delivered suprachoroidally is mostly compartmentalized in the posterior segment tissues, sparing the anterior segment, including the lens, as well as the vitreous humor. In PEACHTREE, the safety profile for CLS-TA was favorable overall, with low rates of cataract and IOP elevation. Across all studies, there were no serious adverse events involving lens injury, suprachoroidal hemorrhage, endophthalmitis, or retinal tear.
3.2.1.3. Duration
An observational extension study, MAGNOLIA, was conducted to determine the maintenance of efficacy of suprachoroidally administered CLS-TA for up to 24-weeks following exit from the PEACHTREE study. Approximately half of the patients in MAGNOLIA did not require rescue therapy for uveitis during the 9 months following the second suprachoroidal injection of CLS-TA administered at Week 12 of PEACHTREE. These results support the preclinical data which demonstrated sustained ocular distribution profiles up to 90 days, and suggest the potential to address the treatment burden of current therapies.
3.2.2. Clinical SCS closure kinetics
In a Phase 1/2 trial for diabetic macular edema, HULK (NCT02949024), eyes receiving suprachoroidal injections of CLS-TA were imaged using anterior segment OCT (AS-OCT). Images were taken before and 30 minutes after suprachoroidal injection of CLS-TA. In the study, the SCS was measured and showed significant expansion 30 minutes post-injection but returned to baseline measurements within 1 month, the next time point assessed [Citation54]. These results suggest that suprachoroidal injection using the a microinjector does not permanently alter the anatomy of the SCS, which is consistent with preclinical models. As previously described, preclinical models show similar expansion post-injection followed by a return to baseline thickness with timing depending on formulation factors such as viscosity.
3.2.3. Effect of microneedle length on suprachoroidal injection
When using the SCS Microinjector, it is recommended that the injection be attempted first with a 900 µm length needle. A second needle, 1100 µm in length, is provided in the event that the space cannot be accessed with the 900 µm length needle. A post hoc analysis was conducted across six clinical trials (PEACHTREE, AZALEA, TANZANITE, TOPAZ, SAPPHIRE, and TYBEE) focusing on baseline injections, the first suprachoroidal injection administered by the investigator to a patient. In this analysis, the 900 µm needle was used for 71% (412 of 581) of suprachoroidal injections; the 1100 µm needle was subsequently used to complete the remaining injections [Citation55,Citation56]. Additionally, in response to the prompt ‘Was the suprachoroidal injection administered?’, 98.1% of injecting physicians responded ‘Yes’ (with no specifics reported from those who responded ‘No’) [Citation33].
Further analysis revealed moderate correlations between the site of injection and the ultimate needle length used to deliver study drug, with the 900 µm needle being most commonly used in the superotemporal quadrant and the 1100 µm needle being most frequently used in the inferonasal quadrant [Citation56]. This aligns with known scleral thickness variations, with the superotemporal quadrant generally showing the thinnest scleral of the quadrants [Citation19,Citation22]. Additionally, there were no statistically significant correlations between the needle length used for suprachoroidal injection and patient variables such as age, gender, race, indication, refraction, visual acuity, IOP, retinal central subfield thickness, or lens status. Overall, the needle usage analysis demonstrated that the two microneedle lengths can reproducibly accommodate a wide range of anatomic and demographic variables.
4. Observations post suprachoroidal injections
This section summarizes post-injection observations, including patience experience and repeat dosing.
4.1. Patient experience
The patient experience for suprachoroidal injection of CLS-TA with the SCS Microinjector is similar to other office-based ocular injection procedures, such as intravitreal injection of anti-VEGFs [Citation57–61]. The rate of eye pain is similar to those reported for other ocular injection procedures [Citation58,Citation59,Citation62,Citation63].
As with any procedure that injects a fixed volume into a closed system like the eye, acute and transient increases in IOP following suprachoroidal injection are an anticipated risk to the patient immediately following a suprachoroidal injection. On the day of the suprachoroidal injection procedure in the PEACTHREE trial, elevated IOP pertaining to the injection was noted in 8.3% of patients, and overall, there were no serious adverse events related to IOP elevation [Citation63].
4.2. Re-accessing the SCS of one eye
Repeat suprachoroidal injections within a study eye have also been clinically evaluated. In the HULK trial, up to 5 monthly suprachoroidal injections were administered over a 6-month period. These multiple injections were well tolerated with a low incidence of adverse events [Citation6]. As with previous trials, the safety profile remained favorable.
5. Conclusion
There has been a rapid evolution in ocular drug delivery technology targeting common blinding retinal disorders. Until recently, chorio-retinal diseases have been treated with pharmacological therapies using periocular, intravitreal, and subretinal administrations. Periocular injection yields less efficacy when compared the intravitreal injection, likely due to limited and variable scleral penetration [Citation1]. Intravitreal injection yields broad diffusion of pharmacological agents throughout the vitreous chamber, but also expose the anterior segment, posing additional potential safety risks, especially for corticosteroids. Suprachoroidal injection represents an alternative procedure that leverages a potential space to target high levels of therapies to affected chorio-retinal tissues to potentially maximize efficacy, while compartmentalizing them away from the unaffected vitreous and anterior segment to potentially enhance safety. Small molecule suspensions also exhibit multi-month duration in preclinical studies as well as in clinical trials assessing suprachoroidally injected CLS-TA. Suprachoroidal injection via the SCS Microinjector has been studied extensively preclinically and demonstrated satisfactory procedural safety and efficacy outcomes clinically, which has led to clinical studies of a tyrosine kinase inhibitor small molecule suspension, as well as viral vector gene therapies and viral nanoparticle conjugates [Citation64,Citation65]. By leveraging biomechanical considerations discussed herein, therapies such as these can be optimized to maximize therapeutic outcomes and patient safety.
6. Expert opinion
Suprachoroidal injection represents a potential new paradigm in ocular drug delivery, possibly addressing key limitations of intravitreal, periocular, and subretinal injection without sacrificing advantages of local ocular therapy. Previous significant office-based ophthalmic innovations, such as the intravitreal injection, revolutionized the treatment of retinal diseases, facilitating high intraocular levels of effective therapies such as anti-VEGF agents, while limiting systemic exposure and resulting adverse events. Suprachoroidal injection has the potential to have a similar impact in ophthalmology by providing additional targeting and compartmentalization benefits.
Suprachoroidal injection of small molecule suspensions target chorio-retinal tissues while maintaining a sustained pharmacokinetic profile. Clinically, evaluations with suprachoroidal injection of triamcinolone acetonide have validated the potential of the SCS. These initial studies have paved the way for additional drug candidates to be evaluated in the SCS, from small molecule suspensions of a tyrosine kinase inhibitor, to oncological therapies, to viral gene therapy, all of which are currently undergoing clinical investigations. While promising clinical results were observed with a corticosteroid, this represents an early juncture in leveraging the benefits of suprachoroidal drug delivery. Consequently, an understanding of the biomechanics of the SCS is crucial to further leveraging this potential space for ocular drug delivery.
With any novel drug delivery platform, comprehensive characterization of the suprachoroidal drug delivery platform is warranted to answer questions such as: ‘Is there an ideal viscosity for specific indications? Is the pars plana the optimal location for suprachoroidal injections? What is the effect of volume on the fluid spread in the SCS? What is the best method to maintain the drug candidate in the SCS? Does the drug candidate reach the posterior pole?’ etc. Existing preclinical studies have answered many of these important questions. However, additional work is necessary to address particular issues related to each potential drug candidate. It will be paramount to design further studies with clinical translation in mind to inform clinical trial design and to understand the potential impacts on clinical efficacy and safety.
The ultimate goal of optimizing this platform for a variety of disease states and therapeutics should be to provide a tailored map of technical specifications that accurately forecast clinical drug delivery outcomes. For example, in the treatment of peripheral choroidal diseases, widespread drug delivery around the entire globe is important. In this case, a low-viscosity, high-volume formulation may be preferred. In contrast, for the treatment of diseases focused on the macula, it is critical to ensure the drug reaches the posterior pole. In this case, strategies employing a low-viscosity high-volume formulation may be best; alternatively, hydrogel pushing or iontophoresis might be developed to ensure the drug candidate preferentially reaches the back of the eye. For glaucoma therapies that decrease aqueous production, delivery in the anterior SCS adjacent to the ciliary body is preferred. This delivery target may require a high-viscosity, low degrading polymeric formulation. As elucidated in these examples, drug delivery in the SCS represents both a flexible and effective platform, that can be tailored to specific applications and indications,
One such application of suprachoroidal delivery is gene therapy, currently being investigated in Phase 2 clinical trials. Subretinal administration of gene therapy has been undergoing optimization for over a decade, and currently the procedure for approved ocular gene therapy treatments is performed in a limited number of regional ocular gene therapy surgical treatment centers. An office-based suprachoroidal delivery of gene therapy would represent another therapeutic advancement, limiting the risks associated with pars plana vitrectomy, retinotomy, and subretinal injection. The suprachoroidal injection can expose more surface area to the gene therapy, providing potential to treat large amounts of retina less invasively. Furthermore, office-based gene therapy would dramatically increase patient access to care, compared to the current model of regional ocular gene therapy surgical treatment centers. Suprachoroidal injection of gene therapy is in its infancy, and additional optimization work will likely be needed to address such factors as the spread of gene therapy vectors around the globe of the eye and appropriate transfection through ocular layers to reach the desired cells of the retina.
In the future, suprachoroidal injection may represent a common route of administration that could complement intravitreal injections, depending on the specific disease and therapeutic agent. The suprachoroidal drug delivery platform holds promise to be the next frontier of ocular drug delivery.
Article highlights
The suprachoroidal space (SCS) is a potential space in the eye, opened by drug candidate injection, between the sclera and the choroid tissue layers.
Injected fluids spread circumferentially and posteriorly within the SCS, targeting chorio-retinal tissues to potentially maximize efficacy, while compartmentalizing therapies away from the unaffected vitreous and anterior segment to potentially enhance safety.
Microneedle delivery to the SCS can be accomplished in an office-based, non-surgical setting by a physician trained in the suprachoroidal injection technique.
Delivery to the SCS via microneedles has been shown to be safe and effective preclinically and clinically in a Phase 3 trial of CLS-TA, a proprietary investigational suspension of triamcinolone acetonide.
Suspension formulations with low solubility exhibit sustained pharmacokinetics in the SCS, demonstrated by the clinical trials involving the use of suprachoroidally injected CLS-TA.
Drug formulations can be tailored to the desired target product profile. Physical and fluidic properties of injectates into the SCS, including volume, viscosity, particle size, and injection pressure, can impact the spread and extent of opening of the SCS. For instance, drug spread is directly proportional to the injected volume and inversely proportional to the viscosity.
This box summarizes key points contained in the article.
List of abbreviations
| SCS, | = | Suprachoroidal Space |
| PK, | = | Pharmacokinetic |
| CLS-TA, | = | Investigational formulation, triamcinolone acetonide |
| IOP, | = | Intraocular pressure |
| UBM, | = | Ultrasound biomicroscopy |
| OCT, | = | Optical coherence tomography |
| HBSS, | = | Hank’s Balanced Salt Solution |
| CMC,Carboxymethyl cellulose | = | |
| PLGA, | = | Poly(lacto-co-glyolic acid) |
| ACF, | = | Acriflavine |
| CNV, | = | Choroidal neovascularization |
| MW, | = | Molecular weight |
| HA, | = | Hyaluronic acid |
| RPE, | = | Retinal pigment epithelium |
| CST, | = | Central subfield retinal thickness |
| SD-OCT, | = | Spectral-domain OCT |
| AS-OCT, | = | Anterior segment OCT. |
Declaration of interest
SE Hancock, TA Ciulla, CR Wan, NE Fisher, RV Andino are employees of, and hold stock option with, Clearside Biomedical, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Thorne JE, Sugar EA, Holbrook JT, et al. Periocular Triamcinolone vs. Intravitreal Triamcinolone vs. Intravitreal Dexamethasone Implant for the Treatment of Uveitic Macular Edema: the PeriOcular vs. INTravitreal corticosteroids for uveitic macular edema (POINT) trial. Ophthalmology. 2019;126(2):283–295.
- Hartman RR, Kompella UB. Intravitreal, subretinal, and suprachoroidal injections: evolution of microneedles for drug delivery. J Ocul Pharmacol Ther. 2018;34(1–2):141–153.
- Campochiaro PA, Wykoff CC, Brown DM, et al. Suprachoroidal triamcinolone acetonide for retinal vein occlusion: results of the tanzanite study. Ophthalmol Retina. 2018 Apr;2(4):320–328.
- Chen M, Li X, Liu J, et al. Safety and pharmacodynamics of suprachoroidal injection of triamcinolone acetonide as a controlled ocular drug release model. J Control Release. 2015;203:109–117.
- Chiang B, Jung JH, Prausnitz MR. The suprachoroidal space as a route of administration to the posterior segment of the eye. Adv Drug Deliv Rev. 2018 Feb;15(126):58–66.
- Wykoff CC, Khurana RN, Lampen SIR, et al. Suprachoroidal triamcinolone acetonide for diabetic macular edema: the HULK trial. Ophthalmol Retina. 2018 Aug;2(8):874–877.
- Yeh S, Khurana RN, Shah M, et al. Efficacy and Safety of Suprachoroidal CLS-TA for macular edema secondary to noninfectious uveitis: phase 3 randomized trial. Ophthalmology. 2020;127(7):948–955.
- Moisseiev E, Loewenstein A, Yiu G. The suprachoroidal space: from potential space to a space with potential. Clin Ophthalmol. 2016;10:173–178.
- Rai Udo J, Young SA, Thrimawithana TR, et al. The suprachoroidal pathway: a new drug delivery route to the back of the eye. Drug Discov Today. 2015 Apr;20(4):491–495.
- Habot‐Wilner Z, Noronha G, Wykoff CC. Suprachoroidally injected pharmacological agents for the treatment of chorio-retinal diseases: a targeted approach. Acta Ophthalmologica. 2019 Aug;97(5):460–472.
- Jung JH, Chae JJ, Prausnitz MR. Targeting drug delivery within the suprachoroidal space. Drug Discovery Today. 2019 Aug;24(8):1654–1659.
- Pearce W, Hsu J, Yeh S. Advances in drug delivery to the posterior segment. Current Opinion in Ophthalmology. 2015 May;26(3):233–239.
- Goldstein DA, Do D, Noronha G, et al. Suprachoroidal corticosteroid administration: a novel route for local treatment of noninfectious uveitis. Transl Vision Sci Technol. 2016 Dec;5(6):14.
- Olsen TW, Feng X, Wabner K, et al. Cannulation of the suprachoroidal space: a novel drug delivery methodology to the posterior segment. Am J Ophthalmol. 2019 Nov;97(5):460–472.
- Peden MC, Min J, Meyers C, et al. Ab-externo AAV-mediated gene delivery to the suprachoroidal space using a 250 micron flexible microcatheter. PLoS One. 2011;6(2):e17140.
- Yeh S, Kurup SK, Wang RC, et al. suprachoroidal injection of triamcinolone acetonide, cls-ta, for macular edema due to noninfectious uveitis: a randomized, Phase 2 Study (DOGWOOD). Retina. 2019;39(10):1880–1888.
- Worthington KS, Wiley LA, Bartlett AM, et al. Mechanical properties of murine and porcine ocular tissues in compression. Exp Eye Res. 2014;121:194–199.
- Krohn J, Bertelsen T. Light microscopy of uveoscleral drainage routes after gelatine injections into the suprachoroidal space. Acta Ophthalmol Scand. 1998 Oct;76(5):521–527.
- Vurgese S, Panda-Jonas S, Jonas JB. Scleral thickness in human eyes. PLoS One. 2012;7(1):e29692.
- Olsen TW, Aaberg SY, Geroski DH, et al. Human sclera: thickness and surface area. Am J Ophthalmol. 1998 Feb;125(2):237–241.
- Oliveira C, Tello C, Liebmann J, et al. Central corneal thickness is not related to anterior scleral thickness or axial length. J Glaucoma. 2006 Jun;15(3):190–194.
- Norman RE, Flanagan JG, Rausch SM, et al. Dimensions of the human sclera: thickness measurement and regional changes with axial length. Exp Eye Res. 2010 Feb;90(2):277–284.
- Patel SR, Berezovsky DE, McCarey BE, et al. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sci. 2012;53(8):4433–4441.
- Emi K, Pederson JE, Toris CB. Hydrostatic pressure of the suprachoroidal space. Invest Ophthalmol Vis Sci. 1989 Feb;30(2):233–238.
- Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010 Mar;29(2):144–168.
- Sarin H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenes Res. 2010 Aug;11(2):14.
- Chiang B, Kim YC, Edelhauser HF, et al. Circumferential flow of particles in the suprachoroidal space is impeded by the posterior ciliary arteries. Exp Eye Res. 2016;145:424–431.
- Krohn J, Bertelsen T. Corrosion casts of the suprachoroidal space and uveoscleral drainage routes in the human eye. Acta Ophthalmol Scand. 1997 Feb;75(1):32–35.
- Patel SR, Lin AS, Edelhauser HF, et al. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm Res. 2011 Jan;28(1):166–176.
- Ding K, Shen J, Hafiz Z, et al. AAV8-vectored suprachoroidal gene transfer produces widespread ocular transgene expression. J Clin Invest. 2019;129(11):4901–4911.
- Shen J, Kim J, Tzeng SY, et al. Suprachoroidal gene transfer with nonviral nanoparticles. Sci Adv. 2020;6(27):eaba1606.
- Kansara V, Muya L, Wan CR, et al. Suprachoroidal delivery of viral and nonviral gene therapy for retinal diseases. J Ocul Pharmacol Ther. 2020;36(6):384–392.
- Henry CR, Schefler AC, Wan CR, et al. Post Hoc Analysis of Clinical Suprachoroidal Injection Experience Across Retinal Disease Indications Retina Society Annual Meeting 2020. [cited 2020 Oct 22]. Available from: https://www.clearsidebio.com/pdf/2020/henry-christopher-post-hoc-analysis.pdf
- Fisher N, Wan C. Suprachoroidal delivery with the SCS Microinjector™: characterization of Operational Forces. Invest Ophthalmol Vis Sci. 2020;61(7):24.
- Fisher N, Yoo J, Hancock SE, et al. A Novel Technique to Characterize Key Fluid Mechanic Properties of the Suprachoroidal Injection Procedure in an In Vivo Model Association for Research in Vision and Ophthalmology Annual Meeting 2018. [cited 2020 Oct 28]. Available from: https://www.clearsidebio.com/?articleattachment=49
- Chiang B, Venugopal N, Grossniklaus HE, et al. Thickness and closure kinetics of the suprachoroidal space following microneedle injection of liquid formulations. Invest Ophthalmol Vis Sci. 2017;58(1):555–564.
- Savinainen A, Grossniklaus H, Kang S, et al. Ocular distribution and efficacy after suprachoroidal injection of AU-011 for treatment of ocular melanoma. Invest Ophthalmol Vis Sci. 2020;61(7):3615.
- Nork TM, Katz AW, Kim CBY, et al. Distribution of aqueous solutions injected suprachoroidally (SC) in rabbits. Invest Ophthalmol Vis Sci. 2020;61(7):320.
- Oshika T, Bissen-Miyajima H, Fujita Y, et al. Prospective randomized comparison of DisCoVisc and Healon5 in phacoemulsification and intraocular lens implantation. Eye. 2010;24(8):1376–1381.
- Kim YC, Oh KH, Edelhauser HF, et al. Formulation to target delivery to the ciliary body and choroid via the suprachoroidal space of the eye using microneedles. Eur J Pharm Biopharm. 2015 Sep;95(Pt B):398–406.
- Chiang B, Venugopal N, Edelhauser HF, et al. Distribution of particles, small molecules and polymeric formulation excipients in the suprachoroidal space after microneedle injection. Exp Eye Res. 2016;153:101–109.
- Hackett SF, Fu J, Kim YC, et al. Sustained delivery of acriflavine from the suprachoroidal space provides long term suppression of choroidal neovascularization. Biomaterials. 2020;243:119935.
- Chiang B, Wang K, Ethier CR, et al. Clearance kinetics and clearance routes of molecules from the suprachoroidal space after microneedle injection. Invest Ophthalmol Vis Sci. 2017;58(1):545–554.
- Mustafa MB, Tipton DL, Barkley MD, et al. Dye diffusion in isotropic and liquid-crystalline aqueous (hydroxypropyl)cellulose. Macromolecules. 1993;26(2):370–378.
- FITC-Dextran Fluorescein isothiocyanate dextran. 2011.
- Jung JH, Desit P, Prausnitz MR. Targeted drug delivery in the suprachoroidal space by swollen hydrogel pushing. Invest Ophthalmol Vis Sci. 2018;59(5):2069–2079.
- Jung JH, Chiang B, Grossniklaus HE, et al. Ocular drug delivery targeted by iontophoresis in the suprachoroidal space using a microneedle. J Control Release. 2018 May;10(277):14–22.
- Touchard E, Berdugo M, Bigey P, et al. Suprachoroidal electrotransfer: a nonviral gene delivery method to transfect the choroid and the retina without detaching the retina. Mol Ther. 2012;20(8):1559–1570.
- Edelhauser HF, Verhoeven RS, Burke B, et al. Intraocular distribution and targeting of triamcinolone acetonide suspension administered into the suprachoroidal space. Invest Ophthalmol Vis Sci. 2014;55(13):5259.
- Hancock SE, Phadke A, Kansara V, et al. Ocular pharmacokinetics and safety of suprachoroidal A01017, small molecule complement inhibitor, injectable suspension in rabbits. Invest Ophthalmol Vis Sci. 2020;61(7):3694.
- Kaiser PK, Ciulla T, Kansara V. Suprachoroidal CLS-AX (axitinib injectable suspension), as a Potential Long-Acting Therapy for Neovascular Age-Related Macular Degeneration (nAMD). Invest Ophthalmol Vis Sci. 2020;61(7):3977.
- Singer MA IOP Following Administration of Suprachoroidal Triamcinolone Acetonide Suspension (CLS-TA): Results from the Phase 3 PEACHTREE Clinical Trial for Uveitis Macula Society Annual Meeting 2020[cited 2020 Oct 12]. Available from: https://www.clearsidebio.com/?articleattachment=83
- Merrill P. Suprachoroidally Injected CLS-TA in Uveitis Maintains Efficacy Outcomes Through 48 Weeks: Results of the MAGNOLIA Phase 3 Extension Study American Society of Retina Specialists Annual Meeting 2019[cited 2020 Oct 12]. Available from: https://www.clearsidebio.com/?articleattachment=64
- Lampen SIR, Khurana RN, Noronha G, et al. Suprachoroidal space alterations following delivery of triamcinolone acetonide: post-hoc analysis of the Phase 1/2 HULK Study of patients with diabetic macular edema. Ophthalmic Surg Lasers Imaging Retina. 2018;49(9):692–697.
- Barakat M, Wan C, Kapik B. Post hoc analysis of clinical suprachoroidal injection experience across indications. Invest Ophthalmol Vis Sci. 2020;61(7):4954.
- Wan C-R, Kapik B, Wykoff CC, et al. Clinical characterization of suprachoroidal injection procedure utilizing a microinjector across three retinal disorders. Trans Vision Sci Technol. 2020;9(11):27.
- Lee JW, Park H, Choi JH, et al. Short-term changes of intraocular pressure and ocular perfusion pressure after intravitreal injection of bevacizumab or ranibizumab. BMC Ophthalmol. 2016 May;31(16):69.
- Lowder C, Belfort R Jr., Lightman S, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011 May;129(5):545–553.
- Shiroma HF, Takaschima AKK, Farah ME, et al. Patient pain during intravitreal injections under topical anesthesia: a systematic review. Int J Retina Vitreous. 2017;3:23.
- EYLEA (aflibcercept) [prescribing information]. Tarrytown, NY: Regeneron Pharmaceuticals, Inc; 2019.
- LUCENTIS (ranibizumab) [prescribing information]. South San Francisco, CA: Genentech, Inc; 2018.
- Doguizi S, Sekeroglu MA, Inanc M, et al. Evaluation of pain during intravitreal aflibercept injections. Eur J Ophthalmol. 2018 Jan;28(1):63–67.
- Yeh S Suprachoroidally Injected CLS-TA Improves Visual Acuity and Macular Edema in Noninfectious Uveitis: Results of the Phase 3 PEACHTREE Study American Society of Retina Specialists Annual Meeting 2018. [cited 2020 Oct 28]. Available from: https://www.clearsidebio.com/Yeh__S._Suprachoroidally_Injected_CLS-TA_Improves_Visual_Acuity_and_Macular_Edema_in_Noninfectious_Uveities_Results_of_the_Phase_3_PEACHTREE_Study.pdf
- Regenxbio Reports First Quarter 2020. Financial Results and Operational HighlightsNews Releases. Rockville, MD: REGENXBIO, 2020. [cited 2020 Dec 23]. Available from: https://regenxbio.gcs-web.com/news-releases/news-release-details/regenxbio-reports-first-quarter-2020-financial-results-and
- Aura Biosciences Presents Updated AU-011 Clinical Data at ARVO 2020.Press Releases. Cambridge, MA: Aura Biosciences, 2020. [cited 2020 Dec 23]. Available from: http://www.aurabiosciences.com/news-archive/2020/6/12/aura-biosciences-presents-updated-au-011-clinical-data-at-arvo–2020
