ABSTRACT
Introduction
Disease due to pulmonary infection with Aspergillus, and other emerging opportunistic fungi remains a significant unmet need. Existing antifungal medicines are predominantly dosed either orally or systemically, but because of limited exposure to the lung lumen, adverse events, and problematic drug-drug interactions, inhaled treatment could provide an attractive option.
Area covered
This review summarizes 1) the limitations of current antifungal therapy, 2) the beneficial effects of inhaled antifungal agents, 3) the clinical development of inhaled antifungal triazoles (repurposed with an innovative inhalation system or a novel inhaled agent) for the treatment of pulmonary fungal infections, and 4) the difficulties and challenges of inhaled antifungal agent development. Regrettably, details of novel inhaled devices or formulations were not covered.
Expert opinion
Inhaled antifungal treatment could provide an attractive option by shifting the risk benefit ratio of treatment favorably. Preclinical and clinical studies with inhaled antifungal agents (off-label use) are encouraging so far. New inhaled antifungal triazoles are well tolerated in early clinical studies and warrant further clinical development. However, challenges remain and many unaddressed issues including required preclinical studies, appropriate clinical design, pharmacokinetics, delivery system(s) and regulatory process need to be resolved. Early communication with regulatory authorities is therefore recommended.
1. Introduction
The incidence of fungal infections has increased substantially over the past two decades. Currently, over 300 million people are afflicted with a serious fungal infection and the annual mortality associated with fungal infections is more than 1.6 million worldwide [Citation1]. For pulmonary mycoses, invasive forms are associated with high morbidity and mortality rates [Citation2]. Those who are non-immunocompetent or have impaired lung function such as patients who suffer from Coronavirus disease 2019 (COVID19), influenza, human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS), tuberculosis (TB), cancers, chronic obstructive pulmonary disease (COPD), and asthma are at high risk of fungal infection [Citation3,Citation4]. The primary cause of pulmonary fungal infections consists of several fungal species including Aspergillus spp., Mucor spp., Fusarium spp., Scedosporium spp., Blastomyces dermatitidis, Coccidioides spp., Cryptococcus spp., Histoplasma spp., Paracoccidioides brasiliensis, and Pneumocystis jirovecii [Citation5–7]. This review will largely focus on cases of Aspergillus spp.
Pulmonary aspergillosis is an angio-invasive and tissue-invasive conditions. A further sub-division is used to characterize patients who have an allergic component (known as allergic bronchopulmonary aspergillosis [ABPA]) compared with those who do not [Citation8]. The risk factors for invasive pulmonary aspergillosis (IPA) may be exposure to immunosuppressive medicines or intubation in an intensive care unit. Alternatively, chronic colonization might happen after a previous infection with tuberculosis [Citation9]. Chronic lung infections with Aspergillus can leave patients with extensive and permanent lung damage, requiring lifetime treatment with antifungal drugs [Citation10]. There is also accumulating evidence that Aspergillus infection may play an important role in clinical COPD [Citation11] and asthma [Citation12]. In addition, associations between the presence of Aspergillus spp. or Candida spp. in the sputum, and worsened lung function were shown in cross-sectional studies [Citation13,Citation14]. Furthermore, 69% of sputum samples from cystic fibrosis (CF) adult patients showed chronic colonization with Candida and 60% showed colonization with Aspergillus. In these patients, Aspergillus but not Candida sensitization was reported to be associated with greater decline of lung function as well as frequent pulmonary exacerbations [Citation15].
Current antifungal azole agents are predominantly dosed either systemically or orally. The routes of delivery are often found to be poor for treating airway infections, as drug concentrations tend to be lower at the site of infection than those in other organs including the liver, kidney, and brain. The liver in particular is known to be a site of triazole toxicity [Citation16] as well as the site exhibiting significant drug-drug interactions (DDIs) due to the inhibition of hepatic P450 enzymes [Citation17,Citation18]. Isavuconazole, a recently approved triazole, offers several advantages over other antifungal agents, including a better adverse event profile with respect to hepatotoxicity, neuro-visual toxicity, QTc prolongation, as well as a stable pharmacokinetic (PK) profile obviating the need for therapeutic drug monitoring. However, this still potentially affects activity of CYP3A4 CYP2C8, 2C9, 2C19, and 2D6. Sub-optimum exposure of antifungal agents can cause the induction of resistant mutants to antifungal agents, consequently leading to therapeutic failure. Therefore, due to the limited exposure of the lung lumen (inadequate local bioavailability), several adverse effects and problematic DDIs, which are associated with current systemic triazole therapy, inhaled treatment route would be an attractive option for favorable risk benefit ratio. Currently, several systemic antifungal agents are in clinical trials for invasive fungal infections [Citation19,Citation20], but inhaled administration is an underutilized delivery route because the drug discovery, formulation and delivery system development process are viewed as challenging, risky, and expensive. Therefore, currently, their clinical utility of inhaled medicine is limited to conditions such as asthma, COPD, and CF only. However, unmet medical need continues to grow, and a significant opportunity exists for the development of inhaled antifungals to treat a range of life-threatening or chronic pulmonary diseases. Several new inhalable antifungal agents are currently in clinical development. New inhaled devices and novel formulations are key factors for successful treatment strategy, and those were well summarized by published review articles [Citation21,Citation22].
2. Fungal infection in the lung
The lungs are one of the most common sites that can be infected by a variety of microorganisms including viruses, bacteria, viruses, fungi and parasites. Fungi are important causes of pulmonary diseases, as fungal spores exist ubiquitously in the environment and the small size of fungus spores can be inhaled and easily reach the distal airways [Citation23]. These small diameter (3–5 µm) conidia are usually cleared by the local and systemic innate defense mechanisms in healthy subjects. However, when a substantial amount of the conidia or the fungi spores are inhaled and the exposure is repeated, the innate defenses of individuals become overwhelmed, especially in patients who are non-immunocompetent. In addition, inhaled spores can persist and spread easily within the airways of patients exhibiting impaired mucociliary clearance and/or macrophage/neutrophil function [Citation24]. Aspergillus is a thermotolerant species which can germinate and colonize the lower conducting airways, becoming a persistent allergen source due to its cell wall constituents and proteases [Citation25] (). In addition, fungi can generate an extracellular matrix and can form ‘biofilms’ serving to protect them from the host immune system and to resist antimicrobial killing, allowing them to eventually form a fungal ball (). Fungal colonization will ultimately lead to the development of the disease, associated with local inflammation, pleural thickening, and parenchymal fibrosis. Where immunity is compromised, tissue invasion and dissemination can occur (). As Gago and colleagues mentioned [Citation26], the definition of clinical criteria to evaluate fungal colonization of the respiratory airways differs between experts, but it normally includes the presence of a single or serial positive tests for the detection of fungal species in a lower airways clinical sample such as sputum or bronchoalveolar lavage fluid (BALF). Thus, fungi can cause and complicate lung disease. Despite the use of antifungal treatment, morbidity, and mortality due to IPA remains significantly high among immune-suppressed and critically ill patients requiring admission to intensive care, post-COVID19 or influenza infections or following high dose of steroids [Citation27–29].
Figure 1. Schematic illustration of the fate of inhaled Aspergillus conidia, and opportunity of inhaled antifungal therapy. Inhaled conidia or germinated hypha are often cleared up by resident macrophages or neutrophils. Survived hypha is branching and grows, forming biofilm. On some occasions, fungus forms fungal ball in lung lumen, and in immunocompromised condition, fungus invades systemically. There are different treatment options/regimen at different infection stage.
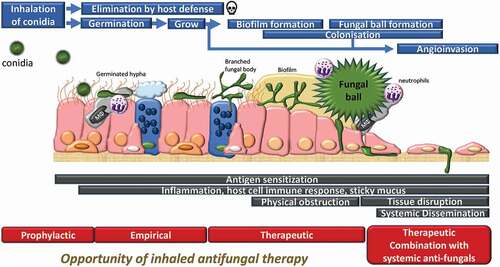
In a meta-analysis with 24 articles for antifungal prophylaxis in lung transplant recipients, antifungal prophylaxis did not significantly reduce the risk of Aspergillus colonization or disease compared to those who did not receive prophylaxis although they have insufficient evidence to support or exclude a benefit of antifungal prophylaxis [Citation30]. As well as Aspergillus spp., Mucor and Rhizopus infection causes detrimental effects on the airway. For example, they secrete ricin-like peptides (mucoricin) that cause extensive tissue damage [Citation6]. These organisms are notorious for causing upper airway disease (sinusitis) as well, but it is believed that this site is highly amenable to inhaled antifungals.
Fungi also play an important role in allergic airway disease. ABPA is a well described but relatively uncommon endotype of severe asthma. Also, up to 70% of patients with severe asthma are reported to be sensitized to different fungi [Citation31]. This heterogeneous population has been termed ‘severe asthma with fungal sensitisation’ (SAFS) [Citation8]. The key allergen is suggested to be a product of active metabolism, a protease [Citation32], which is continuously produced by colonized live fungus in the airways which induces an immuno-inflammatory response. Therefore, these are amenable by an appropriate antifungal agent. For antifungal agents to have potential to treat allergic diseases induced by Aspergillus, the key allergens must depend upon the fungus being alive. In the case of Aspergillus, in preclinical studies, Namvar and colleagues [Citation33] provided evidence that protease activity made a significant contribution to the development of airway inflammation and remodeling in mice. Others have reported that inhalation of live A. fumigatus spores to the sensitized airways of BALB/c mice led to consistent development of fibrosis and smooth muscle changes [Citation34]. More recently, Druey and colleagues [Citation35] have suggested that Aspergillus fumigatus protease alkaline protease 1 (Alp1) is a credible therapeutic target for treatment of fungal asthma. Other reports suggest that the host immunological response to Aspergillus may be complex. The secondary metabolite, gliotoxin, has been reported to stimulate IgE-independent degranulation of mast cells [Citation36], while chitin, a component of the fungal cell wall, may act as an adjuvant in a murine model of A. fumigatus protease induced allergy [Citation37]. As well as Aspergillus, other fungi including Candida spp, Penicillium and Rhizopus cause a hypersensitivity-mediated disease [Citation38]. Thus, (inhaled) antifungal treatment will be beneficial for fungal allergen-sensitized diseases by eliminating live fungus, which causes the antigenic stimulus as well as inflammatory response and Th2 immune activity.
3. Limitations of current antifungal agents in the treatment of pulmonary fungal infection
The antifungal agents approved by United States Food and Drug Administration (FDA) and potentially used for treatment of aspergillosis are liposomal Amphotericin B (AmB), AmB lipid complex (ABLC), voriconazole, itraconazole, posaconazole, isavuconazole, caspofungin, and micafungin. Terbinafine is often used with triazoles as a synergistic combination. Currently available systemic antifungal azole therapies have important limitations when treating airway infections in this way. Tissue penetration of antifungal agents is well summarized by the review article by Fenton et al, and there are big variabilities of lung concentrations between reports. This lack of penetration can lead to sub-optimum level of antifungal agents at the site of infection due to poor solubility, poor permeability, and rapid clearance. Hepatic and cardiac toxicity are also common with the azoles [Citation39] and systemic AmB treatments has the risk of nephrotoxicity. DDIs are also a significant challenge, particularly for the azoles, for lung transplant recipients, patients undergoing treatment for acute lymphoblastic/lymphocytic/lymphoid leukemia (ALL) or patients with acute myeloid leukemia (AML), when treated with calcineurin, vincristine or checkpoint inhibitors [Citation40]. In CF patients, ivacaftor interacts with azole antifungals, such as voriconazole or posaconazole, causing clinical challenge and frustration when the treatment is required for ABPA or fungal infection [Citation41]. Corticosteroids are also a standard therapy for ABPA, but corticosteroid in combination with itraconazole is reported to result in adrenal suppression in both asthma and CF patients with ABPA, because metabolic clearance of corticosteroids is disturbed by itraconazole via inhibition of the hepatic cytochrome P450 3A4 [Citation42]. Adrenal suppression is also reported to occur due to triazole therapy whether on a corticosteroid or not. Although DDIs occur less frequently with echinocandins, these are not recommended as monotherapy for the primary treatment of IPA due to their relatively modest inherent antifungal effects [Citation43].
As a result of these limitations, on top of other issues such as antifungal resistance, shorter local residency and lack of fungicidal activity of most antifungals, current rates of response to treatment of IPA are low (≤50%) and pulmonary fungal infection remains an important cause of morbidity and mortality. There is therefore a critical unmet need for improved antifungal agents for the treatment of pulmonary fungus infection. Thus, the idea of delivering a high concentration of antifungal agent directly to the airway (the infected area) with limited systemic exposure, avoiding systemic toxicity is appealing.
4. Potential beneficial effects of inhaled antifungal agents in pulmonary fungal infections
Over the past decade the use of inhaled anti-infectives for bacterial infection has become standard of care, for example, inhaled tobramycin for the treatment of Pseudomonas infection in patients with CF [Citation44], and inhaled amikacin for the treatment of atypical mycobacterial pulmonary infections [Citation45]. However, there is no inhaled antifungal agent formally approved by the FDA although inhaled AmB is widely used. As mentioned above, delivery of inhaled agents for the treatment of pulmonary fungal infections has many advantages, including avoiding systematic side effects, avoiding unnecessary DDIs and sustained high local concentrations continuously in the airways. These all would lead to higher antifungal efficacy and minimize the risk of emergence of resistance. However, currently there is limited evidence of the beneficial effects of inhaled antifungals in either preclinical studies () or clinical studies of off-label use of intravenous (IV) formulations (). There are however overall encouraging results from these studies which warrant further evaluation.
Table 1. Preclinical evidence of beneficial effects of inhaled antifungals.
Table 2. Clinical evidence of beneficial effects of inhaled antifungals by off-label use.
AmB is an antifungal agent which is being extensively explored for inhaled delivery (). However, in general, compliance with nebulized AmB therapy is poor as nebulization causes airway irritation, coughing, nausea and vomiting. Patients find it particularly unpleasant to inhale due to its smell and taste [Citation66]. For example, Chishimba and colleagues demonstrated that inhaled AmB is reasonably tolerated in lung transplant patients with limited interaction with immunosuppressive drugs, although it is reported to cause bronchospasm in severe asthmatics [Citation67]. However, PKs and safety issue seemed to be improved by liposomal formulation of AmB [Citation68].
In addition, nebulized voriconazole has been reported to produce improved therapeutic outcomes in preclinical in vivo studies and patients (). For example, Aspergillus fumigatus-infected mice treatment with aerosolized voriconazole has significant survival advantages as compared to treatment with AmB. The inhalation of aerosolized formulation was found to be well tolerated with the absence of lung injury or inflammatory changes based on histological findings in uninfected mice [Citation69]. Hilberg and colleagues presented three cases of inhaled voriconazole solution treatment in patients with life-threatening IPA, who previously had systemic voriconazole treatment (which had been withdrawn due to unacceptable adverse effects and as no other conventional treatment options were available) [Citation70]. The report showed that inhaled voriconazole was safer and exhibited better therapeutic effectiveness compared with oral administration. However, as reflected by systemic concentrations, it was absorbed quickly because voriconazole is originally designed to be rapidly and extensively absorbed from the gut, and therefore unlikely to provide sustained exposure in the lung and airways [Citation70,Citation71]. Furthermore, inhaled voriconazole was also tested on severe pulmonary infection with Scedosporium apiospermum in an adolescent with CF and confirmed to be safe and effective [Citation72]. Inhaled itraconazole is another triazole tested to treat Aspergillus infection. McConville and colleagues demonstrated that single dose nebulized itraconazole maintained high concentrations in the lungs of mice for more than 24 h [Citation73] with confirmation in rats [Citation74]. Furthermore the survival rates of mice receiving nebulized itraconazole were significantly higher than the mice given itraconazole orally [Citation75]. Curran and colleagues reported the beneficial effects of inhaled itraconazole in Aspergillus-infected Guinea pigs [Citation76].
Thus, commercially available IV formulations of antifungal agents can be aerosolized for nebulization and the reported data encouraged further studies with the nebulized IV formulations (). However, both voriconazole and AmB have a very short duration of action in the lungs (short lung residence times) and require high doses and frequent administration, and therefore further PK studies are required to ensure the efficacy and safety of inhaled antifungal agents.
To overcome the limitations associated with IV formulation, and to meet the requirements for an inhaled antifungal agent, a significant investment in delivery system/formulation development has been made. This includes dry powder inhalers (DPIs), improved nebulized formulations or the utilization of nanocarriers, for improving efficacy, duration of action, stability, aqueous solubility, PK profiles, and penetration through tissues as well as safety profile. One of the drug delivery systems, DPI, has several advantages in drug delivery to the lungs [Citation77], which include high-dose delivery, propellant-free delivery, portability, ease of operation as well as relatively lower-cost devices. The powder blends in DPI are usually a mixture of micronized drug with coarse carrier particles such as lactose, mannitol and sucrose or drug particles encapsulated in nanocarrier particles. An important factor to determine the deposition and exposure is a particle size [Citation78]. As Aspergillus spores potentially penetrate deep into the lungs, the inhaled antifungal drug particles should be smaller than 5 µm (or 1–3 µm) to ensure deposition in smaller airways. For example, AmB-loaded DPI formulations with cholesteryl carbonate esters, achieving 3.8 µm particle size showed 2–4 times greater in vitro antifungal activity against Cryptococcus neoformans and Candida albicans compared to intact AmB. A dry powder formulation or surface-active hybrid nanoparticles of voriconazole was also developed for treatment of respiratory fungal infection [Citation79,Citation80]. New formulations of itraconazole for topical treatment such as nanoparticles [Citation81], polymeric micelles [Citation82], itraconazole-loaded nanostructured lipid carrier [Citation83] and dry powder [Citation84] were also explored. The milling behavior of voriconazole subjected to particle size reduction using air jet mill was recently investigated [Citation85]. However, the in vivo antifungal activity of these inhaled products has not been reported formally in preclinical studies or clinical studies.
5. Currently developing inhaled antifungal agents
Currently, two approaches are taken for developing inhaled antifungals. One is a repurposed approach with innovative delivery system or formulations, which includes inhaled forms of itraconazole (PUR1900 [Pulmatrix Inc.]) and voriconazole (ZP059 [Zambon group], TFF-VORI [TFF Pharmaceuticals]). Another approach is to develop compounds fully optimized for inhaled route, such as opelconazole (PC-945, Pulmocide Ltd.). [Summarized in ].
Table 3. Novel inhaled antifungals in clinical phase.
5.1. Repurposed antifungals with innovative delivery system
There are 3 repurposed drugs with innovative delivery systems or formulation in clinical development. Firstly, PUR1900 (PulmazoleTM), currently under development byPulmatrix Inc. (Lexington, MA, USA), is a dry powder formulation of itraconazole formulated in a particle engineered dry powder platform called iSPERSE [Inhaled small particles easily respirable and emitted], which allows highly efficient delivery of high drug loads to the lungs via inhalation with a small aerodynamic particle size. Preclinical study with aerosolized investigational formulation of itraconazole have demonstrated higher lung exposure and lower systemic exposure than oral dosing in rats [Citation86] and higher antifungal activity than oral treatment in A. fumigatus infected Guinea pigs [Citation76]. The product has undergone Phase 1 clinical trials [Citation87] and was found to be safe and well‐tolerated under study conditions. After 14 days, PUR1900 resulted in 106‐ to 400‐fold lower plasma exposure across inhaled doses tested (10–35 mg) than steady‐state exposure reported for oral Sporanox 200 mg. In asthmatics, PUR1900 maximum sputum concentrations were 70‐fold higher and mean plasma concentrations were 66‐fold lower than with oral Sporanox. Thus, PK data indicate that PUR1900 results in higher and more sustained lung exposure of itraconazole relative to oral dosingThe results of this Phase 1 study supported the advancement of PUR1900 into the next clinical study in patients with ABPA (ClinicalTrials.gov Identifier: NCT03960606), but it was halted due to the COVID-19 pandemic. TFF Pharmaceuticals Inc. is developing an inhaled voriconazole DPI formulation using the thin film freezing (TFF) method which is used in producing DPI formulations rather than the spray-drying method. Beinborn et al., demonstrated that the microstructured crystalline TFF-voriconazole formulation showed higher area under the dissolution curve, higher fine particle fraction (FPF) (37.8% to 32.4%) and smaller median mass aerodynamic diameter (MMAD)(4.2 µm to 5.2 µm), and also higher lung bioavailability of voriconazole, and longer lung retention in mice [Citation88]. Thus, the microstructured crystalline TFF-voriconazole formulation can achieve high and prolonged concentrations in the lungs with low systemic bioavailability and may potentially be more favorable for pulmonary delivery of the antifungal agent. The phase 1 study (TFF-V1-001) demonstrated that voriconazole inhalation powder at the 80 mg dose level (Multiple dose) was well tolerated (ClinicalTrials.gov Identifier: NCT04872231, NCT04576325).
Zambon SpA is also developing a dry powder formulation of voriconazole (ZP059) using an innovative Edry® spray drying technology [Citation89] for ABPA and the Phase 1 study has been completed (ClinicalTrials.gov Identifier: NTC04229303) although no results have been reported to date.
5.2. The compound fully optimized for inhalation treatment
Early indication of the preclinical or off-label use clinical efficacy shown by known antifungal agents suggests that considerable scope remains for the discovery of bespoke, potent antifungal agents which exhibit optimal characteristics for inhaled delivery. To date, only one antifungal agent fully optimized for inhaled delivery is reported. Opelconazole (PC945, Pulmocide Ltd.) is the first inhaled antifungal triazole, specifically designed to deliver high local concentrations, longer cell retention (offering a longer duration of action), minimal systemic exposure with poor oral availability and high protein plasma binding (to minimize the systemic free drug concentration) [Citation90]. This profile might result in minimal adverse effects, coupled with the potential to eradicate IPA due to high concentrations in the lungs as well as minimizing induction of resistant mutants. Opelconazole had no safety concerns in vitro or in vivo preclinical studies [Citation90], including; human embryonic kidney cells stably transfected with the human ether-a-go-go related gene (hERG), adult toxicology studies (inhaled delivery) in 14-day and 13-week studies in rats and dogs, reproductive performance and embryo-fetal development studies in rats and rabbits [Citation90].
Opelconazole has been shown to have persistent action on epithelial cells and hypha in vitro, and intranasally treated opelconazole showed superior antifungal activity in A. fumigatus infected mice to either intranasally treated voriconazole or posaconazole [Citation91,Citation92]. Antifungal effects against Aspergillus and Candida accumulate on repeat dosing (extended prophylactic treatment) [Citation92], and the observation was repeated in vitro using the human alveolus bilayer cell model [Citation93]. In addition, improved efficacy has been demonstrated when opelconazole is dosed in combination with systemic antifungal agents. In fact, a case report describing the successful use of opelconazole to treat a refractory Aspergillus bronchial anastomotic infection and tracheobronchitis in a lung transplant patient on top of standard care, without any compound-related adverse event has recently been published [Citation94]. In the First in Human (FIH) study (ClinicalTrials.gov Identifier: NCT02715570), opelconazole treatment was found to be well tolerated in both healthy subjects and subjects with mild asthma and, importantly for the inhaled route, no clinically significant lung function changes (defined as >15% change from baseline), nor evidence of acute bronchospasm/irritancy, were observed [Citation95]. The PK profile was translated from nonclinical species to humans, showing slow absorption from lungs and low systemic exposure [Citation95], thereby limiting the potential for adverse side effects and drug interactions commonly seen with systemically delivered azoles. Opelconazole has been studied in patients with asthma and CF (ClinicalTrials.gov Identifier: NCT03745196, NCT03870841) although those studies were halted due to the COVID-19 pandemic. Currently, phase 2 clinical trials with lung transplant patients are ongoing in the United States (NCT05037851).
6. Difficulties and challenges in the development of inhaled antifungal agents
Although over the past decade the use of inhaled anti-infectives for bacterial infection has become standard of care [Citation44], no inhaled antifungal agents are fully developed. Therefore, many unknown factors, unaddressed issues and challenges remain for the development of inhaled antifungal agents. Recently, the FDA organized a workshop entitled ‘Addressing Challenges in Inhaled Antifungal Drug Development,’ to discuss the challenges and clinical trial design considerations for developing inhaled antifungal drugs [Citation96].
6.1. Preclinical work
For preclinical pharmacology, the development of novel antifungal agents relies heavily on broth microdilution susceptibility testing (Minimum inhibitory concentration [MIC] assay). This system is useful to understand the potency against particular fungal species but does not guarantee adequate potency in relevant cells and is not an appropriate model to understand the advantage of inhaled treatment route. For example, to understand the beneficial effects of a combination treatment of an inhaled antifungal agent and a systemic antifungal treatment, usually the additive or synergic effects of the combination treatment of the two drugs are tested by a MIC assay, which ignores treatment route, and is therefore not appropriate to investigate the benefits of inhaled therapy. To differentiate treatment routes double chamber culture systems are useful (). One option is air-liquid interface (ALI) cultured pseudostratified nasal, bronchial and small airway epithelium. The characteristic apical facing surface of ALI cultures allows for direct application of topical agents, replicating topical delivery in vivo. Furthermore, as the cells are highly differentiated with modest turn-over (unlike the aggressive replication of cancer cell lines), the epithelium is long lasting and more similar to what is found in humans. Fungus infection was demonstrated in ALI epithelium [Citation97], but no pharmacological intervention studies have been reported. Another well-established cell system is a bilayer alveolus model, originally reported by Gregson and colleagues [Citation98] . These double chamber systems enable to detect local fungus infection (apical side) and invasion through epithelium (basolateral side). This has been utilized to evaluate pharmacodynamics (PD) (antifungal), potential local PK and also PK-PD relationship for systemic antifungal agents. The antifungal effects of apically treated opelconazole were also evaluated, which was more potent than posaconazole and voriconazole and showed minimum exposure to basolateral chamber (mimicking systemic exposure) [Citation93]. In addition, combination benefits of apically treated opelconazole and basolateral treated posaconazole, voriconazole, itraconazole or echinocandins were demonstrated [Citation93,Citation99]. Furthermore, cells from subjects with diseases such as asthma, COPD or CF, which potentially produce more mucus or where host defense is impaired, should be taken into account and investigated further.
Figure 2. Preclinical in vitro assay method for inhaled antifungals. a) air-liquid interface cultured pseudostratified epithelium (nasal, bronchial, small airway). b) bilayer alveolus model [Citation98]. Black freeform scribble: fungus body. c) A. fumigatus infection in bilayer (apical phase). Histology of alveolus model d) non-infected, e) 48 h post infection with A. fumigatus conidia. Solid arrow: Conidial head and conidiophore, Dotted arrow: hyphae and foot cell, White arrow: original endothelial and epithelial cell bilayer.
![Figure 2. Preclinical in vitro assay method for inhaled antifungals. a) air-liquid interface cultured pseudostratified epithelium (nasal, bronchial, small airway). b) bilayer alveolus model [Citation98]. Black freeform scribble: fungus body. c) A. fumigatus infection in bilayer (apical phase). Histology of alveolus model d) non-infected, e) 48 h post infection with A. fumigatus conidia. Solid arrow: Conidial head and conidiophore, Dotted arrow: hyphae and foot cell, White arrow: original endothelial and epithelial cell bilayer.](/cms/asset/36058f16-4e7e-4ee2-b37a-f3b377158080/iedd_a_2084530_f0002_oc.jpg)
For preclinical in vivo pharmacological studies, the challenge is mimicking the inhalation treatment in patients. Even when the intended route of treatment in clinical study was inhalation of the nebulized suspension, occasionally, antifungal agents were administered intranasally or intra-tracheally to avoid any loss in animals. If the inhalation route is used, nostril size and tidal volume are different between humans and animals, and the exposure (and potential loss) cannot be standardized. In addition, the exposure level varies in different exposure systems, such as whole-body aerosol exposure box versus directly connected to nose via a mask. In early preclinical pharmacology studies, clinical inhalation formulations are not often used. Thus, the absolute amount exposed to the lung will differ due to different treatment methods and formulations, and due to inter-animal differences. The use of radiolabelled compound would provide insight into lung exposure in individual animals.
Based on standard PK approaches in clinics, lung exposure after inhalation is predicted based on plasma time-concentration profiles observed in animal PK studies. Therefore, the PK profiles in the target organ (lung) and plasma should be well established in two or more animal species. However, as limited systemic exposure is expected, for a fully optimized inhaled molecule, an ultra-sensitive drug assay method to measure the extremely low plasma concentrations (possibly pg/mL) following inhaled administration is required. This is an important consideration since the assays take time to establish and can be relatively expensive. After inhalation of aerosolized agents, approximately 40–90% of the administered dose can be swallowed and potentially absorbed from the gastro-intestinal tract. For a fully optimized inhaled molecule exhibiting little or no oral availability, oral absorption will not contribute to therapeutic efficacy, but care should be taken when orally active compounds are repurposed.
To measure lung deposition, epithelium lining fluid (ELF) is usually used to understand the exposure, however lipophilic compounds are potentially deposited in lung resident cells such as epithelium and macrophages. ELF is not necessary to predict compound exposure of antifungal agents. If the aqueous solubility of an antifungal agent is low and its rate of dissolution is slow, it is highly likely that solid particles are deposited in the lung after inhalation of the aqueous suspension. Consequently, it is not possible to differentiate between dissolved and solid antifungal agent during ELF (or BAL) concentration measurements. Thus, failing to differentiate dissolved from undissolved compound means that the total drug concentrations measured will not be accurate estimates of physiological concentrations. Therefore, care should be taken for interpreting the data in ELF after inhalation.
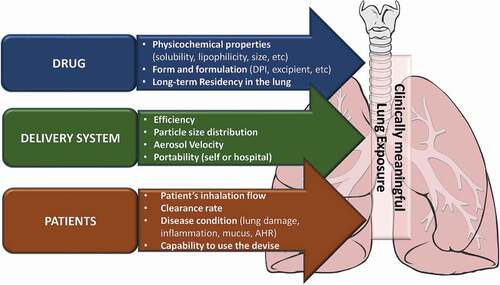
Another important factor to affect compound exposure is patient disease status (). Compounds are potentially taken into fungus cells in the lungs, but also into nonresident inflammatory cells such as macrophages and neutrophils. Both posaconazole and opelconazole are known to accumulate in neutrophils and macrophages [Citation100,Citation101]. Biofilms formed by recurrent infections and mucus production stimulated by infection/inflammation are also factors affecting local exposure levels of antifungal agents when inhaled. In fact, Aspergillus infected mice showed slightly different exposure levels of opelconazole when compared with intact mice. This might be due to different cell components in the samples as opelconazole was found to show a better PK-PD relationship when PK was normalized to cell numbers [Citation101]. After A. fumigatus infection, the number and type of cells present in the lung change over time, and therefore, further correction of concentrations with cell number based on cell type is technically challenging. There is no information that any antifungal agent preferentially accumulates in certain cells, so further studies are required. Despite these challenges, developing an appropriate PK/PD model would be useful to predict the best dosage regimen for achieving adequate protection against fungal complications to aid clinical development.
Figure 3. Factors affecting lung exposure and distribution of inhaled-antifungal agent. AHR: airway hyperresponsiveness.
Preclinical toxicology for an inhaled drug is also challenging. Limited expertise, relevant facilities and assay services are available. There is, however, useful guidance published by the FDA (Nonclinical Safety Evaluation of Reformulated Drug Products and Products Intended for Administration by an Alternate Route Guidance for Industry and Review Staff by FDA) [Citation102]. These toxicology studies are suggested to consist of short-term studies (2 to 4 weeks) in two species (at least one non-rodent) and up to a 6- month study in the most appropriate species with the inhaled formulation. No matter if the drug being tested for inhalation treatment is repurposed for inhalation use or a newly created substance or formulations, the appropriate inhalation toxicity studies should be conducted. Estimation of exposure level (or pulmonary deposited dose) is also challenging, furthermore particle size, aerosol drug concentration, respiratory minute volume (L/min), duration of inhalation, and deposition factor (which is different in different animals) should be taken into consideration (). Furthermore, infected animal model can be used to enhance the predictive ability of toxicity testing in these agents, which are not required for regulatory submission at this time.
6.2. Early clinical studies
6.2.1. Target population
Patient population is often very heterogeneous regarding severity of illness, underlying patient characteristics (comorbidities) and microbiologic etiology. The different presentations of pulmonary fungal infections, for instance, classical IPF, necrotizing aspergillus pneumonia, etc. will be differently affected by inhaled antifungal therapy. Incidences of fungal infections are relatively low, and there can be limited cases of certain target population such as lung transplant and ABPA cohorts. In addition, little or no (large) natural history nor interventional studies have been reported in pulmonary fungal disease in general.
6.2.2. Dosing period and dosing
The expected treatment duration for the eradication of fungus by antifungal agents is generally longer ranging from 6 weeks to more than 12 months, although it depends on fungus species, the severity of the illness, and the immune status of the patient. Optimal duration of therapy and length of follow up to determine the treatment benefit is not clear. Dose-Response and/or Dose-Finding should be tested in the Phase 2 drug development programmes. Several factors will affect lung deposition site and total exposure, such as the drug’s physico-chemical properties, delivery system (particle size, velocity etc) and patient matter (lung function, inflammatory condition etc) (). To-be-marketed inhaled drug formulations and associated medical devices need to be used in Phase 2/3 clinical trials. Moreover, medical devices need to be safe and effective, and components such as spacers, holding chambers, facemasks, mouthpieces etc need to be subject to strict regulation.
6.2.3. Endpoint
A clinically meaningful endpoint (for example, mortality, lung function, symptom score, number/severity of exacerbation, time to event) are needed but are difficult to pinpoint. For example, for persistent symptoms related to underlying clinical condition, microbiologic endpoints do not necessarily correlate with clinical outcomes. A surrogate endpoint such as a laboratory measurement or radiographic image might be useful to predict clinical benefit but are not themselves measures of clinical benefit. For a target engagement study in early clinical trials, microbiologic assessments are still useful, but the goal is likely reduction of burden rather than eradication as technically fungal spores are continuously ubiquitous in the environment (less likely in ICU), so environmental contamination would be an issue in respiratory samples.
For mycology testing, a selection of sample is also important. Blood and BAL can be used to detect galactomannan as Aspergillus systemic invasion or local biomarker. Sputum is also useful as the samples are taken in a noninvasive manner. Usually, very small volume (such as 1 µL) of samples is used for quantification on fungal culture agar, which is the method used for classic clinical bacteria culture testing, but the detection rate is low. Instead, high volume culture is reported to show higher detection rate [Citation103]. Polymerase chain reaction (PCR) is also used for detection although sensitivity and specificity are lower, suggesting a combination with other methods such as colony forming unit (CFU) or galactomannan (GM) is more effective [Citation104].
6.2.4. Exposure to human (Dosing and PK issue)
The exposure calculation will be different when using different inhalation methods and devices (nebulizer, DPI etc), and disease status (vs Healthy evaluated in Phase 1). PK parameters are not directly measured in the lung but estimated. If the relationship between local exposure and plasma level (dose proportionality, consistent elimination half-life) is well established in a preclinical study, lung exposure level in human can be estimated using plasma data. Systemic PK (if measurable), along with in vitro metabolism data, can be used to evaluate potential clinical DDIs.
Direct evaluation of exposure can also be considered, including measurement of compound concentration in sputum, BAL, ELF, BAL cell pellets as well as positron emission tomography (PET), and nuclear magnetic resonance (NMR) spectroscopy. As discussed in the pre-clinical section, evaluation of ELF and alveolar macrophage drug concentrations provide information on drug penetration into the lungs. Interpretation of sputum, ELF, and/or alveolar macrophage antifungal drug concentrations are challenging due to high degree of variability, especially sputum, which is not always reflective of lung target-site of action.
7. Conclusion
The current standard treatment by systemic administration is limited by inadequate local bioavailability and systemic toxic effects. Treatment success rates for pulmonary Aspergillus infections remain disappointingly low, compared with antibiotics against pulmonary bacterial infections. Aspergillus infections in patients with chronic lung diseases including CF, severe asthma and COPD are currently under-diagnosed and under-treated due to the poor risk-benefit profiles of currently available therapies. There is therefore a significant unmet need for an antifungal that can effectively treat pulmonary Aspergillus infections that has a lower risk of significant side effects and drug interactions than currently approved antifungals. Thus, inhalation of antifungals is an attractive approach to overcome these problems, but there are uncertain and unaddressed issues, such as (an Investigational New Drug/Clinical Trial Authorization Application (IND/CTA)-enabling) preclinical and non-clinical study package, clinical design, and respirable antifungal formulations. Although preclinical and clinical studies of nebulized or lung delivered antifungals show promising therapeutic effects with fewer adverse events, there is no consensus on dosage regimen (e.g. dose, duration, frequency, and monotherapy/add-on to systemic antifungals, prophylaxis/empirical/therapeutic). Interest in the potential of inhaled antifungal agents is demonstrated by the FDA’s decision to recently hold a symposium to discuss their clinical development [Citation96]. Currently inhaled antifungal agents in development, which are expected to be available in clinical practice in the next 3–5 years, have the potential to maximize the likelihood of clearing pulmonary fungal infection, while minimizing the risk of either direct toxicity or toxicity/medical complications arising from unwanted DDIs.
8. Expert opinion
The inhaled medicines in regular clinical use today have transformed the lives of millions of patients suffering from respiratory diseases. Inhaled antifungal treatment could provide an attractive option by shifting the risk benefit ratio of treatment favorably as existing oral or systemic antifungal medicines have adverse effects, problematic DDIs and limited exposure of the lung lumen. Inhaled anti-bacterial therapy has been established over the last decade, but no inhaled antifungal therapy has yet been launched. Although clinical studies with inhaled antifungal agents (off-label use) show promising therapeutic effects with fewer adverse effects, there is no consensus regarding the dosage regimen (dosing period, frequency), monotherapy or in conjunction with standard systemic antifungal agents, or treatment regimen (prophylaxis, empirical and therapeutic). On top of dosage regimen, there are a lot of uncertainty and unaddressed issues. Firstly, preclinical and non-clinical study lists specific to inhaled antifungal agents should be established. This includes in vitro translational cell model and in vivo inhaled delivery in healthy and infected animals, helping to predict beneficial effects by inhaled route and to predict human dose. Preclinical inhalation PK is also important to establish the relationship between systemic exposure and lung exposure as well as for bridging to human PK. Secondarily, clinical trials should be carefully and specifically designed for inhaled medicine, including target population (including inclusion and exclusion criteria), dosing regimen (as discussed above), dosing device/equipment and sample size. It is also important to select a clinically meaningful endpoint and not one based solely on a biomarker, laboratory or mycology test. A target engagement clinical study with shorter treatment period is also important. Noninvasive sampling and sensitive/specific biomarker or improved microbiological assay methods are keys to obtain meaningful results with limited number of subjects. For systemic PK, where inhaled drug is expected to have limited systemic exposure, high sensitivity assay systems are also required. Extensive research is being carried out for innovative delivery devices, but which system is the best option for inhaled antifungal therapy is still inconclusive (and also dependent on target population). Although not discussed in this review, novel inhalation devices and formulations would be able to bridge the gap. Because of the uncertainty, it is important to communicate with regulatory authorities such as FDA as early as possible during development through various interactive processes.
Article highlights
Pulmonary fungal infections are associated with high morbidity and mortality and remains a significant unmet need.
Inhaled antifungal treatment could provide an attractive option by shifting the risk benefit ratio of treatment favorably.
Preclinical and clinical (off-label use) studies with aerosolized antifungal agents are encouraging.
There are a lot of challenges and unaddressed issues during development of inhaled antifungals including preclinical package, clinical design, PK, delivery system and regulatory process.
Early communication with authority is recommended.
Four inhaled antifungal triazoles are currently in early phase clinical trials.
Declaration of interest
K Ito serves as a consultant for and is a shareholder of Pulmocide Ltd. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Gaffi L. Global action fund for fungal infections. 2021 [ cited 26 May 2022]; Available from: https://gaffi.org/
- Latge JP, Chamilos G. Aspergillus fumigatus and Aspergillosis in 2019. Clin Microbiol Rev. 2019 Dec 18;33(1):e00140–18.
- Moldoveanu B, Gearhart AM, Jalil BA, et al. Pulmonary Aspergillosis: spectrum of Disease. Am J Med Sci. 2021 Apr;361(4):411–419.
- van de Veerdonk FL, Bruggemann RJM, Vos S, et al. COVID-19-associated Aspergillus tracheobronchitis: the interplay between viral tropism, host defence, and fungal invasion. Lancet Respir Med. 2021 Jul;9(7):795–802.
- Lortholary O, Denning DW, Dupont B. Endemic mycoses: a treatment update. J Antimicrob Chemother. 1999 Mar;43(3):321–331.
- Soliman SSM, Baldin C, Gu Y, et al. Mucoricin is a ricin-like toxin that is critical for the pathogenesis of mucormycosis. Nat Microbiol. 2021 Mar;6(3):313–326.
- Reid G, Lynch JP 3rd, Fishbein MC, et al. Mucormycosis. Semin Respir Crit Care Med. 2020 Feb;41(1):99–114.
- Rapeport WG, Ito K, Denning DW. The role of antifungals in the management of patients with severe asthma. Clin Transl Allergy. 2020 Nov 6;10(1):46.
- Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ. 2011 Dec 01;89(12):864–872.
- Limper AH, Knox KS, Sarosi GA, et al. An official American Thoracic Society statement: treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011 Jan 1;183(1):96–128.
- Bafadhel M, McKenna S, Agbetile J, et al. Aspergillus fumigatus during stable state and exacerbations of COPD. Eur Respir J. 2014 Jan;43(1):64–71.
- Chishimba L, Niven RM, Cooley J, et al. Voriconazole and posaconazole improve asthma severity in allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitization. J Asthma. 2012 May;49(4):423–433.
- Chotirmall SH, O’Donoghue E, Bennett K, et al. Sputum Candida albicans presages FEV(1) decline and hospital-treated exacerbations in cystic fibrosis. Chest. 2010 Nov;138(5):1186–1195.
- Agbetile J, Fairs A, Desai D, et al. Isolation of filamentous fungi from sputum in asthma is associated with reduced post-bronchodilator FEV1. Clinical and Experimental Allergy: Journal of the British Society for Allergy and Clinical Immunology. 2012 May;42(5):782–791.
- Baxter CG, Moore CB, Jones AM, et al. IgE-mediated immune responses and airway detection of Aspergillus and Candida in adult cystic fibrosis. Chest. 2013 May;143(5):1351–1357.
- Kyriakidis I, Tragiannidis A, Munchen S, et al. Clinical hepatotoxicity associated with antifungal agents. Expert Opin Drug Saf. 2017 Feb;16(2):149–165.
- Jeong S, Nguyen PD, Desta Z. Comprehensive in vitro analysis of voriconazole inhibition of eight cytochrome P450 (CYP) enzymes: major effect on CYPs 2B6, 2C9, 2C19, and 3A. Antimicrob Agents Chemother. 2009 Feb;53(2):541–551.
- Bruggemann RJ, Verheggen R, Boerrigter E, et al. Management of drug-drug interactions of targeted therapies for haematological malignancies and triazole antifungal drugs. Lancet Haematol. 2022 Jan;9(1):e58–e72.
- Johnson MD. Antifungals in Clinical Use and the Pipeline. Infect Dis Clin North Am. 2021 Jun;35(2):341–371.
- Waterer G. Advances in anti-fungal therapies. Mycopathologia. 2021 Oct;186(5):665–672.
- Liao Q, Lam JKW. Inhaled Antifungal Agents for the Treatment and Prophylaxis of Pulmonary Mycoses. Curr Pharm Des. 2021;27(12):1453–1468.
- Kaur R, Kaur R, Singh C, et al. Inhalational Drug Delivery in Pulmonary Aspergillosis. Crit Rev Ther Drug Carrier Syst. 2019;36(3):183–217.
- Fairs A, Agbetile J, Bourne M, et al. Isolation of Aspergillus fumigatus from sputum is associated with elevated airborne levels in homes of patients with asthma. Indoor Air. 2013 Aug;23(4):275–284.
- Takazono T, Sheppard DC. Aspergillus in chronic lung disease: modeling what goes on in the airways. Med Mycol. 2017 Jan 1;55(1):39–47.
- Denning DW, Pashley C, Hartl D, et al. Fungal allergy in asthma-state of the art and research needs. Clin Transl Allergy. 2014;4:14.
- Gago S, Denning DW, Bowyer P. Pathophysiological aspects of Aspergillus colonization in disease. Med Mycol. 2019 Apr 1;57(Supplement_2):S219–S27.
- Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24 Suppl 1:e1–e38.
- Thompson IIIGR, Cornely OA, Pappas PG, et al. Invasive Aspergillosis as an Under-recognized Superinfection in COVID-19. Open Forum Infect Dis. 2020;7(7):548.
- Kistemann T, Hüneburg H, Exner M, et al. Role of increased environmental Aspergillus exposure for patients with chronic obstructive pulmonary disease (COPD) treated with corticosteroids in an intensive care unit. Int J Hyg Environ Health. 2002 Feb;204(5–6):347–351.
- Pennington KM, Baqir M, Erwin PJ, et al. Antifungal prophylaxis in lung transplant recipients: a systematic review and meta-analysis. Transpl Infect Dis. 2020 May;25:e13333.
- Denning DW, O’Driscoll BR, Hogaboam CM, et al. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J. 2006 Mar;27(3):615–626.
- Matsumura Y. Role of Allergen Source-Derived Proteases in Sensitization via Airway Epithelial Cells. J Allergy (Cairo). 2012;2012:903659.
- Namvar S, Warn P, Farnell E, et al. Aspergillus fumigatus proteases, Asp f 5 and Asp f 13, are essential for airway inflammation and remodelling in a murine inhalation model. Clinical and Experimental Allergy: Journal of the British Society for Allergy and Clinical Immunology. 2015 May;45(5):982–993.
- Hoselton SA, Samarasinghe AE, Seydel JM, et al. An inhalation model of airway allergic response to inhalation of environmental Aspergillus fumigatus conidia in sensitized BALB/c mice. Med Mycol. 2010 Dec;48(8):1056–1065.
- Druey KM, McCullough M, Krishnan R. Aspergillus fumigatus Protease Alkaline Protease 1 (Alp1): a New Therapeutic Target for Fungal Asthma. J Fungi (Basel). 2020 Jun 16;6(2):48.
- Urb M, Pouliot P, Gravelat FN, et al. Aspergillus fumigatus induces immunoglobulin E-independent mast cell degranulation. J Infect Dis. 2009 Aug 1;200(3):464–472.
- Dubey LK, Moeller JB, Schlosser A, et al. Chitin enhances serum IgE in Aspergillus fumigatus induced allergy in mice. Immunobiology. 2015 Jun;220(6):714–721.
- Chowdhary A, Agarwal K, Kathuria S, et al. Allergic bronchopulmonary mycosis due to fungi other than Aspergillus: a global overview. Crit Rev Microbiol. 2014 Feb;40(1):30–48.
- Benitez LL, Carver PL. Adverse Effects Associated with Long-Term Administration of Azole Antifungal Agents. Drugs. 2019 Jun;79(8):833–853.
- Cornely OA, Kontoyiannis DP. How to prophylax against invasive fungal infections in adult ALL? An unmet need. Mycoses. 2018 Sep;61(9):646–649.
- Jordan CL, Noah TL, Henry MM. Therapeutic challenges posed by critical drug-drug interactions in cystic fibrosis. Pediatr Pulmonol. 2016 Oct;51(S44):S61–S70.
- Skov M, Main KM, Sillesen IB, et al. Iatrogenic adrenal insufficiency as a side-effect of combined treatment of itraconazole and budesonide. Eur Respir J. 2002 Jul;20(1):127–133.
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1–e60.
- Mukker JK, Singh RS, Derendorf H. Pharmacokinetic and pharmacodynamic implications in inhalable antimicrobial therapy. Adv Drug Deliv Rev. 2015 May;85:57–64.
- Olivier KN, Shaw PA, Glaser TS, et al. Inhaled amikacin for treatment of refractory pulmonary nontuberculous mycobacterial disease. Ann Am Thorac Soc. 2014 Jan;11(1):30–35.
- Kirkpatrick WR, Najvar LK, Vallor AC, et al. Prophylactic efficacy of single dose pulmonary administration of amphotericin B inhalation powder in a Guinea pig model of invasive pulmonary aspergillosis. J Antimicrob Chemother. 2012 Apr;67(4):970–976.
- Wlaz P, Knaga S, Kasperek K, et al. Activity and Safety of Inhaled Itraconazole Nanosuspension in a Model Pulmonary Aspergillus fumigatus Infection in Inoculated Young Quails. Mycopathologia. 2015 Aug;180(1–2):35–42.
- Nishimoto Y, Ito K, Kimura G, et al. Antifungal Effects of PC945, a Novel Inhaled Triazole, on Candida albicans Pulmonary Infection in Immunocompromised Mice. J Exp Pathol. 2021;2(1):69.
- Kimura G, Nakaoki T, Colley T, et al. Biomarker Analysis of the Effects of Intranasally Dosed PC945, a Novel Antifungal Triazole, on Aspergillus fumigatus Infection in Immunocompromised Mice. Antimicrob Agents Chemother. 2017 Sep;61(9):548.
- Colley T, Alanio A, Kelly SL, et al. In vitro and in vivo antifungal profile of a novel and long acting inhaled azole, PC945, on Aspergillus fumigatus infection. Antimicrob Agents Chemother. 2017;1:87.
- Colley T, Sharma C, Alanio A, et al. Anti-fungal activity of a novel triazole, PC1244, against emerging azole-resistant Aspergillus fumigatus and other species of Aspergillus. J Antimicrob Chemother. 2019 Oct 1;74(10):2950–2958.
- Colley T, Sehra G, Chowdhary A, et al. In Vitro and In Vivo Efficacy of a Novel and Long-Acting Fungicidal Azole, PC1244, on Aspergillus fumigatus Infection. Antimicrob Agents Chemother. 2018 May;62(5):e01941–17.
- Kurtz MB, Bernard EM, Edwards FF, et al. Aerosol and parenteral pneumocandins are effective in a rat model of pulmonary aspergillosis. Antimicrob Agents Chemother. 1995 Aug;39(8):1784–1789.
- Kimura G, Nakaoki T, Nishimoto Y, et al. Effects of intranasally dosed posaconazole on fungal load and biomarkers in Aspergillus fumigatus infected immunocompromised mice. Mycoses. 2017 Nov;60(11):728–735.
- Van Ackerbroeck S, Rutsaert L, Roelant E, et al. Inhaled liposomal amphotericin-B as a prophylactic treatment for COVID-19-associated pulmonary aspergillosis/aspergillus tracheobronchitis. Crit Care. 2021 Aug 19;25(1):298.
- Otu AA, Langridge P, Denning DW. An evaluation of nebulized amphotericin B deoxycholate (Fungizone((R))) for treatment of pulmonary aspergillosis in the UK National Aspergillosis Centre. Mycoses. 2019 Nov;62(11):1049–1055.
- Ram B, Aggarwal AN, Dhooria S, et al. A pilot randomized trial of nebulized amphotericin in patients with allergic bronchopulmonary aspergillosis. J Asthma. 2016 Jun;53(5):517–524.
- Chong GL, Broekman F, Polinder S, et al. Aerosolised liposomal amphotericin B to prevent aspergillosis in acute myeloid leukaemia: efficacy and cost effectiveness in real-life. Int J Antimicrob Agents. 2015 Jul;46(1):82–87.
- Nihtinen A, Anttila VJ, Ruutu T, et al. Low incidence of invasive aspergillosis in allogeneic stem cell transplant recipients receiving amphotericin B inhalation prophylaxis. Transpl Infect Dis. 2012 Feb;14(1):24–32.
- Hullard-Pulstinger A, Holler E, Hahn J, et al. Prophylactic application of nebulized liposomal amphotericin B in hematologic patients with neutropenia. Onkologie. 2011;34(5):254–258.
- Monforte V, Ussetti P, Gavalda J, et al. Feasibility, tolerability, and outcomes of nebulized liposomal amphotericin B for Aspergillus infection prevention in lung transplantation. J heart lung transplantation. 2010May; 29(5): 523–530
- Rijnders BJ, Cornelissen JJ, Slobbe L, et al. Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: a randomized, placebo-controlled trial. Clin Infect Dis. 2008 May 01;46(9):1401–1408.
- Currie DC, Lueck C, Milburn HJ, et al. Controlled trial of natamycin in the treatment of allergic bronchopulmonary aspergillosis. Thorax. 1990 Jun;45(6):447–450.
- Pagani N, Armstrong-James D, Reed A. Successful salvage therapy for fungal bronchial anastomotic infection after -lung transplantation with an inhaled triazole anti-fungal PC945. J heart lung transplantation. 2020;39(12):1505–1506.
- Sole A, Garcia-Robles AA, Jorda C, et al. Salvage therapy with topical posaconazole in lung transplant recipients with invasive Scedosporium infection. Am J Transplant. 2018 Feb;18(2):504–509.
- Schwartz S, Behre G, Heinemann V, et al. Aerosolized amphotericin B inhalations as prophylaxis of invasive aspergillus infections during prolonged neutropenia: results of a prospective randomized multicenter trial. Blood. 1999 Jun 1;93(11):3654–3661.
- Chishimba L, Langridge P, Powell G, et al. Efficacy and safety of nebulized amphotericin B (NAB) in severe asthma with fungal sensitisation (SAFS) and allergic bronchopulmonary aspergillosis (ABPA). J Asthma. 2015 Apr;52(3):289–295.
- Monforte V, Ussetti P, Lopez R, et al. Nebulized liposomal amphotericin B prophylaxis for Aspergillus infection in lung transplantation: pharmacokinetics and safety. J heart lung transplantation. 2009Feb; 282: 170–175
- Tolman JA, Wiederhold NP, McConville JT, et al. Inhaled voriconazole for prevention of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2009 Jun;53(6):2613–2615.
- Hilberg O, Andersen CU, Henning O, et al. Remarkably efficient inhaled antifungal monotherapy for invasive pulmonary aspergillosis. Eur Respir J. 2012;40(1):271–273.
- Andersen CU, Sonderskov LD, Bendstrup E, et al. Voriconazole Concentrations in Plasma and Epithelial Lining Fluid after Inhalation and Oral Treatment. Basic Clin Pharmacol Toxicol. 2017 Nov;121(5):430–434.
- Holle J, Leichsenring M, Meer PE. Nebulized voriconazole in infections with Scedosporium apiospermum–case report and review of the literature. J Cyst Fibros. 2014 Jul;13(4):400–402.
- McConville JT, Overhoff KA, Sinswat P, et al. Targeted high lung concentrations of itraconazole using nebulized dispersions in a murine model. Pharm Res. 2006 May;23(5):901–911.
- Rundfeldt C, Steckel H, Scherliess H, et al. Inhalable highly concentrated itraconazole nanosuspension for the treatment of bronchopulmonary aspergillosis. Eur J Pharm Biopharm. 2013 Jan;83(1):44–53.
- Hirano K, Izumikawa M, Yoshida K. Efficacy of Antifungal Therapy with Inhaled Itraconazole against Murine Invasive Pulmonary Aspergillosis with Itraconazole-Low Susceptible Aspergillus fumigatus. 6th Advances Against Aspergillosis 2014. Madrid 2014:9.
- Curran A, Perry J, Hava D. Efficacy of PUR1900, an inhaled antifungal therapy, in a Guinea pig model of invasive pulmonary aspergillosis. 8th Advances Against Aspergillosis. Lisbon 2018:147.
- Cheng SN, Tan ZG, Pandey M, et al. A Critical Review on Emerging Trends in Dry Powder Inhaler Formulation for the Treatment of Pulmonary Aspergillosis. Pharmaceutics. 2020 Nov 28;12(12):78.
- Strong P, Ito K, Murray J, et al. Current approaches to the discovery of novel inhaled medicines. Drug Discov Today. 2018 May;23(10):1705–1717.
- Arora S, Haghi M, Young PM, et al. Highly respirable dry powder inhalable formulation of voriconazole with enhanced pulmonary bioavailability. Expert Opin Drug Deliv. 2016;13(2):183–193.
- Kaur R, Dennison SR, Burrow AJ, et al. Nebulized surface-active hybrid nanoparticles of voriconazole for pulmonary Aspergillosis demonstrate clathrin-mediated cellular uptake, improved antifungal efficacy and lung retention. J Nanobiotechnology. 2021 Jan 11;19(1):19.
- Pornputtapitak W, El-Gendy N, Berkland C. NanoCluster Itraconazole Formulations Provide a Potential Engineered Drug Particle Approach to Generate Effective Dry Powder Aerosols. J Aerosol Med Pulm Drug Deliv. 2015 Oct;28(5):341–352.
- Moazeni E, Gilani K, Najafabadi AR, et al. Preparation and evaluation of inhalable itraconazole chitosan based polymeric micelles. Daru. 2012 Dec 3;20(1):85.
- Pardeike J, Weber S, Zarfl HP, et al. Itraconazole-loaded nanostructured lipid carriers (NLC) for pulmonary treatment of aspergillosis in falcons. Eur J Pharm Biopharm. 2016;1:87.
- Hassanpour Aghdam M, Ghanbarzadeh S, Javadzadeh Y, et al. Aggregated Nanotransfersomal Dry Powder Inhalation of Itraconazole for Pulmonary Drug Delivery. Adv Pharm Bull. 2016 Mar;6(1):57–64.
- Kaur A, Yadav JP, Sathe RY, et al. Understanding Poor Milling Behavior of Voriconazole from Crystal Structure and Intermolecular Interactions. Mol Pharm. 2022 Mar 7;19(3):985–997.
- Curran AK, McIntyre KT, Tracy HS, et al. DELIVERY OF PUR1900 ENABLES HIGH LUNG EXPOSURE OF ITRACONAZOLE RELATIVE TO ORAL DOSING. Pediatr Pulmonol. 2016;51(S45):S333.
- Hava DL, Tan L, Johnson P, et al. A phase 1/1b study of PUR1900, an inhaled formulation of itraconazole, in healthy volunteers and asthmatics to study safety, tolerability and pharmacokinetics. Br J Clin Pharmacol. 2020 Apr;86(4):723–733.
- Beinborn NA, Du J, Wiederhold NP, et al. Dry powder insufflation of crystalline and amorphous voriconazole formulations produced by thin film freezing to mice. Eur J Pharm Biopharm. 2012 Aug;81(3):600–608.
- Caponetti G, Maggi L, Sardina M, et al. Edry™ an innovative dry powder technology evaluated via functional respiratory imaging (FRI). Eur Respir J. 2016;48:A958.
- Murray A, Cass L, Ito K, et al. PC945, a Novel Inhaled Antifungal Agent, for the Treatment of Respiratory Fungal Infections. J Fungi (Basel). 2020 Dec 17;6(4):965.
- Colley T, Alanio A, Kelly SL, et al. In Vitro and In Vivo Antifungal Profile of a Novel and Long-Acting Inhaled Azole, PC945, on Aspergillus fumigatus Infection. Antimicrob Agents Chemother. 2017 May;61(5):e02280–16.
- Kimura G, Nakaoki T, Colley T, et al. In Vivo Biomarker Analysis of the Effects of Intranasally Dosed PC945, a Novel Antifungal Triazole, on Aspergillus fumigatus Infection in Immunocompromised Mice. Antimicrob Agents Chemother. 2017 Sep;61(9):e00124–17.
- Colley T, Sehra G, Daly L, et al. Antifungal synergy of a topical triazole, PC945, with a systemic triazole against respiratory Aspergillus fumigatus infection. Sci Rep. 2019 Jul 1;9(1):9482.
- Pagani N, Armstrong-James D, Reed A. Successful salvage therapy for fungal bronchial anastomotic infection post-lung transplantation with a novel inhaled triazole antifungal PC945. J Heart Lung Transplant. 2020;39(12):1505–1506.
- Cass L, Murray A, Davis A, et al. Safety and nonclinical and clinical pharmacokinetics of PC945, a novel inhaled triazole antifungal agent. Pharmacol Res Perspect. 2021 Feb;9(1):e00690.
- FDA. Workshop: addressing Challenges in Inhaled Antifungal Drug Development. 2020 [ cited 26 May 2022]; Available from: https://www.fda.gov/drugs/news-events-human-drugs/addressing-challenges-inhaled-antifungal-drug-development-09252020-09252020.
- Toor A, Culibrk L, Singhera GK, et al. Transcriptomic and proteomic host response to Aspergillus fumigatus conidia in an air-liquid interface model of human bronchial epithelium. PloS One. 2018;13(12):e0209652.
- Gregson L, Hope WW, Howard SJ. In vitro model of invasive pulmonary aspergillosis in the human alveolus. Methods Mol Biol. 2012;845:361–367.
- Daly L, Lucas KA, Colley T, et al. Synergic anti-fungal activity of topical PC945, a novel inhaled azole, with systemic echinocandin on Aspergillus fumigatus in vitro human alveoli model. AAAM2020. Lugano. 2020;abstract 33.
- Baistrocchi SR, Lee MJ, Lehoux M, et al. Posaconazole-Loaded Leukocytes as a Novel Treatment Strategy Targeting Invasive Pulmonary Aspergillosis. J Infect Dis. 2016;5:7.
- Ito K, Kizawa Y, Kimura G, et al. Accumulation of a novel inhaled azole, PC945 in alveolar cells in temporally neutropenic immunocompromised mice infected with Aspergillus fumigatus. AAAM2020. Lugano. 2020:abstract 68.
- FDA. Nonclinical Safety Evaluation of Reformulated Drug Products and Products Intended for Administration by an Alternate Route, Guidance for Industry and Review Staff. 2015. [Cited 26 May 2022]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/nonclinical-safety-evaluation-reformulated-drug-products-and-products-intended-administration
- Vergidis P, Moore CB, Novak-Frazer L, et al. High-volume culture and quantitative real-time PCR for the detection of Aspergillus in sputum. Clin Microbiol Infect. 2019. S1198-743X(19)30619–6
- Ye F, Zeng P, Li Z, et al. Detection of Aspergillus DNA in BALF by Real-time PCR and Galactomannan Antigen for the Early Diagnosis of Chronic Pulmonary Aspergillosis. Ann Clin Lab Sci. 2021 Sep;51(5):698–704.
