ABSTRACT
Introduction
The glycosaminoglycan hyaluronan forms a gel-like substance, which presents a barrier to bulk fluid flow in the subcutaneous (SC) space, limiting SC drug delivery volume and administration rates. Recombinant human hyaluronidase PH20 (rHuPH20) acts locally to temporarily remove this barrier, facilitating rapid SC delivery of large volumes and/or high doses of sequentially or co-administered therapeutics.
Areas covered
An extensive clinical and post-marketing dataset of safety and immunogenicity of rHuPH20 in its current applications with approved therapeutics demonstrates that rHuPH20 acts locally, without measurable systemic absorption at the SC doses used in the approved products, and is well tolerated in combination with several co-administered therapeutic agents across diverse patient groups. The immunogenicity profile demonstrates no adverse effects associated with treatment-emergent rHuPH20 antibody responses. Immunogenicity to monoclonal antibodies co-formulated with rHuPH20 shows no clinical difference between SC and intravenous administration. Safety assessments of patient subsets for special populations, including children, elderly patients, and pregnant women, raise no additional safety concerns.
Expert opinion
The benefits of SC administration for patients and healthcare systems often outweigh those of intravenous administration, driving future initiation of SC-only drug development programs. Injection devices allowing large-volume SC administration could be facilitated by incorporating co-formulated biologics containing rHuPH20.
1. Introduction
Hyaluronan (HA) is a naturally occurring glycosaminoglycan and key component of the extracellular matrix that is distributed widely throughout the body and in the skin [Citation1–3]. HA forms a highly hydrated viscous gel [Citation2,Citation4,Citation5], which acts as a lubricant and also plays a role in maintaining cell architecture, water homeostasis, macromolecular filtering, and viscoelasticity [Citation1,Citation6]. In the hypodermis of the skin, also known as the subcutaneous (SC) space, the visco elastic HA gel presents a barrier to bulk fluid flow, limiting rapid, large-volume SC drug delivery, and restricting drug dispersion and absorption [Citation5]. As a result, SC administration of fluids and therapeutics is usually limited to volumes of <2 mL, or requires long infusion times or multiple injections to deliver the dose [Citation5,Citation7,Citation8].
Animal-derived hyaluronidases have been used for more than 70 years to modify tissue permeability through depolymerization of HA and to increase dispersion of other infused drugs [Citation9–11]. In line with the World Health Organization recommendation to avoid animal-derived materials in the manufacturing of pharmaceutical products wherever possible [Citation12], a highly purified recombinant form of human hyaluronidase PH20 enzyme (rHuPH20) comprising human, rather than animal, amino acid sequences was developed more recently [Citation8]. rHuPH20 facilitates the SC delivery of co-administered therapeutics by locally depolymerizing HA in the SC space, temporarily removing the barrier to SC administration of relatively large volumes [Citation8]. The application of rHuPH20 to existing SC drug formulations can optimize dosing and administration, remove the volume limitations associated with conventional SC delivery, and also enable reformulation of existing intravenous (IV) drugs for rapid SC delivery [Citation8].
rHuPH20 has been available as the active pharmaceutical ingredient in HYLENEX® recombinant hyaluronidase human injection since 2005 in the USA, indicated as an adjuvant in SC fluid administration for achieving hydration, for increasing the dispersion and absorption of other injected drugs, and in SC urography for improving resorption of radiopaque agents [Citation13]. In addition, rHuPH20 is available as ENHANZE® drug delivery technology, which is used to facilitate and optimize the SC administration of co-administered biotherapeutics. In this setting, rHuPH20 is approved for use as a component in a range of products in different therapeutic areas [Citation8,Citation14]. shows products currently approved for use with rHuPH20.
Table 1. Products approved for use with rHuPH20
2. Mechanism of action, pharmacodynamics, and pharmacokinetics of rHuPH20
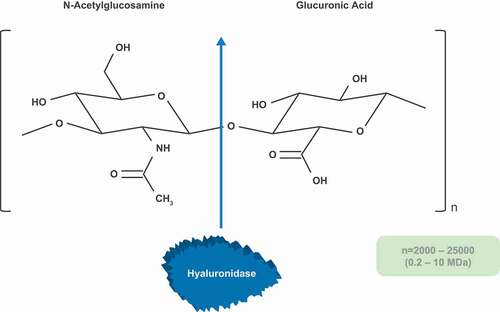
The mechanism of action of rHuPH20 is well-characterized in the literature. Hyaluronidases are a family of enzymes that degrade HA in the body as part of normal physiological processes, with approximately one-third of the total HA content in the body turning over every 24 hours [Citation3,Citation18–20]. There are five functional mammalian hyaluronidases: HYAL1, HYAL2, HYAL3, HYAL4, and the SPAM1 gene product, posterior head protein 20 (PH20) [Citation20,Citation21]. PH20 is synthesized in the epididymis and localized to the posterior head region of immature sperm, where it degrades HA to facilitate sperm penetration of the cumulus matrix surrounding an oocyte and aids fertilization [Citation22]. Hyaluronidases generally depolymerize HA by hydrolysis of the β (1→4) linkage between the C1 position of N-acetylglucosamine and the C4 position of glucuronic acid [Citation21,Citation23,Citation24], producing fragments of approximately 20 kDa (50 disaccharide units) with HYAL2 as the main enzyme, and lower molecular weight fragments with the other hyaluronidases [Citation3,Citation20,Citation21].
rHuPH20 is a recombinant human form of the PH20 enzyme, which lacks the carboxy-terminal amino acids that mediate attachment to the plasma membrane via a lipid anchor, resulting in a soluble form of the human PH20 protein [Citation24]. rHuPH20 acts by depolymerizing HA in the extracellular matrix (), reducing the viscosity of the SC space and enabling bulk fluid flow, and dispersion and absorption of sequentially or co-administered therapeutic agents [Citation5,Citation8].
It is important to note that rHuPH20, when used to facilitate SC administration, has no therapeutic activity as a single agent. Consequently, the pharmacodynamic profile of rHuPH20 is assessed through its effects on the absorption of co-administered active agents, or changes in flow rate, pharmacokinetics (PK)/bioavailability, tolerability, and general ability to rapidly deliver large volumes subcutaneously [Citation25,Citation26]. Studies have demonstrated that the application of rHuPH20 to co-administered SC agents may impact their PK profile in terms of increased absorption rate, increased bioavailability, increased maximum concentration (Cmax), and accelerated time to reach Cmax (tmax) as compared to SC therapeutics without rHuPH20 [Citation26–28]. Co-administration of rHuPH20 can also reduce intra- and inter-individual PK variability [Citation29,Citation30].
When administered subcutaneously, rHuPH20 has no measurable systemic exposure at the doses used in currently approved products [Citation31–33]. In a series of phase Ib dose-ranging studies of rHuPH20 co-administered subcutaneously with various monoclonal antibody therapeutics in healthy volunteers, rHuPH20 plasma concentrations remained below the limit of quantification (<61.4 pg/mL, contra values in Kirschbrown et al. 2019 [Citation31]) for all rHuPH20 doses used (1350 U up to 30,000 U) [Citation31–33]. The clinical observation of no measurable systemic exposure at the doses tested is supported by the observed low SC bioavailability (<10%) in non-human primates as well as modeling studies that predict low systemic exposure after SC administration in humans [Citation34]. In addition, a study in healthy volunteers demonstrated that intravenously administered rHuPH20 is rapidly cleared from the plasma [Citation14]. Plasma concentrations of rHuPH20, quantified as both rHuPH20 hyaluronidase activity (enzymatic rHuPH20) and as immunoreactive rHuPH20 plasma concentration, increased during a single 5-min IV infusion of 10,000 U (n = 11) or 30,000 U (n = 12) rHuPH20 (median tmax = 6 min from IV initiation for both doses of rHuPH20 and both enzymatic and immunoreactive assays, ) [Citation14]. This was followed by an early rapid decline in plasma concentrations of rHuPH20 that were below the limit of quantification within 2 hours [Citation14]. The apparent mean half-life for plasma rHuPH20 concentration was 3.7 and 5.6 min for the enzymatic assay with 10,000 U and 30,000 U rHuPH20 respectively, and 6.6 and 10.4 min for the immunoreactive assay with 10,000 U and 30,000 U, respectively () [Citation14].
Figure 2. Plasma rHuPH20 concentration following a single 5-minute IV infusion of 10,000 U (n = 11) or 30,000 U (n = 12) rHuPH20 in healthy volunteers. rHuPH20 was quantified as (A) plasma rHuPH20 hyaluronidase activity (enzymatic rHuPH20), and (B) immunoreactive rHuPH20 plasma concentration [Citation14]
![Figure 2. Plasma rHuPH20 concentration following a single 5-minute IV infusion of 10,000 U (n = 11) or 30,000 U (n = 12) rHuPH20 in healthy volunteers. rHuPH20 was quantified as (A) plasma rHuPH20 hyaluronidase activity (enzymatic rHuPH20), and (B) immunoreactive rHuPH20 plasma concentration [Citation14]](/cms/asset/12bb3242-607d-435d-b731-3d13a95c9a7e/iedd_a_1981286_f0002_oc.jpg)
3. Patient exposure to rHuPH20
There has been extensive clinical exposure to rHuPH20 across a broad range of studies and in the post-marketing setting with HYLENEX and other products using rHuPH20 (as ENHANZE drug delivery technology). Studies have assessed the safety of rHuPH20 as a single agent for intradermal injection, as well as when administered subcutaneously immediately prior to another agent, mixed with other agents and co-administered subcutaneously, or co-formulated with other agents. rHuPH20 has been assessed for use with a range of therapeutics, including insulin and insulin analogs [Citation28,Citation35,Citation36], small molecules including morphine, ondansetron and ceftriaxone [Citation27,Citation37,Citation38], human blood-derived polyclonal immunoglobulin [Citation26], human plasma-derived C1 esterase inhibitor [Citation39], monoclonal antibodies [Citation33,Citation40–45], and hydration fluids [Citation46]. The safety of rHuPH20 has also been evaluated across age groups, including in healthy adults [Citation14], in pediatric and elderly populations [Citation46–49] and in pregnant women [Citation50].
In total, approximately 1600 patients have been exposed to rHuPH20 in 30 studies conducted under the HYLENEX investigational new drug application (IND) or as post-marketing non-IND studies, and over 5000 patients have been exposed to rHuPH20 co-administered with monoclonal antibodies in studies conducted by ENHANZE partners. Based on units sold, assuming one vial per patient, nearly 3 million patients have received rHuPH20 as HYLENEX recombinant. Products containing rHuPH20 have been approved in many countries worldwide [Citation51,Citation52], with trastuzumab co-formulated with rHuPH20 approved in more than 100 countries [Citation51]. The commercial products are HYQVIA®/HyQvia® (Immune Globulin Infusion 10% [Human] with sequential SC administration of rHuPH20) [Citation53,Citation54], Herceptin® SC/HERCEPTIN HYLECTA™ (trastuzumab co-formulated with rHuPH20) [Citation55–57], MabThera® SC/RITUXAN HYCELA™/RITUXAN® SC (rituximab co-formulated with rHuPH20) [Citation58–60], DARZALEX® SC/DARZALEX FASPRO™ (daratumumab co-formulated with rHuPH20) [Citation61,Citation62], and PHESGO™ (pertuzumab and trastuzumab co-formulated with rHuPH20) [Citation63,Citation64] (). As of January 2021, more than 400,000 patients had received these products. Therefore, both regulatory authorities and prescribers have considerable familiarity with the use and safety profile of rHuPH20-containing products [Citation13,Citation53–56,Citation58,Citation59,Citation61–63].
4. Safety of rHuPH20
4.1. General safety aspects
Although patients receiving SC rHuPH20 undergo multiple injections, rHuPH20 has a short half-life and its effect in the SC space is local and transient. HA fully regenerates within 24–48 hours with no associated histological alterations or inflammatory changes observed in animal or human skin biopsies [Citation25,Citation35,Citation65]. In the setting of an insulin pump study in humans, biopsies of the infusion site were taken up to 48 hours after removal of the cannula. The results showed modest inflammatory changes consistent with a foreign body reaction to the cannula and there were no differences between infusions with or without rHuPH20 [Citation30]. Furthermore, preclinical data have shown no evidence of intrinsic skin aging affecting either the efficacy and safety of rHuPH20 or the reconstitution of HA in the SC space, which suggests that the SC delivery of co-formulated therapeutics with rHuPH20 should also not be affected [Citation66]. In clinical practice, the profiles of adverse events (AEs) seen with SC injection of co-formulations of rHuPH20 and therapeutic drugs are the same as those observed with the individual use of each agent or the AEs are associated with the rapid introduction of a large volume into the SC space [Citation8]. In addition, the safety profile of rHuPH20 is consistent across different age groups and unaffected by the speed of the injection, and skin care is not required routinely after the injection, with the only caution being to avoid injection into abnormal or scarred skin [Citation53–56,Citation58,Citation59,Citation61–63].
The approval of HYLENEX by the US Food and Drug Administration (FDA) in 2005 was in part reliant on the FDA’s finding of the effectiveness of hyaluronidases from the Drug Efficacy Study Implementation (DESI) review in 1970, and the results from a study of healthy volunteers that assessed hypersensitivity [Citation5]. The DESI review established the efficacy of three animal-derived hyaluronidases based on many years of clinical use, and this formed the basis of the HYLENEX label [Citation5]. The safety information in the HYLENEX label, based on the DESI review, is consistent with that of the currently marketed animal-derived hyaluronidases VITRASE® (an ovine-derived hyaluronidase) and AMPHADASE® (a bovine-derived hyaluronidase) [Citation10,Citation11,Citation13].
In clinical studies, subcutaneously administered rHuPH20 has been generally well tolerated across diverse study populations, including healthy volunteers [Citation29,Citation33,Citation36,Citation38,Citation67,Citation68], children [Citation46–48,Citation69], the elderly [Citation49], and hospice and palliative care patients [Citation37], as well as patients with cancer [Citation40,Citation42,Citation44,Citation45], primary immune deficiencies [Citation26,Citation48,Citation69], type 1 and 2 diabetes [Citation35], rheumatoid arthritis, hereditary angioedema [Citation39], and hyperlipidemia [Citation70]. rHuPH20 is also well tolerated when given subcutaneously in combination with other agents, including Ringer’s lactate solution [Citation71], normal saline [Citation71], co-injected drugs (morphine, ceftriaxone, and ondansetron) [Citation27,Citation37,Citation38,Citation67], insulin and rapid-acting insulin analogs [Citation28,Citation29,Citation35,Citation36], human blood-derived polyclonal immunoglobulin G [Citation26,Citation48,Citation69], human plasma-derived C1 esterase inhibitor [Citation39], and monoclonal antibodies (adalimumab [Citation72,Citation73], rituximab [Citation40], trastuzumab [Citation42,Citation43], daratumumab [Citation41,Citation45], bococizumab [Citation68,Citation70], and combined pertuzumab and trastuzumab [Citation44]). In most clinical studies, the AEs have usually reflected the safety profile of the co-administered agent or have been associated with the rapid introduction of a relatively large volume of fluid in the SC space and the administration of multiple SC injections [Citation8]. The large body of clinical data and experience to date have not demonstrated any long-term safety concerns from repeated injections [Citation40,Citation42,Citation69,Citation74–78]. Indeed, with repeated large volume SC infusions of HyQvia over several years (mean volume 292.2 mL per infusion, median maximum rate of 300 mL/hr), the rate of related local AEs decreased over time from 3.68/subject-year in months 1–12 to approximately 1.50/subject-year after 30 months of treatment [Citation70].
In a study evaluating IV administration of rHuPH20 in 24 healthy volunteers, both tested doses of 10,000 U and 30,000 U administered over 5 min to 12 volunteers in each dose group were well tolerated [Citation14]. No serious AEs (SAEs) were reported in either treatment group, and the two AEs that occurred in the study were not attributable to rHuPH20 (Grade 1 catheter site pain in the 10,000 U group and Grade 1 hypotension in the 30,000 U group) [Citation14]. The study provided reassurance that should accidental IV exposure to rHuPH20 occur, it would be unlikely to cause clinically significant AEs at the doses used in approved SC co-formulated products (range 10,000–30,000 U) [Citation55,Citation61,Citation63].
4.2. Local reactions
The most common AEs seen in the HYLENEX clinical studies were mild, transient, and self-limiting local administration-site reactions including erythema, pain, ecchymosis, bruising, pruritus, burning, tenderness, edema, induration, irritation, paresthesia, numbness, and rash [Citation8,Citation79]. Moderate administration-site reactions were less frequent and included burning, erythema, pain, and numbness [Citation8]. One clinical study evaluated sensitivity to a single intradermal dose of rHuPH20 vs saline in healthy volunteers [Citation79]. No allergic reactions were observed in the 100 volunteers receiving rHuPH20. A total of 11 AEs were reported in 9% of the volunteers; none of the AEs were considered related to the study treatment, and all events resolved without sequelae. No SAEs were reported in this study [Citation79].
In clinical studies where rHuPH20 was combined with other agents, local administration-site reactions were more common with SC in comparison to IV formulation but were mostly mild-to-moderate reactions such as swelling, erythema, and mild pain [Citation40,Citation53,Citation63]. Rates of administration-site reactions for approved monoclonal antibodies and other therapeutics, when co-administered subcutaneously with rHuPH20 and when administered intravenously, are shown in . Rates of administration-site reactions with SC formulations ranged from 7‒91% of patients ().
Table 2. Rates of local administration-site reactions for approved monoclonal antibodies and other therapeutics, when co-administered subcutaneously with rHuPH20 and when administered intravenously
4.3. Systemic infusion-related reactions, administration-related reactions, and hypersensitivity
Administration of biotherapeutics can cause systemic infusion-related reactions (IRRs) characterized by flushing, rash, fever, rigor, chills, dyspnea, hypotension, cardiac dysfunction, and/or anaphylaxis [Citation80]. Administration-related reactions (ARRs) include all AEs related to administration and may include both local and systemic reactions [Citation58]. Incidences of IRRs, ARRs, and hypersensitivity reactions for different administration routes (SC or IV) have been compared for several approved products where rHuPH20 is combined with other agents (). In a phase III trial in patients with HER2-positive early breast cancer receiving trastuzumab and a phase III trial in patients with follicular lymphoma receiving rituximab, greater incidences of ARRs were observed in patients receiving therapeutics subcutaneously in comparison with those receiving therapeutics intravenously (ARR incidence was 48% vs 37% with trastuzumab SC vs trastuzumab IV and 48% vs 35% with rituximab SC vs rituximab IV, respectively, significance not reported) [Citation40,Citation55]. In contrast, in other studies, incidences of IRRs and/or ARRs were found to be similar for IV and SC administration routes, or SC administration was associated with lower IRR or ARR rates than IV administration (). For example, in a phase III trial in patients with relapsed or refractory multiple myeloma, the incidence of systemic IRRs was found to be significantly lower among patients receiving daratumumab SC (13% of patients) in comparison with daratumumab IV (34% of patients, odds ratio 0.28, 95% confidence interval 0.18–0.44, P< 0∙0001) [Citation45]. There are no reports from clinical trials of severe hypersensitivity reactions or allergic reactions associated with rHuPH20 alone [Citation14,Citation79].
Table 3. Incidences of systemic infusion-related reactions and administration-related reactions in patients treated with therapeutics co-administered with rHuPH20
4.4. Immunogenicity of rHuPH20
The immunogenicity profile of rHuPH20 has been characterized in more than 1900 patients from 16 clinical trials. rHuPH20-reactive antibodies are present in around 5% of the adult population in the absence of exposure to rHuPH20 [Citation83]. Prevalence of rHuPH20-reactive antibodies increased significantly with age (P= 0.0006) and was significantly higher in males than females (P= 0.0007) [Citation83]. In a study of rHuPH20 administered intravenously, rHuPH20-reactive antibodies at baseline were detected in 2 of 24 volunteers (both male), and were not associated with any unusual safety observations during the study [Citation14].
The prevalence of preexisting rHuPH20-reactive antibodies has also been evaluated in clinical studies of rHuPH20 co-administered with SC human blood-derived polyclonal immunoglobulin and human plasma-derived C1 esterase inhibitor, trastuzumab, rituximab, or insulin. In these studies, the prevalence of preexisting rHuPH20-reactive antibodies ranged from 3‒13% (except in one study with SC human blood-derived polyclonal immunoglobulin, where only 1 of 87 patients was positive for rHuPH20-reactive antibodies at baseline) [Citation39,Citation41,Citation84]. SC dosing of rHuPH20 co-formulated with monoclonal antibody products was associated with treatment-emergent rHuPH20-reactive antibodies in 1‒21% of treated individuals [Citation41,Citation55,Citation61,Citation63,Citation84]. Exposure to rHuPH20 in combination with human blood-derived polyclonal immunoglobulin or C1 esterase inhibitor was associated with treatment-emergent rHuPH20-reactive antibodies in 18% [Citation53] and 45% [Citation39] of patients, respectively. summarizes the incidence rate of treatment-induced or treatment-enhanced rHuPH20-reactive antibodies.
Table 4. Incidences of treatment-induced/enhanced rHuPH20-reactive antibodies in patients treated with therapeutics co-administered with rHuPH20
Endogenous PH20 is primarily expressed in the male reproductive tract. Although the product labels for HyQvia include a warning of the potential for rHuPH20-reactive antibodies to cross-react with endogenous PH20, animal studies have shown no evidence of any adverse effect of antibodies to rHuPH20 on male and female fertility, embryo-fetal development, or offspring development. In human studies, antibodies to rHuPH20 have not been associated with AEs [Citation8,Citation53,Citation54].
Overall, in investigations of the potential immunogenicity of rHuPH20, no clinical signs or symptoms have been associated with a positive rHuPH20 antibody response and no rHuPH20-reactive antibodies capable of neutralizing hyaluronidase activity have been confirmed to date [Citation26,Citation40,Citation41,Citation48,Citation84]. Possible explanations for a lack of clinical significance for rHuPH20-reactive antibodies include the highly restricted expression pattern of the endogenous enzyme [Citation85], and the local and transient effect of rHuPH20 in the SC space [Citation25].
4.5. Immunogenicity of co-formulated monoclonal antibodies
Despite a common belief that SC delivery elicits higher rates of immunogenicity than the IV route of administration, the immunogenicity of co-formulated monoclonal antibodies was similar with SC delivery in comparison with IV administration [Citation45,Citation55,Citation58,Citation61,Citation63,Citation80,Citation86]. The incidences of treatment-emergent rituximab-reactive antibodies in a phase III study of rituximab in patients with follicular lymphoma were 2% in the SC group and 1.5% of patients in the IV group. No evidence for neutralizing rituximab-reactive antibodies was found [Citation58]. A phase III study of trastuzumab in patients with HER2-positive early breast cancer reported that trastuzumab-reactive antibodies developed in 16% of patients (47 of 295) receiving trastuzumab SC and in 10% of patients (30 of 296) receiving treatment by the IV route. Neutralizing trastuzumab-reactive antibodies were detected in 6% of patients (3 of 47) receiving trastuzumab SC and in 7% of patients (2 of 30) receiving trastuzumab IV [Citation55].
In studies of patients with multiple myeloma treated with daratumumab, either as monotherapy or in combination with other chemotherapy agents, of the 451 patients receiving daratumumab SC (either as monotherapy or in combination), 1 patient (<1%) receiving daratumumab monotherapy developed treatment-emergent daratumumab-reactive antibodies and transient neutralizing antibodies [Citation61]. Treatment-emergent daratumumab-reactive antibodies were not present in any of the patients who were treated with monotherapy IV (0 of 111) and were found in <1% of patients who were treated intravenously with combination therapy (2 of 1050) [Citation86]. One patient administered combination therapy intravenously developed transient neutralizing antibodies against daratumumab [Citation86].
In a phase III study of the combination therapy, trastuzumab and pertuzumab, in patients with operable or locally advanced HER2-positive early breast cancer, treatment-emergent pertuzumab-reactive antibodies were found in 5% of patients (11 of 231) when treatment was administered subcutaneously and in 3% of patients (7 of 237) when treatment was administered intravenously. Treatment-emergent trastuzumab-reactive antibodies were found in 1% of patients (2 of 232) when treatment was administered subcutaneously and in <1% of patients (1 of 237) when treatment was administered intravenously [Citation63]. Among patients who tested positive to pertuzumab-reactive antibodies, neutralizing pertuzumab-reactive antibodies were detected in 1 patient receiving SC treatment and in 1 patient receiving IV treatment. Among patients who tested positive to trastuzumab-reactive antibodies, neutralizing trastuzumab-reactive antibodies were detected in 1 patient receiving SC treatment [Citation63].
4.6. Long-term treatment and follow-up
Long-term assessment of rHuPH20 safety has found rHuPH20 use to be well tolerated in the long term and not associated with systemic AEs related to the length of treatment [Citation69]. Long-term safety outcomes for trastuzumab SC are available from four studies: a phase III study in 294 patients with HER2-positive early breast cancer (18 cycles of treatment and 5.9 years median follow-up) [Citation42]; a phase III study in 102 patients with early breast cancer (9 cycles of treatment, 24 months of safety follow-up) [Citation74]; a phase IIIb study in 240 patients with early or locally advanced breast cancer (18 cycles of treatment over 1 year, 2 years of follow-up) [Citation75]; and a phase IIIb study in 50 patients with metastatic breast cancer (median of 19 cycles of treatment [maximum 49 cycles], minimum of 24 months of safety follow-up) [Citation76]. Long-term data for rituximab SC are available from a phase III study in 205 patients with follicular lymphoma (up to 19 cycles of SC treatment, median follow-up of 37 months) [Citation40]; from a phase IIIb study in 381 patients with diffuse large B-cell lymphoma (DLBCL; 1 initial cycle of IV treatment followed by 7 cycles of SC treatment, median follow-up of 35 months) [Citation77]; and from a single arm in a phase IIIb study in which 140 patients with DLBCL or follicular lymphoma switched from IV to SC administration (up to 19 cycles of SC treatment, median follow-up of 33.5 months) [Citation78].
The results from the above studies, which address long-term treatment and/or safety follow-up after the end of treatment, confirm the acceptable safety and tolerability profile of rHuPH20 in patients receiving trastuzumab SC or rituximab SC, with no new safety concerns [Citation40,Citation42,Citation74–78]. In addition, in an analysis of two phase III studies encompassing 188 patient-years of subcutaneous immunoglobulin (SCIG) exposure, long-term treatment with SCIG was well tolerated in both adults and children. The incidence of systemic and local AEs during SCIG exposure was low (8.03 systemic AEs per patient-year and 2.65 local AEs per patient-year), and the rate of treatment-related local AEs declined during the treatment course from 3.68 per patient-year in months 1–12 to 1.51 per patient-year after 30 months of treatment [Citation69].
4.6.1. Special populations
The safety of rHuPH20 has been assessed in special populations, including pediatric patients (infants and children) participating in rehydration studies [Citation46,Citation47], a subgroup of pediatric patients from two consecutive SCIG phase III studies [Citation48], patients participating in SCIG post-marketing analyses in elderly patients [Citation49], and a pregnancy registry [Citation50]. In two studies of rHuPH20 to aid SC administration of hydration fluids in infants and children with mild-to-moderate dehydration, rHuPH20 was well tolerated. Most AEs were related to infusion-site reactions (i.e. erythema, swelling, and edema) [Citation46,Citation47]. rHuPH20 was also well tolerated when administered in a subset of children with primary immunodeficiency diseases who received human blood-derived polyclonal immunoglobulin therapy [Citation48]. No treatment-related SAEs were reported, and the incidence of local and systemic treatment-related AEs was low (0.09/infusion and 0.10/infusion, respectively) [Citation48].
In a post-marketing study of 16 elderly patients with primary or secondary immunodeficiencies, rHuPH20-facilitated SC 10% human blood-derived polyclonal immunoglobulin was well tolerated [Citation49]. Six patients reported local adverse drug reactions: redness (n = 2), rash (n = 2), pain at the infusion site (n = 2), and feeling of a bloated abdomen (n = 1). Two patients reported systemic adverse drug reactions: a single night of sleeplessness (n = 1) and slight malaise on the day of the infusion (n = 1) [Citation49]. In another study, 32 patients in a hospice setting received rHuPH20 to enhance hypodermoclysis with standard hydration fluids (n = 26) or to enhance SC infusion of nine medications (n = 6). Higher than expected serum lidocaine concentration was observed in 1 patient, but no other significant AEs were reported [Citation87].
While the safety of rHuPH20 during pregnancy has been examined in only a pregnancy registry to date, there is a low risk of rHuPH20-related AEs on human embryo–fetal development given that the doses of rHuPH20 used in patients are unlikely to achieve measurable systemic exposure [Citation31–33]. During a post-authorization pregnancy registry of women who become pregnant during or after treatment with rHuPH20-facilitated SCIG, 9 women and 7 infants were exposed to SCIG. No AEs related to SCIG were reported, and all infants had normal appearance, pulse, grimace, activity, and respiration cores [Citation50]. In addition, there were no reports of AEs related to rHuPH20 associated with pregnancy in the Halozyme safety database.
5. Conclusions
Recombinant human hyaluronidase PH20 has a well-understood mechanism of action and facilitates rapid, large volume SC delivery of co-administered therapeutics by locally depolymerizing HA in the SC space, transiently removing the barrier to SC administration of larger volumes. Extensive clinical experience demonstrates that rHuPH20 is well tolerated as a single agent, as well as when combined with a range of co-administered therapeutic agents, across diverse patient groups. The immunogenicity profile of rHuPH20 has been examined in more than 20 clinical studies to date, with no confirmed rHuPH20-neutralizing antibody activity detected and no AEs associated with positive rHuPH20 antibody titers. No clinical difference was observed in the immunogenicity of co-formulated monoclonal antibodies when delivered subcutaneously in comparison with IV administration.
6. Expert opinion
Although SC delivery of biologic drugs is common across many disease states, many drugs are still administered intravenously, particularly in oncology. Part of the explanation for this is that, historically, oncology drugs were predominately small molecules administered intravenously by healthcare practitioners. In addition, there are recognized challenges in delivering SC injections of volumes >2 mL due to the presence of HA in the extracellular matrix, which limits bulk fluid flow in the SC space and impacts the ability to deliver large-volume, high-dose biologic drugs [Citation5]. Rapid large-volume SC injections may cause swelling, induration, and pain at the injection site, which can be addressed by slow infusion rates, multiple injection sites, or frequent dosing [Citation5,Citation7,Citation8]. The alternative is to administer biologics intravenously, which often requires infusion times of several hours. IV infusions require patients to visit hospitals, adding to the burden on both the patient and healthcare services, as patients and caregivers need to travel and spend considerable time at a medical facility, and healthcare resources are needed to administer and monitor the infusions [Citation80,Citation88]. In addition, in times of a global pandemic exposing patients to a high-risk environment in a hospital is to be avoided.
The co-formulation of rHuPH20 with therapeutic monoclonal antibodies may help address these issues by facilitating the administration of large-volume, high-dose biologics over a period of minutes [Citation89]. This approach is supported by the safety profile of rHuPH20 which is well characterized, well tolerated, and consistent across multiple products, including four approved oncology products and an approved immunoglobulin G (IgG) product [Citation53,Citation55,Citation58,Citation61,Citation63].
There are benefits to patients and healthcare systems from developing SC formulations of therapeutics that are conventionally administered intravenously. The switch from IV to SC administration is associated with a reduction in treatment burden for patients by reducing both hospital visits and lengthy infusion times, as well as reducing time and cost burden on the healthcare system [Citation80,Citation88,Citation90,Citation91]. Studies have shown that SC administration is generally preferred by patients, predominately due to the shorter infusion times and reduced pain compared with IV administration [Citation89,Citation92].
The proven, safe use of rHuPH20 to facilitate SC injections also introduces the option of at home, self-, or caregiver-administration of large-volume drugs, which represents an opportunity for the future. HyQvia, a life-long IgG replacement therapy given sequentially with rHuPH20, is approved for self-administration at home and studies have shown the long-term acceptability of this approach [Citation48,Citation49,Citation69]. With longer-term use associated with maintenance treatment in oncology patients, this option of chronic treatment at home will become an increasingly attractive option.
Nonetheless, barriers remain to the more widespread adoption of facilitated large volume SC injections. One barrier is suboptimal awareness by healthcare practitioners and patients of the ability to administer large volumes subcutaneously, safely, and repeatedly. The increasing use of approved rHuPH20 products, along with future approvals of new products, will improve this awareness. In addition, a realization by drug developers, particularly in oncology, that the benefits of SC administration to patients and healthcare systems often outweigh the benefits of IV administration, will drive the development of SC-only programs. These programs are already the norm for many therapies in the autoimmune disease area.
The use of devices for delivering volumes of <2 mL is becoming more common in diabetes and autoimmune disease areas [Citation93]. These devices improve the ease of administration for patients and caregivers over needle and syringe delivery systems. Preclinical studies have shown that the addition of rHuPH20 to a SC injection via an autoinjector resulted in improved SC injection performance compared with placebo for volumes up to 2 mL even at high delivery rates [Citation94]. It is a reasonable hypothesis to suggest that similar results are likely to be seen when rHuPH20 is used in a device in a human study.
With an increased focus on SC delivery for patients in various therapeutic areas, there is a need for devices that can administer volumes larger than 2 mL, either by a large volume autoinjector or an on-body device, where rHuPH20 may improve the injection performance. This is an active area of research, and data on the use of rHuPH20 co-formulated products in large volume (5 and 10 mL) devices are beginning to emerge. Future clinical studies should continue to evaluate the possibility of incorporating co-formulated biologics containing rHuPH20 into these autoinjectors and on-body devices to determine whether rHuPH20 in these devices can safely enable injection over a shorter period of time and achieve consistency of injection experience from dose to dose.
Article highlights
Recombinant human hyaluronidase PH20 (rHuPH20) acts locally in the subcutaneous (SC) space to temporarily remove hyaluronan, thus facilitating the rapid SC delivery of large volumes of co-administered therapeutics.
There is an extensive safety database for globally approved rHuPH20-containing products.
rHuPH20 is well tolerated and the safety profile is consistent across the approved products and across different age groups.
The adverse effects associated with rHuPH20 are local injection-site reactions, predominately mild/moderate erythema, which is transient, self-limiting and does not lead to discontinuation.
Treatment-emergent antibodies to rHuPH20 are not associated with adverse effects and do not interfere with the activity of rHuPH20.
This box summarizes key points contained in the article.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Medical writing support, including assisting authors with the development of the outline and initial draft, incorporation of comments, and fact checking, was provided by Talya Underwood, MSc, and Rachel O’Meara, PhD; editorial support, including formatting, proofreading, and submission, was provided by Travis Taylor, BA, all of Paragon, Knutsford, UK, supported by Halozyme Therapeutics, Inc. according to Good Publication Practice guidelines.
Disclosure statement
All the authors are employees of Halozyme Therapeutics, Inc., the sponsor for this study, and hold shares in the company. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242(1):27–33.
- Cowman MK, Lee HG, Schwertfeger KL, et al. The content and size of hyaluronan in biological fluids and tissues. Front Immunol. 2015;6:261.
- Stern R. Devising a pathway for hyaluronan catabolism: are we there yet? Glycobiology. 2003;13(12):105R–115R.
- Dicker KT, Gurski LA, Pradhan-Bhatt S, et al. Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater. 2014;10(4):1558–1570.
- Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv. 2007;4(4):427–440.
- Evanko SP, Tammi MI, Tammi RH, et al. Hyaluronan-dependent pericellular matrix. Adv Drug Deliv Rev. 2007;59(13):1351–1365.
- Wasserman RL. Overview of recombinant human hyaluronidase-facilitated subcutaneous infusion of IgG in primary immunodeficiencies. Immunotherapy. 2014;6(5):553–567.
- Locke KW, Maneval DC, LaBarre MJ. ENHANZE® drug delivery technology: a novel approach to subcutaneous administration using recombinant human hyaluronidase PH20. Drug Deliv. 2019;26(1):98–106.
- Atkinson WS. Use of hyaluronidase with local anesthesia in ophthalmology: preliminary report. Arch Ophthalmol. 1949;42(5):628–633.
- US Food and Drug Administration. VITRASE prescribing information 2004 [cited 2020 Feb 14]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021640s016lbl.pdf.
- US Food and Drug Administration. AMPHADASE prescribing information 2005 [cited 2020 Feb 14]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021665s005lbl.pdf.
- World Health Organization. WHO Guidelines on transmissible spongiform encephalopathies in relation to biological and pharmaceutical products 2003 [cited 2020 Feb 14]. Available from: https://www.who.int/biologicals/publications/en/whotse2003.pdf?ua%C2%BC1&ua%C2%BC1.
- Halozyme Therapeutics Inc. HYLENEX recombinant prescribing information 2016 [cited 2020 Feb 14]. Available from: http://hylenex.com/downloads/approved-uspi-lbl301feb2016.pdf.
- Printz MA, Dychter SS, DeNoia EP, et al. A phase I study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of recombinant human hyaluronidase PH20 administered intravenously in healthy volunteers. Curr Ther Res Clin Exp. 2020;93:100604.
- US Food and Drug Administration. Herceptin prescribing information 2018 [cited 2020 Mar 25]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103792s5345lbl.pdf.
- Modelevsky L, Tizon R, Reiss SN, et al. Rapid infusion rituximab is well tolerated in patients with primary CNS lymphoma. CNS Oncol. 2018;7(3):CNS19.
- Genentech Inc. PERJETA® prescribing information South San Francisco, CA 2020 [cited 2020 May 28]. Available from: https://www.gene.com/download/pdf/perjeta_prescribing.pdf.
- Jadin L, Bookbinder LH, Frost GI. A comprehensive model of hyaluronan turnover in the mouse. Matrix Biol. 2012;31(2):81–89.
- Vigetti D, Viola M, Karousou E, et al. Metabolic control of hyaluronan synthases. Matrix Biol. 2014;35:8–13.
- Csoka AB, Frost GI, Stern R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001;20(8):499–508.
- Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev. 2006;106(3):818–839.
- Evans EA, Zhang H, Martin-deleon PA. SPAM1 (PH-20) protein and mRNA expression in the epididymides of humans and macaques: utilizing laser microdissection/RT-PCR. Reprod Biol Endocrinol. 2003;1(1):54.
- US Food and Drug Administration. HYDASE Prescribing Information 2005 [cited 2020 Feb 14]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021716s003lbl.pdf.
- Wasserman RL. Progress in gammaglobulin therapy for immunodeficiency: from subcutaneous to intravenous infusions and back again. J Clin Immunol. 2012;32(6):1153–1164.
- Bookbinder LH, Hofer A, Haller MF, et al. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Control Release. 2006;114(2):230–241.
- Wasserman RL, Melamed I, Stein MR, et al. Recombinant human hyaluronidase-facilitated subcutaneous infusion of human immunoglobulins for primary immunodeficiency. J Allergy Clin Immunol. 2012;130(4):951–957.
- Thomas JR, Yocum RC, Haller MF, et al. The INFUSE-Morphine IIB study: use of recombinant human hyaluronidase (rHuPH20) to enhance the absorption of subcutaneous morphine in healthy volunteers. J Pain Symptom Manage. 2009;38(5):673–682.
- Vaughn DE, Yocum RC, Muchmore DB, et al. Accelerated pharmacokinetics and glucodynamics of prandial insulins injected with recombinant human hyaluronidase. Diabetes Technol Ther. 2009;11(6):345–352.
- Morrow L, Muchmore DB, Ludington EA, et al. Reduction in intrasubject variability in the pharmacokinetic response to insulin after subcutaneous co-administration with recombinant human hyaluronidase in healthy volunteers. Diabetes Technol Ther. 2011;13(10):1039–1045.
- Muchmore DB, Vaughn DE. Accelerating and improving the consistency of rapid-acting analog insulin absorption and action for both subcutaneous injection and continuous subcutaneous infusion using recombinant human hyaluronidase. J Diabetes Sci Technol. 2012;6(4):764–772.
- Kirschbrown WP, Wynne C, Kagedal M, et al. Development of a subcutaneous fixed-dose combination of pertuzumab and trastuzumab: results from the phase Ib dose-finding study. J Clin Pharmacol. 2019;59(5):702–716.
- Wynne C, Harvey V, Schwabe C, et al. Comparison of subcutaneous and intravenous administration of trastuzumab: a phase I/Ib trial in healthy male volunteers and patients with HER2-positive breast cancer. J Clin Pharmacol. 2013;53(2):192–201.
- Morcos PN, Zhang X, McIntyre C, et al. Pharmacokinetics and pharmacodynamics of single subcutaneous doses of tocilizumab administered with or without rHuPH20. Int J Clin Pharmacol Ther. 2013;51(7):537–548.
- Fathallah AM, Printz MA, Sugarman BJ, et al. Modeling the pharmacokinetics and pharmacodynamics of ENHANZE(TM) (rHuPH20) platform on the absorption of therapeutic antibodies after SC administration in preclinical and clinical settings. Presented at: AAPS NBC; 2015.
- Vaughn DE, Muchmore DB. Use of recombinant human hyaluronidase to accelerate rapid insulin analogue absorption: experience with subcutaneous injection and continuous infusion. Endocr Pract. 2011;17(6):914–921.
- Morrow L, Muchmore DB, Hompesch M, et al. Comparative pharmacokinetics and insulin action for three rapid-acting insulin analogs injected subcutaneously with and without hyaluronidase. Diabetes Care. 2013;36(2):273–275.
- Thomas JR, Wallace MS, Yocum RC, et al. The INFUSE-Morphine study: use of recombinant human hyaluronidase (rHuPH20) to enhance the absorption of subcutaneously administered morphine in patients with advanced illness. J Pain Symptom Manage. 2009;38(5):663–672.
- Harb G, Lebel F, Battikha J, et al. Safety and pharmacokinetics of subcutaneous ceftriaxone administered with or without recombinant human hyaluronidase (rHuPH20) versus intravenous ceftriaxone administration in adult volunteers. Curr Med Res Opin. 2010;26(2):279–288.
- Riedl MA, Lumry WR, Li HH, et al. Subcutaneous administration of human C1 inhibitor with recombinant human hyaluronidase in patients with hereditary angioedema. Allergy Asthma Proc. 2016;37(6):489–500.
- Davies A, Merli F, Mihaljevic B, et al. Efficacy and safety of subcutaneous rituximab versus intravenous rituximab for first-line treatment of follicular lymphoma (SABRINA): a randomised, open-label, phase 3 trial. Lancet Haematol. 2017;4(6):e272–e282.
- Usmani SZ, Nahi H, Mateos MV, et al. Subcutaneous delivery of daratumumab in relapsed or refractory multiple myeloma. Blood. 2019;134(8):668–677.
- Jackisch C, Stroyakovskiy D, Pivot X, et al. Subcutaneous vs intravenous trastuzumab for patients with ERBB2-positive early breast cancer: final analysis of the HannaH phase 3 randomized clinical trial. JAMA Oncol. 2019;5(5):e190339.
- Ismael G, Hegg R, Muehlbauer S, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13(9):869–878.
- Tan AR, Im SA, Mattar A, et al. Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection plus chemotherapy in HER2-positive early breast cancer (FeDeriCa): a randomised, open-label, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2021;22(1):85–97.
- Mateos MV, Nahi H, Legiec W, et al. Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): a multicentre, open-label, non-inferiority, randomised, phase 3 trial. Lancet Haematol. 2020;7(5):e370–e380.
- Spandorfer PR, Mace SE, Okada PJ, et al. A randomized clinical trial of recombinant human hyaluronidase-facilitated subcutaneous versus intravenous rehydration in mild to moderately dehydrated children in the emergency department. Clin Ther. 2012;34(11):2232–2245.
- Allen CH, Etzwiler LS, Miller MK, et al. Recombinant human hyaluronidase-enabled subcutaneous pediatric rehydration. Pediatrics. 2009;124(5):e858–867.
- Wasserman RL, Melamed I, Kobrynski L, et al. Recombinant human hyaluronidase facilitated subcutaneous immunoglobulin treatment in pediatric patients with primary immunodeficiencies: long-term efficacy, safety and tolerability. Immunotherapy. 2016;8(10):1175–1186.
- van Paassen P, Pittrow D, Scheidegger C, et al. Use of recombinant human hyaluronidase-facilitated subcutaneous immunoglobulin in elderly patients. Immunotherapy. 2020;12(2):131–139.
- Jahnz-Rozyk K. Registry to collect long term safety data from pregnant women treated with recombinant human hyaluronidase (rHuPH20)-facilitated subcutaneous immunoglobulin (FSCIG): interim results. Presented at: ESID; 2019 18-21 September.
- Hoffmann-La Roche Ltd. FDA approves Herceptin Hylecta for subcutaneous injection in certain HER2-positive breast cancers 2019 [cited 2019 Dec 3]. Available from: https://www.globenewswire.com/news-release/2019/02/28/1744585/0/en/FDA-approves-Herceptin-Hylecta-for-subcutaneous-injection-in-certain-HER2-positive-breast-cancers.html.
- Halozyme Therapeutics. FDA advisory committee provides unanimous recommendation for subcutaneous rituximab coformulated with halozyme enhanze technology 2017 [cited 2020 Dec 8]. Available from: https://www.1stoncology.com/blog/fda-advisory-committee-provides-unanimous-recommendation-for-subcutaneous-rituximab-coformulated-with-halozyme-enhanze-technology/.
- Baxalta US Inc. HYQVIA prescribing information for HyQvia: immune globulin infusion 10% (human) with recombinant human hyaluronidase 2020 [cited 2020 Jul 7]. Available from: https://www.shirecontent.com/PI/PDFs/HYQVIA_USA_ENG.pdf.
- European Medicines Agency. HyQvia summary of product characteristics 2018 [cited 2020 Apr 27]. Available from: https://www.ema.europa.eu/en/documents/product-information/hyqvia-epar-product-information_en.pdf.
- Roche Products Ltd. HERCEPTIN HYLECTA™ prescribing information for subcutaneous use 2019 [cited 2019 Mar 28]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761106s000lbl.pdf.
- European Medicines Agency. Herceptin summary of product characteristics 2018 [cited 2019 Dec 19]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__Product_Information/human/000278/WC500074922.pdf.
- Hoffmann-La Roche Ltd. Pr HERCEPTIN® SC trastuzumab injection 600 mg/5ml single dose vial. Product monograph 2020 [cited 2020 Dec 2]. Available from: https://www.rochecanada.com/PMs/Herceptin/HerceptinSC_PM_E.pdf.
- Genentech Inc. RITUXAN HYCELA™ prescribing information for subcutaneous use 2020 [cited 2021 Mar 29]. Available from: https://www.gene.com/download/pdf/rituxan_hycela_prescribing.pdf.
- European Medicines Agency. MabThera summary of product characteristics 2019 [cited 2019 Mar 28]. Available from: https://www.ema.europa.eu/en/documents/product-information/mabthera-epar-product-information_en.pdf.
- Hoffmann-La Roche Ltd. RITUXAN SC rituximab 120 mg/mL solution for subcutaneous injection. Product monograph 2018 [cited 2021 Mar 28]. Available from: http://www.rochecanada.com/content/dam/roche_canada/en_CA/documents/Research/ClinicalTrialsForms/Products/ConsumerInformation/MonographsandPublicAdvisories/RituxanSC/RituxanSC_PM_E.pdf.
- Janssen Biotech Inc. DARZALEX FASPRO™ prescribing information for subcutaneous use 2020 [cited 2021 Mar 29]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761145s000lbl.pdf.
- European Medicines Agency. Darzalex summary of product characteristics 2018 [cited 2019 Dec 19]. Available from: https://www.ema.europa.eu/documents/product-information/darzalex-epar-product-information_en.pdf.
- Genentech Inc. PHESGOTM prescribing information, for subcutaneous use 2020 [cited 2021 Mar 29]. Available from: https://www.gene.com/download/pdf/phesgo_prescribing.pdf.
- European Medicines Agency. PHESGO summary of product characteristics 2021 [cited 2021 Feb 21]. Available from: https://www.ema.europa.eu/en/documents/product-information/phesgo-epar-product-information_en.pdf.
- Richter WF, Bhansali SG, Morris ME. Mechanistic determinants of biotherapeutics absorption following SC administration. AAPS J. 2012;14(3):559–570.
- Connor RJ, Blouw B, Cowell J, et al. A preclinical investigation into the effects of aging on dermal hyaluronan properties and reconstitution following recombinant human hyaluronidase PH20 administration. Dermatol Ther (Heidelb). 2020;10(3):503–513.
- Dychter SS, Harrigan R, Bahn JD, et al. Tolerability and pharmacokinetic properties of ondansetron administered subcutaneously with recombinant human hyaluronidase in minipigs and healthy volunteers. Clin Ther. 2014;36(2):211–224.
- Bass A, Plotka A, Mridha K, et al. Pharmacokinetics, pharmacodynamics, and safety of bococizumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, in healthy subjects when administered in co-mixture with recombinant human hyaluronidase: a phase 1 randomized trial. Health Sci Rep. 2018;1:e61.
- Wasserman RL, Melamed I, Stein MR, et al. Long-term tolerability, safety, and efficacy of recombinant human hyaluronidase-facilitated subcutaneous infusion of human immunoglobulin for primary immunodeficiency. J Clin Immunol. 2016;36(6):571–582.
- Ridker PM, Tardif JC, Amarenco P, et al. Lipid-reduction variability and antidrug-antibody formation with bococizumab. N Engl J Med. 2017;376(16):1517–1526.
- Dychter SS, Ebel D, Mead TR, et al. Comparison of the tolerability of recombinant human hyaluronidase + normal saline and recombinant human hyaluronidase + lactated ringer’s solution administered subcutaneously: a phase IV, double-blind, randomized pilot study in healthy volunteers. Curr Ther Res Clin Exp. 2009;70(6):421–438.
- Kivitz A, Cohen S, Dowd JE, et al. Clinical assessment of pain, tolerability, and preference of an autoinjection pen versus a prefilled syringe for patient self-administration of the fully human, monoclonal antibody adalimumab: the TOUCH trial. Clin Ther. 2006;28(10):1619–1629.
- Kivitz A, Segurado OG. HUMIRA® Pen: a novel autoinjection device for subcutaneous injection of the fully human monoclonal antibody adalimumab. Expert Rev Med Devices. 2007;4(2):109–116.
- Denys H, Martinez-Mena CL, Martens MT, et al. Safety and tolerability of subcutaneous trastuzumab at home administration, results of the phase IIIb open-label BELIS study in HER2-positive early breast cancer. Breast Cancer Res Treat. 2020;181(1):97–105.
- Zambetti M, Montemurro F, Morandi P, et al. Safety profile of subcutaneous trastuzumab for the treatment of patients with HER2-positive early or locally advanced breast cancer: primary analysis of the SCHEARLY study. Eur J Cancer. 2018;105:61–70.
- Woodward N, De Boer RH, Redfern A, et al. Results from the first multicenter, open-label, phase IIIb study investigating the combination of pertuzumab with subcutaneous trastuzumab and a taxane in patients with HER2-positive metastatic breast cancer (SAPPHIRE). Clin Breast Cancer. 2019;19(3):216–224.
- Lugtenburg P, Avivi I, Berenschot H, et al. Efficacy and safety of subcutaneous and intravenous rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in first-line diffuse large B-cell lymphoma: the randomized MabEase study. Haematologica. 2017;102(11):1913–1922.
- García-Muñoz R, Quero C, Pérez-Persona E, et al. Safety of switching from intravenous to subcutaneous rituximab during first-line treatment of patients with non-Hodgkin lymphoma: the Spanish population of the MabRella study. Br J Haematol. 2020;188(5):661–673.
- Yocum RC, Kennard D, Heiner LS. Assessment and implication of the allergic sensitivity to a single dose of recombinant human hyaluronidase injection: a double-blind, placebo-controlled clinical trial. J Infus Nurs. 2007;30(5):293–299.
- Bittner B, Richter W, Schmidt J. Subcutaneous administration of biotherapeutics: an overview of current challenges and opportunities. BioDrugs. 2018;32(5):425–440.
- Jackisch C, Kim SB, Semiglazov V, et al. Subcutaneous versus intravenous formulation of trastuzumab for HER2-positive early breast cancer: updated results from the phase III HannaH study. Ann Oncol. 2015;26(2):320–325.
- Gligorov J, Ataseven B, Verrill M, et al. Safety and tolerability of subcutaneous trastuzumab for the adjuvant treatment of human epidermal growth factor receptor 2-positive early breast cancer: safeHer phase III study’s primary analysis of 2573 patients. Eur J Cancer. 2017;82:237–246.
- Rosengren S, Souratha J, Conway D, et al. Recombinant human PH20: baseline analysis of the reactive antibody prevalence in the general population using healthy subjects. BioDrugs. 2018;32(1):83–89.
- Rosengren S, Dychter SS, Printz MA, et al. Clinical immunogenicity of rHuPH20, a hyaluronidase enabling subcutaneous drug administration. AAPS J. 2015;17(5):1144–1156.
- Baxter Healthcare Corporation, Baxter BioScience. HyQvia: immune globulin infusion 10% (human) with recombinant human hyaluronidase, blood products advisory committee briefing book 2014 [cited 2020 Nov 18]. Available from: https://wayback.archive-it.org/7993/20170113014523/http:/www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/BloodProductsAdvisoryCommittee/UCM407013.pdf.
- Janssen Biotech Inc. Darzalex prescribing information for intravenous use 2019 [cited 2020 Jan 22]. Available from: http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/DARZALEX-pi.pdf.
- Pirrello RD, Ting CC, Thomas SH. Initial experiences with subcutaneous recombinant human hyaluronidase. J Palliat Med. 2007;10(4):861–864.
- Burcombe R, Chan S, Simcock R, et al. Subcutaneous trastuzumab (Herceptin®): a UK time and motion study in comparison with intravenous formulation for the treatment of patients with HER2-positive early breast cancer. Adv Breast Cancer Res. 2013;2(4):133–140.
- Pivot X, Gligorov J, Muller V, et al. Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013;14(10):962–970.
- De Cock E, Kritikou P, Sandoval M, et al. Time savings with rituximab subcutaneous injection versus rituximab intravenous infusion: a time and motion study in eight countries. PLoS One. 2016;11(6):e0157957.
- De Cock E, Pivot X, Hauser N, et al. A time and motion study of subcutaneous versus intravenous trastuzumab in patients with HER2-positive early breast cancer. Cancer Med. 2016;5(3):389–397.
- Rummel M, Kim TM, Aversa F, et al. Preference for subcutaneous or intravenous administration of rituximab among patients with untreated CD20+ diffuse large B-cell lymphoma or follicular lymphoma: results from a prospective, randomized, open-label, crossover study (PrefMab). Ann Oncol. 2017;28(4):836–842.
- Wright JM, Jones GB. Developing the subcutaneous drug delivery route. Med Res Arch. 2017;5:1652.
- Shi GH, Connor RJ, Collins DS, et al. Subcutaneous injection performance in yucatan miniature pigs with and without human hyaluronidase and auto-injector tolerability in humans. AAPS PharmSciTech. 2021;22(1):39.