1. Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory condition that mainly affects the lining of the synovial joints. It can progressively cause disability and even premature death. Moreover, it is associated with substantial socioeconomic burdens [Citation1]. Currently, RA affects more than 23 million people globally, including 12.6 million Indians [Citation1].
Recent findings have suggested the involvement of genetic factors in the development of RA. The HLA-DRB1 alleles that code shared epitopes (conserved sequence of amino acids in positions 70–74 of HLA-DRβ chains) are associated with the structural severity of RA. It was reported that more than 80% of the RA-affected individuals carry the HLA-DRB1*04 cluster, in which *04 signifies the allele group which corresponds to the serological type [Citation1].
The specific etiology of RA is under investigation. The RA pathogenesis mainly involves activation of macrophages in the synovial inflammatory joint through the production of the pro-inflammatory cytokines, such as interleukin (IL)-1, IL – 6, and tumor necrosis factor (TNF-α). In addition, the synovial fibroblasts of both the synovial lining and sub-lining layers undergo significant proliferation in RA. As macrophages and fibroblasts tend to be the most active cells in the RA synovium, their interaction is particularly appealing; ultimately resulting in inflammation and tissue damage. These cells elicit the production of IL-6, granulocyte-macrophage colony-stimulating factor, and IL-8 [Citation1].
The RA treatment strategy encompasses expediting the diagnosis and achieving a low disease activity state. To accomplish this, corticosteroids and non-steroidal anti-inflammatory drugs have been used to relieve pain and stiffness universally. However, these therapies provide only symptomatic treatment. Over the past 20 years, disease-modifying anti-rheumatic drugs have gained popularity in RA treatment due to their efficient attenuation of RA activity and substantial delay in deformity of joints [Citation2]. However, these agents are distributed indiscriminately by systemic administration and lack of tissue or organ specificity, causing extra-articular adverse effects. Additionally, the short half-life and suboptimal concentration at the target site elicit the need for frequent and high dosing that results in a higher incidence of side effects and poor patient compliance. Thus, the development of novel targeted therapies for the treatment of RA is essential.
In recent days, the delivery of rheumatic drugs using nanoparticle-based systems has made enormous advancements. The nanoparticles with a size less than 250 nm can reside at the inflammatory site due to the enhanced permeation effect and release the drug in a sustained manner. However, this passive targeting approach lacks the site-specific target drug delivery [Citation3]. The way to wrestle with this confront is to screen inflammatory cells for structural entities expressed on their surface that are engaged in pathological functions; apart from normal cells involved in physiological activities.
The pathophysiology of RA is complex and involves several immune modulators and signaling pathways. A hallmark of the inflammatory responses like RA synovitis involves the overexpression of adhesion molecules including vascular cell adhesion molecule, intercellular adhesion molecule-1, and Cluster Determinant 44 (CD44). The adhesion molecules play a vital role in inflammatory processes by modulating leukocyte endothelial cell adhesion and migration, and T cell-antigen presenting cell interactions. Compared to normal cells, the inflammatory cells express higher levels of folate, CD44 receptors, E-selectin, vasoactive intestinal peptides, and alpha V and beta 3 integrins on their surface. The folic acid and hyaluronic acid are extensively utilized as folate receptor and CD44 receptor-targeted ligands, respectively. Additionally, during the hypoxic state at the inflamed synovium, overexpression of additional markers such as alpha V and beta 3 integrins can be targeted to inhibit angiogenesis and bone resorption [Citation1].
Among the receptors that have been discussed earlier, CD44 has been reported to be over-expressed in the sites of inflammation in RA proportionate to the inflammation intensity [Citation4]. The levels of CD44 in the synovium of patients with RA are 3.5-fold and 10.7-fold higher than that of patients with osteoarthritis and joint trauma, respectively. According to histochemical immunostaining, CD44 expression was greater in RA and osteoarthritis synovium, than in the normal synovium [Citation5]. Thus, there has been a surge in the research that focuses on the CD44 targeted drug delivery strategies to treat RA. CD44 targeting has been attempted using anti-CD44 monoclonal antibodies, DNA vaccines, and CD44 siRNA nanoparticles. This review focuses on the CD44 targeted drug delivery strategies that can be used in the treatment of RA.
2. Cluster determinant 44 (CD44) receptors
CD44 is a multi-structural cell surface glycoprotein for the ligand hyaluronan, which is a major polysaccharide present in the synovium. CD44 can potentially produce over 800 isoforms. The alternatively spliced versions of CD44-like CD44v3, v6–v10 were highly expressed in fibroblasts from RA synovium. These results indicate that joint inflammation activates fibroblasts expressing CD44 variants or CD44 alternatively spliced versions [Citation5]. Also, CD44 expression was found to be higher on synovial lymphocytes and macrophages in rats with adjuvant arthritis. There have been reports of the role of CD44 and hyaluronan in the upregulation of Fas receptor on the RA synovial cells, and the implication of the involvement of CD44 in the pathogenesis of RA [Citation6]. The CD44 receptor, which is overexpressed in synovial macrophages and fibroblasts, binds to hyaluronic acid (HA) [Citation5]. CD44 receptor-mediated active targeting drug delivery systems have been immensely explored among the various targeted delivery systems [Citation7]. CD44 can recognize and bind glycosaminoglycan-based biomaterials such as HA and chondroitin sulfate (CS). Glucosaminoglycans are polysaccharides that typically consist of repeating disaccharide units that have been modified to contain an amino group and negatively charged groups. CD44 consists of binding sites for HA and CS where HA binds to N- terminal of the CD44 and CS binds to the variant stem fibrin region.
3. Hyaluronan based CD44-targeted drug delivery
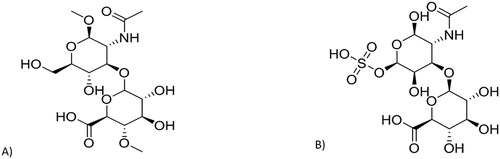
Hyaluronan or hyaluronic acid is a natural, water-soluble, linear, non-sulfated anionic glycosaminoglycan. It consists of repeating β-1, 3-N-acetyl glucosamine, and β-1, 4-glucuronic acid disaccharide units [Citation8]. HA is found in epithelial and connective tissue, synovial fluid, and vitreous humor of the eye. It possesses exclusive properties including, excellent biocompatibility, biodegradability, ease of chemical modification, satisfactory safety for enduring clinical use in human subjects, and less immunogenicity. It also possesses cartilage protective and lubricant effects in synovial fluid [Citation9]. Besides the structural role, HA is a principal counter molecule to HA receptors on CD44 () and ). The synovium of the healthy subject contains the highest concentrations of hyaluronan in the entire human body. During the inflamed joint condition, the depletion of hyaluronan occurs. It was reported that 0.71 ± 0.1 mg/cm3 is found in rheumatoid synovial tissue, whereas 1.07 ± 0.16 mg/cm3 in healthy subject.
Figure 2. Diagrammatic illustration of the CD44 receptor mediated endocytosis of glycosaminoglycan coated nanoparticles by the activated macrophages or lymphocytes and the drug release
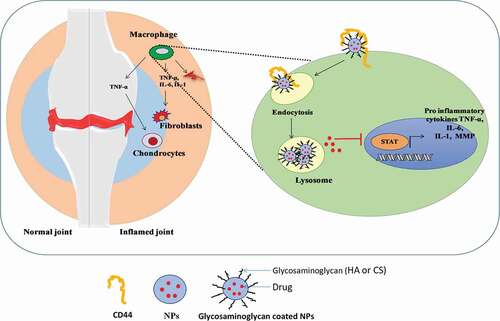
Targeting techniques using HA-modified nanocarriers rely on their preferential binding to the CD44 cell receptor, which is overexpressed in synovial activated cells and plays a role in autoimmune response control. The off-target distribution of the drug could be reduced by targeted delivery to CD44, and by using the pH sensitive lipids to inflamed synovium (acidic environment). Because of this pH sensitivity and target ability, the drug molecules are released efficiently into the cytoplasm. Gouveia and colleagues reported the conjugation of 5 β-cholanic acid to HA via acid-labile ketal linker to produce self-assemble pH-sensitive nanocarriers of methotrexate (MTX). The nanocarrier was found to be highly responsive to the inflamed environment of arthritis (pH 7.4) and the release of MTX was found to be only 30% over 2 days, whereas 80% drug was released at pH 5.0 due to the cleavage of ketal linker. The in vivo biodistribution study results showed an increased fluorescence signal of Cy5.5 dye at the inflamed joint of RA, which confirmed the nanoparticles’ targeted ability to the CD44 [Citation10].
Tamura et al. developed a clinical candidate that is DK226 which was a conjugate of HA and MTX with Peptide (α–Phe–Phe) and linker (–NH–(CH2)2–NH–). The research group appraised the safety and efficacy of intra-articular DK226 in adjuvant-induced arthritis (AIA) model and collagen-induced arthritis (CIA) model. In AIA model, intra-articular injection of DK226 (13.6 μg/week) showed the same efficacy as that of oral administration of MTX (1875 μg/week). Whereas in CIA model, intra-articular injection of DK226 to the right knee significantly reduced the joint swelling, but not in the contralateral untreated knee. DK226 was internalized due to HA receptor and cleaved by lysosomal enzymes. Interestingly, they concluded that upon incubation of DK226 with rabbit synovial fluid no release of MTX was observed. Thus, MTX was distributed and retained in the synovium, which reduced the systemic adverse effects [Citation11].
4. Chondroitin sulfate based CD44-targeted drug delivery
Chondroitin sulfate (CS) is a water-soluble, unbranched sulfated anionic glycosaminoglycan composed of alternating 4-linked β- d-glucuronic acid, and 3-linked N-acetyl galactosamine disaccharide units with varied sulfate positions [Citation12]. The sulfate group imparts hydrophilicity, and was found on the fourth and sixth positions in CS, which are named CS A and CS C, respectively. Serglycin comprises CS-A and CS-C chains bound to a centrally positioned Ser-Gly repeat. Toyama et al. reported that the binding ability of serglycin to CD44 is independent of its CS composition [Citation13].
CS is distributed in cartilage around the joint and ligament. It shows good biocompatibility and biodegradability, and low toxicity. Moreover, it is well known that during arthritis conditions, the CS present at the knee joint decreases the joint space’s discomfort and narrowing. It also protects the joint by lowering the matrix in enzymes in chondrocytes [Citation7]. In addition, CS has chemical structural similarity to HA (); thus CS can specifically target the CD44 receptor (). Due to these reasons, the CS-based nanocarriers were used for passive and active targeted drug delivery. Mahtab group produced a novel Teriflunomide-loaded nanoliposome coated with CS (CS-TEF-LIPO) to efficiently treat RA. The CS-TEF-LIPO showed enhanced long circulation and the targeting ability because of the negatively charged CS layer. The CS-TEF-LIPO significantly enhanced uptake on U937 and MG-63 cell lines compared with the conventional liposomes. The radiographic analysis on the CFA rat model showed that CS-TEF-LIPO (0.61 ± 0.54) showed reduced radiographic scores compared with the conventional liposomes (1.07 ± 0.05) and arthritic control group (2.72 ± 0.28). This proved the active-targeted delivery of CS-coated nanoparticles to the inflamed joint site after lysosomal degradation [Citation7]. In addition, they significantly reduced the TNF-α level and repaired cartilage damage compared to the control group. Therefore, targeting CD44 in RA conditions can be used as a potential target for selective drug delivery.
depicts the case studies of HA and CS based drug delivery systems for the treatment of RA.
Table 1. Hyaluronic acid and chondroitin sulfate based drug delivery systems for the treatment of rheumatoid arthritis
5. Expert opinion and future perspective
The trajectory of RA has been significantly altered by new treatment methods. Early recognition, rapid diagnosis, and immediate initiation of small-molecule therapeutics treatment may improve the chances of remission. For subjects with RA, these treatment strategies can facilitate optimal care and better prospects observed traditionally. Still, there is no complete cure for RA. The most critical aspect of RA treatment is targeted drug delivery to the macrophages and fibroblasts. Targeting these immune cells will regulate the generation of cytokines that augment inflammation and contribute to the destruction of cartilage and bone. The glycosaminoglycans (HA and CS)-coated nanoparticles would be effective in treating RA and can overcome the issue of conventional therapy. However, translation of HA and CS-based nanocarrier systems to the clinical settings is difficult as the chemical modification may affect the structures of HA and CS which may alter the CD44 targeting efficiency. In addition, the protein corona will encumber CD44 targeting due to the degradation of the glycosaminoglycan. Further, there is a need for an appropriate in vivo model to assess the targetability and fate of glucosaminoglycan coated nanoparticles. Developing an animal model that can entirely mimic the RA disease state is still challenging. The models with the least dissimilarity and the greatest similarity are favored. These models were divided into two groups as arthritis-induced models and genetic models (e.g.: K/BxN mice, human tumor necrosis factor gene mice, IL-1ra-/- transgenic mice). The collagen type II generated arthritis in rats or mice and adjuvant-induced arthritis in rats were mostly reported and showed predictability for efficacy in humans. Also, the K/BxN model has proven to help elucidate some of the mechanisms driving RA. There is no doubt that these models have undeniably elevated the status of processes thought to be important in disease onset and progression of RA.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors gratefully acknowledge the Ph.D. fellowship from DST-INSPIRE, Govt. of India [#IF190259].
Additional information
Funding
References
- Gorantla S, Singhvi G, Rapalli VK, et al. Targeted drug-delivery systems in the treatment of rheumatoid arthritis: recent advancement and clinical status. Therape Delivery. 2020;11:269–284. Available from https://doi.org/10.4155/tde-2020-0029
- Sepriano A, Kerschbaumer A, Smolen JS, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2020;79:760–770.
- Heo R, Park JS, Jang HJ, et al. Hyaluronan nanoparticles bearing γ-secretase inhibitor: in vivo therapeutic effects on rheumatoid arthritis. J Control Release. 2014;192:295–300. Available from https://pubmed.ncbi.nlm.nih.gov/25109660/
- Badghaish MMO, Qorban GNM, Albaqami AS. Rheumatoid Arthritis, Pathophysiology and Management. Egypt J Hosp Med. 2018;70:1898–1903.
- Teriete P, Banerji S, Noble M, et al. Structure of the regulatory hyaluronan binding domain in the inflammatory leukocyte homing receptor CD44. Mol Cell. 2004;13:483–496.
- Shin JM, Kim SH, Thambi T, et al. A hyaluronic acid–methotrexate conjugate for targeted therapy of rheumatoid arthritis. Chem Commun. 2014;50:7632–7635. Available from www.rsc.org/chemcomm
- Mahtab A, Rabbani SA, Neupane YR, et al. Facile functionalization of Teriflunomide-loaded nanoliposomes with Chondroitin sulphate for the treatment of Rheumatoid arthritis. Carbohydr Polym. 2020;250:116926.
- Gouveia VM, Lopes-De-Araújo J, Costa Lima SA, et al. Hyaluronic acid-conjugated pH-sensitive liposomes for targeted delivery of prednisolone on rheumatoid arthritis therapy. Nanomedicine. 2018;13:1037–1049. Available from http://www.ncbi.nlm.nih.gov/pubmed/29790395
- Lee H, Lee MY, Bhang SH, et al. Hyaluronate-gold nanoparticle/Tocilizumab complex for the treatment of rheumatoid arthritis. ACS Nano. 2014;8:4790–4798. Available from https://doi.org/10.1021/nn500685h
- Jeon J, Rao NV, Byun JH, et al. pH-responsive hyaluronic acid-based nanocarrier for treatment of rheumatoid arthritis. J Nanosci Nanotechnol. 2016;16:11849–11856.
- Tamura T, Higuchi Y, Kitamura H, et al. Novel hyaluronic acid-methotrexate conjugate suppresses joint inflammation in the rat knee: efficacy and safety evaluation in two rat arthritis models. Arthritis Res Ther. 2016;18:79. Available from: https://doi.org/10.1186/s13075-016-0971-8
- Shilpi S, Upadhaye S, Shivvedi R, et al. Chondroitin sulphate mediated targeted delivery of methotrexate and aceclofenac to the joints for effective management of rheumatoid arthritis. Asian J Pharm Pharmacol. 2019;5:495–502.
- Toyama-Sorimachi N, Kitamura F, Habuchi H, et al. Widespread expression of chondroitin sulfate-type serglycins with CD44 binding ability in hematopoietic cells. J Biol Chem. 1997;272:26714–26719. Available from https://pubmed.ncbi.nlm.nih.gov/9334256/
- Yu C, Li X, Hou Y, et al. Hyaluronic acid coated acid-sensitive nanoparticles for targeted therapy of adjuvant-induced arthritis in rats. Molecules. 2019;24. Available from https://pubmed.ncbi.nlm.nih.gov/30609724/
- Zhou M, Hou J, Zhong Z, et al. Targeted delivery of hyaluronic acid-coated solid lipid nanoparticles for rheumatoid arthritis therapy. Drug Deliv. 2018;25:716–722. Available from https://doi.org/10.1080/10717544.2018.1447050