ABSTRACT
Introduction
Sustainability within the pharmaceutical industry is becoming a focal point for many companies, to improve the longevity and social perception of the industry. Both additive manufacturing (AM) and microfluidics (MFs) are continuously progressing, so are far from their optimization in terms of sustainability; hence, it is the aim of this review to highlight potential gaps alongside their beneficial features. Discussed throughout this review also will be an in-depth discussion on the environmental, legal, economic, and social particulars relating to these emerging technologies.
Areas covered
Additive manufacturing (AM) and microfluidics (MFs) are discussed in depth within this review, drawing from up-to-date literature relating to sustainability and circular economies. This applies to both technologies being utilized for therapeutic and analytical purposes within the pharmaceutical industry.
Expert opinion
It is the role of emerging technologies to be at the forefront of promoting a sustainable message by delivering plausible environmental standards whilst maintaining efficacy and economic viability. AM processes are highly customizable, allowing for their optimization in terms of sustainability, from reducing printing time to reducing material usage by removing supports. MFs too are supporting sustainability via reduced material wastage and providing a sustainable means for point of care analysis.
1. Introduction
The global acceptance of human-driven climate change has reframed the future priorities of many industries. Finding solutions to both limit and counteract the climate emergency has become a pressing matter of increasing concern. This has led to multiple global initiatives and reports, such as the recent Sixth Assessment Report (AR6) produced by the Intergovernmental Panel on Climate Change (IPCC), which highlights the need for radical systems change in all industrial sectors to limit global warming to 1.5°C [Citation1]. Further, the UN Climate Change Conference (COP26) which took place in the UK in 2021 has made the specific goal of achieving a low carbon and sustainable health system as part of the new initiative ‘The COP26 Health Programme’ [Citation2].
The pharmaceutical industry is not immune from either the concerns or innovations that are born from this emergency. Available data suggest that the pharmaceutical manufacturing industry is far from a green industry, with 2018 statistics presenting that the industry produced 52 millions of metric tons of CO2 equivalent (MMt-CO2e) over the year [Citation3]. To provide context to this statistic, in the same year, the automotive manufacturing industry contributed less; 46.4 MMt-CO2e. Contributions to this figure come from various sources, including research, transport, and direct manufacturing emissions [Citation4], all of which can be feasibly targeted for reduction. Whilst there are many anthropogenic substances that contribute greenhouse gas emissions, CO2 is the most widely focussed on when measuring an industry’s effect on the environment. This is not necessarily the case though for the pharmaceutical industry, as there is a plethora of toxic chemicals that are used throughout the production line of various formulations and devices, for example volatile organic compounds (VOCs) [Citation5], pesticides [Citation6] and formaldehyde [Citation7]. Solvent use reduction within the pharmaceutical industry is a main focus to help minimize the effect on the environment; currently, 80–90% of all materials used for manufacturing processes are solvents, which provides a large target for reduction [Citation8].
To promote sustainability during manufacturing, it is important to identify and factor within a supply chain and manufacturing process that could potentially be altered to improve the overall environmental friendliness. Using a designed approach of ‘reduce, re-use, recycle’ [Citation9], has been at the forefront of establishing more eco-friendly processes within the industry, and then further integrating this as part of a circular economy [Citation9]. Reducing or replacing harmful materials is one of the simplest ways of improving the environmental sustainability within an area, which has been done successfully in the pharmaceutical industry in a few examples: the reduction of materials during selective laser sintering (SLS) 3D printing (3DP) via removing printing supports [Citation10], medicines reconciliations performed by pharmacists [Citation11] or the reduced use of solvents via microfluidics (MFs).
The term sustainability covers more than just the contribution to the environment, but also how the area is viewed socially, legally, and economically. These are all important factors to consider for a process, as alongside efficacy, these constituents will determine a process’ longevity.
Addressing the massive consumption of energy/materials and their consequent emissions is now becoming a factor that is being considered by industries and it is important that the emerging technologies (ET) that will become the gold standard of manufacturing will adhere to sufficient green standards for the future. Coupling these factors discussed within this review are expert opinions on both the beneficial factors and drawbacks on the sustainability of additive manufacturing (AM) and MFs, which are quickly coming to be leading technologies within their respective areas. These innovative technologies will be discussed in detail throughout this review to help determine how sustainable each practice can be in the area of pharmaceutical manufacturing.
1.1. PESTLE analysis
The PESTLE tool is extremely useful for summarizing the impacts of various sources upon the progression of a technology. As detailed in , the impact of PESTLE factors are displayed for the pharmaceutical industry as a whole, then further narrowed down toward 3DP and MFs. The most notable matters to draw upon in this analysis are the political, economic, and environmental factors, especially for 3DP. Throughout the review, many of these factors will be discussed in further detail to enlighten the potential of the technologies, specifically from a sustainability standpoint.
Table 1. PESTLE analysis to summarize relevant socioeconomical factors currently influencing the progress of these emerging technologies within the pharmaceutical industry.
2. Additive manufacturing
AM, otherwise known as 3DP, is a phenomenon within the faculty of ET that is gaining great traction both for industrial use, as well as personal use. There are, however, questions relating to the sustainable practice of its use, especially within the area of pharmaceutical manufacturing. As of 2021, it is estimated that 16.4% of revenue collected from AM processes was related to medical implementation [Citation19].
AM allows for a wide variety of materials to be custom printed to form structures within a pre-defined 3D space. The compatibility of the materials used for each printer will depend upon the mechanism by which the printer functionsfor example, SLS fuses powder together via a temperature-based mechanism, whilst stereolithography (SLA) 3DP employs ultraviolet (UV) rays to solidify photosensitive polymeric materials. This highlights a first point, which certain printers may be more environmentally sustainable to use than others, both owing to the materials that can be used, but also the energy and time that are required for completing the print. Compared to the traditional manufacturing method of casting, SLS has been observed to be 50 times more efficient when regarding CO2 emissions per part, as well as negating the need for water [Citation20]. It has been previously noted though that AM methods are more energy demanding for pharmaceutical manufacturing than injection molding [Citation20]. This is mainly due to how time intensive complex medical prints can be, especially if the printed structures are large. Injection molding can lack the specificity required for producing medical devices with high degrees of intricacy, which is something that can be achieved with AM [Citation21]. A comparison of AM methods compared to traditional methods can be seen in . The factor of energy consumption is important for environmental reasons, as well as economic reasons. A process will obviously be attractive if it is environmentally friendly, but even more so if there is an enticing price-tag alongside. The issues surrounding global warming and climate change engross us; and this in turn affects choices that are made when purchasing equipment for industry/research, relating to their energy usage and carbon emissions. The growing competition for retailers within the AM area has aided the reduction of energy usage, whether that be during product distribution, or via the actual mechanisms of the printer [Citation22].
One of the most regarded benefits for the use of AM is the reduction of materials used during the manufacturing process [Citation23]. Since a design is pre-determined before printing, as well as the fact that AM is performed by a layer-by-layer method, the amount of material used to produce a complex structure is streamlined. Niaka et al., referred to this approach as complexity-for-free, as the complexity of an object has little impact upon the production cost, owing to the layer-by-layer printing [Citation24]. Further practices including printing multiple structures upon a single stage can help further optimize the material and time efficiency of the overall print. One criticism of material efficiency for AM is the occasional need for structural supports during the printing process. The supports ensure that during the printing process, all components of the print remain in their desired location throughout printing. The supports are removed during post-processing, but they would be considered as ‘waste’ accumulated during AM. Powder bed processes, such as SLS or fused deposition modeling (FDM) can occasionally be printed without the need for supports, provided that critical print angles [Citation25] are not exceeded. This would be a factor to consider when choosing which printer would be most suitable.
One of the main drawbacks to AM in general, not just regarding sustainability, is the possibility for print failures. If a print is designed poorly, or a wrong material is used, it may result in a print failure. This will, of course, lead to increased wastage of both materials and energy. Operators receiving sufficient training on devices, using well thought-through designs, can reduce the impact of print failures. For pharmaceutical manufacturing, most AM operators will be sufficiently trained, although the complexity of designs may be the cause of print failure. Household 3D printers may be a cause for concern in terms of wastage, due to the ‘recreational’ usages of AM, as well as a potential lack of experience [Citation26].
It is commonly accepted that most materials used for AM are generally safe to handle (), as their inertness is one of the key factors that allows for long timescale prints. It has, however, been suggested that the use of some materials for AM may cause the production of ultrafine particles which can cause inflammatory pulmonary and cardiovascular issues [Citation27]. One such example of this is when using polylactic acid (PLA) filaments in an enclosed space with poor ventilation [Citation28]. The issue of ultrafine particles is not often seen for pharmaceutical manufacturing as workspaces should be optimized with good ventilation throughout.
Table 2. Comparison between different AM methods and traditional methods, documenting their usages within the pharmaceutical industry and their correlating CO2 emissions.
Using AM, it is possible to produce items in situ, as compared to needing a supply chain to procure a product. This is hugely beneficial for endorsing an environmentally beneficial process, and as a way of avoiding any issues obtaining a product. As a prime example of this, supply chain issues caused by the recent covid-19 pandemic saw some industries grind to a halt within weeks [Citation46]. This risk factor of supply chain issues is greatly reduced using AM. The reduction in carbon emissions caused by the transportation of goods is also greatly reduced by manufacturing pharmaceutical devices in situ is greatly reduced also. The digitalization of 3DP means that design files can be distributed worldwide instantaneously, without the need for a supply chain. On average, removing the factor of a supply chain will not only reduce the costs of the service but it could also reduce the carbon footprint of the process by 15 times, including factoring in the storage of the items pre-transit [Citation47].
2.1. AM & circular economy
A further contribution factor to consider if a supply chain is still required post-AM, is that owing to the layer-by-layer approach-printed items frequently weigh less than those manufactured via injection molding or casting. This will affect the weight of goods transported during a supply chain, which in turn will affect the carbon footprint of the chain. The printed items are lighter because printed items can still maintain the structural strength of traditionally manufactured items whilst using reduced materials. The print orientation can be optimized to support the mechanical strength, but also items can be manufactured as a single unit, rather than multiple parts. By printing as a single part, the overall economic sustainability is improved, since there is no need for an extensive assembly line to fashion the final product.
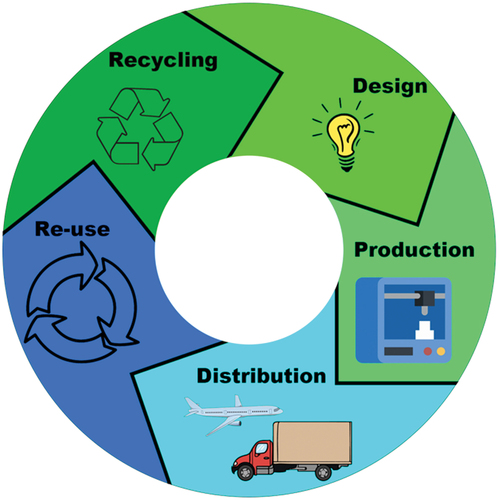
The previous paragraph highlights a poignant example for the promotion of a ‘circular economy’ [Citation27,Citation48]. This term refers to a designed approach strategy placed to benefit the business, society, and the environment within the same cycle. A visual representation of this can be seen in . For example, when applied to pharmaceutical manufacturing using AM, a circular economy can be achieved via changing printing parameters such as the amount of material being used. Reducing the amount of material used in a print by designing the structure to be viably printed with fewer supports will clearly benefit the business as less material is being used, the print may take a shorter time, and the amount of post-processing will also be reduced. In the case of pharmaceutical manufacturing, this will lead to a decreased economic burden befalling the manufacturer, the purchaser (a.k.a. the relevant healthcare system), and the final patient. Socially, the depiction of AM may improve as the decreased cost to the manufacturer should lead to a decrease in the cost for the consumer. Finally, environmentally, less material usage should equate to reduced waste; however, a main principle of circular economy is to endorse the re-use and recycling of unused material, which should be considered upon during the design phase.
Figure 1. Visual representation of a circular economy, which would ideally be implemented within a manufacturing process.
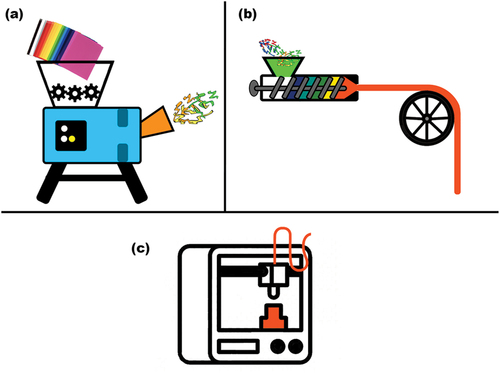
AM frequently allows the practice of material re-use, as especially for powder bed technologies, any material left un-fused will be available for future printing. The practice of material re-use during AM is common for metal powders, UV sensitive powders, and thermoplastics. This principle has been applied multiple timesfor example, for the reuse of polypropylene, using intermediary processes like hot melt extrusion (HME) () [Citation49]. Currently, the re-use of resin-based printing material is not available as to our knowledge, there are no methods to achieve this. The area of material re-use is currently being highly researched, owing to its importance of promoting a circular economy. It is estimated that 5000 tonnes of waste is produced from AM each year [Citation50], which unless addressed, will only grow due to the practice of AM becoming increasingly common. As is well known, plastics are one of the world’s great pollutants owing to their extremely slow degradation rates. This factor also applies to resins, which can also have long degradation periods, which is why further exploration into the practice of resin re-use is essential, before an inordinate amount of resin waste is accumulated from AM.
Figure 2. Diagram representing the reuse of materials to be implemented for AM. (a) depicts the physical shredding of viable thermoplastics, (b) HME being applied to produce filaments that will be compatible with the chosen AM method and (c) AM of the filament produced from the reused materials.
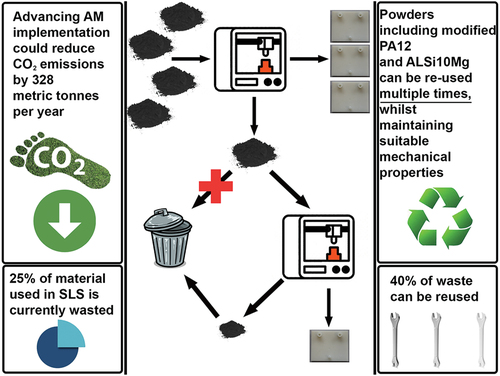
The reuse of powder from powder bed technologies is also possible, to reduce material wastage. It is suggested that currently, on average, 25% of powder used during SLS is wasted [Citation51]; and of this waste, 40% can be reused [Citation52]. Research into this area has investigated the suitability of various powders for reuse, investigating whether their mechanical properties are maintained after their usage. Such materials include polyamide 12 (PA12) [Citation53] and AlSi10Mg [Citation54] (), which are both widely used materials, with PA12 being at the forefront for SLS manufacturing. The mechanical properties of AlSi10Mg were preserved throughout the printing process, leading to an easy reuse. Studies involving virgin PA12 determined that the crystalline structure of the compound was harshly affected during printing, meaning that modifications may need to be performed to ensure that printed objects with reused powder still possessed high-quality attributes. Fortunately, a company called materialise©, has patented a material called Bluesint PA12 which greatly improves the reusability of the material, and its subsequent green impact. According to Kellens et al., increasing the use of AM within all industries would contribute to decreasing the annual CO2 impact by 328 metric tonnes [Citation55] ().
Figure 3. Schematic to represent the multiple beneficial factors relating to the reuse of materials in AM.
When compared to subtractive manufacturing (SM), the amount of waste produced from AM can be regarded as negligible. SM produces 3D structures via the intentional deletion of materials from an overall larger structure. This process naturally produces a far greater amount of waste than AM, which will often not have any chance of being re-used for SM. The complexity of designs that can be produced via SM is also frequently limited due to the lack of a layer-by-layer manufacturing method. It is for these main reasons that AM is deemed both socially and environmentally as the most promising manufacturing method of the two.
A highly researched field of AM, which is being investigated currently, is the printing of MF chips for healthcare purposes. The authors will discuss the sustainability of the emerging technology MFs later in this review, and how combining technologies (3DP and MFs) can improve the sustainability of scientific research in general.
2.2. Social interpretation of AM
AM has gained so much traction over the past 40 years that entire conventions and conferences are being held to ensure the sustainable use of AM worldwide. Conventions including Formnext, 3DP expo and the materials conference. This is to improve the public’s perception of AM, as important discussions are being held to uphold its practice and ensure relevant information about AM sustainability is readily available. Formnext, in particular, is dedicated solely to the practice of sustainability within AM, which had world-renowned experts presenting their thoughts and opinions on the future of the craft and how to reduce its environmental impact.
As with medication and medical devices, for a 3D-printed medical device to be used for patient care, it must adhere to a governing bodies’ regulatory standards [Citation56]; for example the US food and drug administration (FDA) or the medicines and healthcare products regulatory agency (MHRA) in UK. The devices may also need to be proven to display suitable efficacy before being prescribed to a patient. This regulation and standard maintenance have meant that any device being used for patient treatment can be trusted to a high degree by healthcare professionals and patients alike. It is still the case that the greatest use for AM within the medical field is for the manufacturing of anatomical models for the preplanning of surgeries [Citation56,Citation57]. It has been proposed for many uses though, including prosthetics, implants, and individualized tablets. Gauging the opinion of medical professionals on devices and formulations produced via AM is incredibly important relating to the longevity of its use within the medical field. Concerning AM-produce tablets, Beer et al., concluded that pharmacist-trained personnel are generally in favor of their production and distribution from community pharmacies [Citation58]. Owing to their increasing use and efficacy [Citation59,Citation60], it is safe to say that the implementation of AM for the production of anatomical models is a successful one, and one which is likely to be used greatly in the future.
Possibly, the most important perspective to view socially about AM is that of the patient receiving the treatment. Although concrete data is sparsely documented, it is possible to infer information from the data available. It has been acknowledged that the presence of a 3D model improved the patient’s confidence, knowledge, and experience was improved via the availability of a patient-specific 3D model [Citation60]. For applied medical devices, AM has been shown to improve the patient compliance, due to increased comfort, breathability, and esthetics, as was demonstrated in a study highlighting the role of 3D printed devices for scoliosis [Citation61]. The capacity for AM to produce flexible structures has also been noted to improve patient’s experience of 3D printed devices [Citation61].
2.3. Legal concerns of AM
With the steady introduction of blockchain entering the pharmaceutical industry, it is possible that AM will play a part in the fraudulent interjection of supply chains. It is also possible that due to the digital nature of AM, intellectual property (IP) for designs and processes may be at risk. AM has the capacity to replicate designs and devices to a high degree of accuracy and complexity. As discussed in section 3.2, all 3D printed medical devices and formulations must adhere to the standards of their relevant regulatory body. If these specifications were to be matched by a counterfeit supplier with access to AM machinery, then non-legitimate devices could make their way into the supply chain. The introduction of blockchain networks should assist in the reduction of this practice, although it may still be a concern whilst parts of the supply chain network are not covered.
Addressing the second point, safeguarding the IP of print designs can become an issue, owing to the various transactions of CAD files that will occur during research associated with AM. Within the ‘traditional’ laboratory research area, the explicit sharing of raw data is uncommon; whilst for AM, the sharing of complete designs can be a regular occurrencefor example, sending a print design to a specific printer [Citation62]. As a result of this, it is possible for IP theft to occur throughout the stages of research, unless the operators take care as to the destination of their files.
As we enter ‘industry 4.0 [Citation63],’ where the digital revolution now has internet connectivity, it is important to consider the impact that machine learning (ML) will have upon the practice of AM. ML is essentially a subsector of artificial intelligence, whereby computational analysis will help determine optimal digital parameters for printing, based upon a risk/reward basis. The technology functions via various mechanisms, such as supervised learning, where specific input/output parameters are identified by an operator, or by reinforced learning, where pattern-detecting algorithms are scripted, to gradually improve the functionality of a design [Citation64]. Once optimized, ML should be able to improve the design and manufacturing workflow of AM, decreasing printing time and resource use, and also reducing the likelihood of print failures [Citation65]. This, however, may cause legal issues in terms of whether an optimal design be established via ML, then who will the IP ownership belong to. As the usage of ML increases within AM, it will become common practice to establish these intricacies before commencing research.
2.4. Sustainable food via AM
The printing of edible substances via AM has been a concept for almost 20 years [Citation66]; originally implemented to assist maintaining food supply levels to cope with a rapidly expanding population, as well as producing customized foodstuffs. The area has since evolved into various applications, from personalized nutrition to product marketing [Citation67]. In terms of environmental sustainability, it is estimated that 20–30% of global greenhouse emissions can be sourced back to the food supply chain [Citation68], whether that be due to the energy used during harvesting or emissions from animal rearing.
Food AM is being researched within the pharmaceutical industry mainly in relation to the capacity to personalize food to individuals’ specifications. For example, Severini et al. have used AM to enhance the nutritional qualities of cereal-based snacks, enhanced with amino acids from an insect-based source [Citation69]. The enhancements of the items came from the printed microstructures within the baked goods, as well as the specificity of the amount of materials that were incorporated into each item. It is feasible that this process could be applied to other foodstuffs, such as meat, which would also reduce the amount of energy and water consumption that is involved in the mass rearing of livestock.
Whilst the applications within the pharmaceutical industry are still in their initial phases, AM manufacturing of food for medical purposes could have great potential treatments to target; clear targets for future research being areas such as baby food and gluten-free bread production.
3. Microfluidics
MFs is another ET that is showing great promise in terms of its recent usages in a variety of fields such as nanoformulation [Citation70], compound separation [Citation71] and The chips themselves can be manufactured in multiple ways, depending upon which material(s) is required for the chipfor example, glass chips may be fabricated via direct piezoelectric laser etching [Citation72], whilst a polydimethylsiloxane (PDMS) chip may be fashioned more suitably via soft lithography [Citation73]. As mentioned previously in section 3.1, AM is quickly becoming the go-to-choice for the manufacture of many types of chips, as chips can be designed at printed to exact specifications depending on their use. For many pharmaceutical research facilities, AM and MFs are researched together, negating the need to obtain chips from extraneous sources via a supply chain.
3.1. MFs for formulation manufacturing
MFs incorporates fluids within a tightly constricted volumetric area, meaning that milliliter volumes of reagents are involved in the process. This does, however, have drawbacks, which will be discussed later. When using MFs to prepare liposomes, for example, the amount of material being used can be reduced by factors of 250%, in comparison to other methods such as thin film hydration (TFH) [Citation74,Citation75]. As such, the manufacturing of formulations via these methods fully supports the idea of a circular economy. The efficiency of the process will decrease the cost of manufacturing due to reduced material, as well as reducing the amount of waste produced in parallel. As mentioned though, there are drawbacks to manufacturing formulations with MF, including the difficulty in scaling up processes to an industrial level [Citation76]. Whilst the social and environmental aspects of circular economy remain untarnished, without an easy way to scale the process up, the business side may suffer. In terms of the solvent efficiency, MFs is also held in high regards. Solvent waste is a prime causative factor toward the risks posed by volatile organic compounds (VOC) [Citation77], and is the 5th target to alter according to the American chemical society (ACS) 12 principles of green chemistry [Citation78]. VOCs pose a great threat to both the health of the public and of the environment; for human health, they can be carcinogenic, cause allergies and long-term conditions such as asthma, if those in question are subject to prolonged exposure [Citation79]. For the environment, VOCs can cause debilitating environmental pollution and can contribute toward global warming by negatively altering the chemical composition of the atmosphere, in the form of ozone irregularities [Citation80]. MFs allow the reduction of solvent use during manufacturing methods, which is an excellent example of adhering to a circular economy.
Time efficiency is one of the most crucial factors considered when judging the sustainability of a process. The speed at which a formulation can be produced from scratch using MFs is far superior to more traditional methods and can also be performed as a continuous manufacturing process, as opposed to batch. This means that the consumption of energy will also be reduced, further bolstering its claim to becoming a sustainable practice. Faster processes that can produce the same quality of product as slower processes will normally be economically favorable if the energy consumption is not affected. There are a number of many articles available that display the time effectiveness of MFs as a process whilst still maintaining a low-cost manufacturefor example, Bian et al. [Citation81] worked upon colloidal crystal manufacture using MFs which allowed for a cheaper and faster method of production.
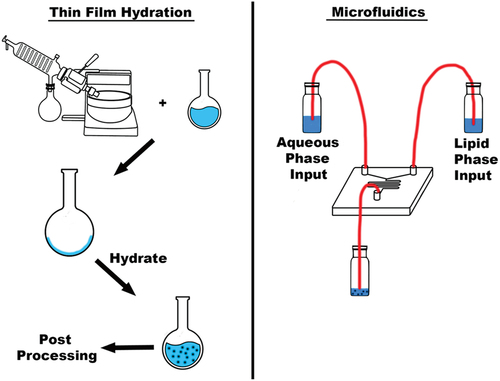
Post-processing can be a long, energy consuming process that is required for many laboratory methods. For nanomedicine (NM) preparation, many initial synthesis methods, e.g., TFH, produce nanoparticles (NPs) of various sizes and polydispersity indexes (PDI), which will require a post-processing method such as extrusion. This is not the case with MFs, as the particles produced directly from manufacturing often have physical properties that are desirable, without the need for post processing. The constrained volumetric availability during manufacturing is the cause of this, alongside the fluid dynamic interactions [Citation82]. depicts the one-step process available to the MF method, as compared to TFH, and to also highlight the reduced materials used in the process.
3.2. MFs for sustainable analysis
‘Standard’ MF setups rely on a source of pressure to combine fluids at a fast, controlled rate during manufacture. However, there are MF setups that can function without the requirement of an external power or pressure source. One of the most promising emerging branches of MFs appears in the study of paper MFs. Paper MF devices come from a renewable source, are transportable, do not require external power sources, and are widely recyclable. The paper MF devices instead often rely solely on capillary action. There already exist paper MF devices to detect and analyze various materials, including DNA, blood/plasma and various biomolecules (e.g. glucose and uric acid) [Citation83]. The capacity for these devices to provide point-of-care testing (POCT) reduces the need for samples to be sent away for further analysis, reducing the carbon footprint of the overall process. Alternative renewable materials have been suggested to act as viable platforms for MFs, including wood and corn proteins [Citation84,Citation85]. Reducing the use of (single use) plastics, which are massive world pollutants, is the goal of this continued branch of research; the results of which a looking incredibly promising. The paper and wood-based MFs are additionally attractive because of their low manufacturing costs, hence the cost of research within the area is drastically reduced.
Location-based testing is not limited to paper and wood devices, as it has also been adapted to resin and PDMS chips, which have been used for environmental assays and monitoring [Citation86]. The MF chips are highly customizable, to meet specific requirements for their processes; this can mean that additional assets can be added alongside the traditional MF channels, such as microreactors, detectors, or microwells [Citation87]. In the theme of promoting sustainability, MF devices have been modified to allow for a portable means of testing pollutant levels in the environment at any desired location [Citation86]. The modifications have incorporated a pre-treatment modules that allows for pollutant samples in the desired medium to be simply concentrated down to an analytical level; due to the restricted size on MFs, the detection level can still be as sparse as parts per million (ppm) [Citation88]. Devices such as these can rely on multiple mechanisms to move fluids, ranging from electrophoresis to simple particle diffusion which allow analytes to move from the original sample location to the detectors.
3.3. Other MF applications for sustainability
Recent innovative uses for MFs have seen the technology acts as energy storage devices, to help reduce the amount of metal waste produced from traditional storage mediums. Paper has been suggested as a suitable short-term energy storage material due to its recent advancements and commercialization [Citation89]. The flexibility of paper is one of the most attractive features of the material as an electronic component, alongside its porosity and roughness [Citation83]. The technology functions via the addition of conductive inks being printed in microchannels on the surface, in addition to an electrolyte medium being absorbed onto the paper, to provide a source of electron gradient throughout. Whilst this technology is innovative, it is hindered by its capacity for long-term functionality caused by the fragility and degradation of the platform. The usage of renewable materials instead of finite metals is a promising step forward though, for sustainability, and the funding available for this kind of research is ever increasing.
3.4. Drawbacks of MFs
Despite the highlighted positive aspects that MFs provides, there are unfortunately a few drawbacks that should be considered in terms of its sustainable use. Firstly, the cost of MF setups that require a pressure source for functionality can be very expensive for the initial setup. This will also limit the portability of the system; however, the pressure-based systems can produce results quickly and accurately.
Secondly, as mentioned in section 3.1, MFs have difficulty being scaled up, due to the innate nature of its micro-sized channels. The simplest way of scaling the process up is via running multiple devices in parallel, as increasing parameters, such as flow rates, may affect the quality of the manufactured material. Scalability is a large factor to consider when taking a process toward industrial usage because of the economic hindrances that may occur. Formulation processes including nanoparticle synthesis may be particularly impacted by scaling a procedure up, owing to the importance of parameter control during manufacturing.
4. Electrospinning
Electrospinning is responsible for a large proportion of the pharmaceutical industry’s impact upon the production of bio-scaffolds as well as regenerative wound dressings [Citation90,Citation91]. Traditional methods of fabricating the scaffolds, such as lyophilization [Citation92], are considered to be well established, but have drawbacks including being time-consuming and highly resource intensive. Electrospinning offers a one-step process capable of fabricating biologically compatible scaffolds and wound dressings within a short amount of time.
The main drawback in terms of sustainability for the use of electrospinning is its high dependency on environmentally unfriendly solvents, for example, chloroform. Given this, a critical parameter that is being investigated is reducing their use by optimizing the solvent choice and the polymer concentration used during the manufacture. A study performed by Agarwal et al. investigated such parameters, delving even into the possibility of using water as the required solvent [Citation93]. Recent advancements in the applications of electrospinning to promote environmental sustainability lie in techniques outside of scaffolding and wound dressings. Electrospinning is currently being explored for applications within the field of eco-friendly air filtration [Citation94], water treatment, energy storage, and gas separation [Citation95]. Melt-electrospinning is one of the methods that has been introduced the recent years, with more researchers currently using this method, which avoids the use of solvents.
5. Conclusion
These ETs mentioned are by no means a final gold standard to address all the factors of sustainability, but they do represent a significant progression in improving the impact that the pharmaceutical industry is having worldwide. AM is nearing the forefront of component and device manufacturing, offering a variety of means to produce highly customized items. The research and competition in its field is attributing to faster, cheaper, and more environmentally friendly ways of producing objects to be used within the pharmaceutical industry. The application of AM within other medical fields such as MFs, surgery, and prosthetics is also opening doors within their respective field, to attain a level of care that has never been reached before.
MFs have been a field of pharmaceutical manufacturing that whilst having existed for almost half a century, has not previously reached its full potential. It is only within the last two decades that it’s utilization is starting to be properly implemented; producing world-leading results for both formulation manufacture and component analysis. Environmentally, the reduction of materials used during the process, and its subsequent reduction in wastage, has solidified MFs as a desirable pharmaceutical method for future investment.
Continued monitoring of energy and input/output statistics will be imperative for adjusting the applications of both technologies to help optimize their sustainable progression; but when compared to previous technologies available, the Ets appear to be far superior.
6. Expert opinion
Addressing the issues of climate change within the pharmaceutical industry is finally being recognized as a priority, owing to the attention being paid and the research that is being performed within the area. It is clear to see both the benefits and drawbacks of the Ets discussed throughout, although it is important for the readers to be aware which may be more important in their respective categories.
AM is guaranteed to be a technology that will constantly evolve throughout the next decade, especially within the pharmaceutical field. Both 3DP and 4D printing are becoming the gold standard of precise manufacturing and with dedicated research into faculties such as ML, the environmental impact and cost of the processes should be reduced also. The flexibility that AM offer in terms of the printers available and their compatible materials allow for sustainable choices to be made throughout the printfor example, choosing SLS over SLM to complete a design, owing to the reduced energy usage of the printer.
Printing structures without supports, or with fewer supports, is also key to reducing material wastage and printing time. This can be achieved via a designed thinking approach, ensuring that the digital blueprint for the structure is optimized to the best capacity. This can be coupled up by establishing that the operator has expertise in AM and is capable of designing print files in such a way.
The reuse of materials for AM (), whilst being an innovative way to reuse waste materials, may not always be appropriate to apply, since the shredding and extrusion processes will require energy to run and produce the AM filament. The amount of waste created will be reduced, which for plastics and their long degradation times will be favorable, however it may be more applicable, in some cases for plastic waste to be sent directly to a recycling center instead.
MFs, despite the frequently high set-up cost, are truly establishing itself as a field-leader for nanoformulation, both from a resource perspective, but also in terms of the quality of product that it can produce. Compared to its traditional counterparts, MFs appears far superior for formulation manufacturing, and is currently being investigated for a wide spectrum of uses, from nanoformulation to microreactors. Research into paper-based microfluidics is also gaining much traction due to its capacity for portability, low cost, and recyclability. The capacity for MFs to act directly as an environmental monitoring system is also an attractive feature to the technology for environmental stability and one that can be directly improved via coupling with AM technology during chip synthesis. The biggest barrier to MFs though is its scalability to an industrial level, which is the main reason that research in the area has been hesitant in the past.
In general, the authors would note that these technologies are likely to expand rapidly within the pharmaceutical industry, and provide sustainable, cost-effective processes, which are only likely to improve given more time and research. Their implementation is easily adapted to suit a circular economy, whereby all parties involved will benefit. AM is more likely to suffer from legal issues, owing to its digitalization, however as the practice becomes more common, clearer legislation will come in place, decreasing the risk of IP conflicts. Environmentally friendly processes are available to both technologies, depending upon the operator’s proficiency and the design of the method being used. It is likely that as the commercial utilization of AM becomes commonplace, so will the skills of the operators, meaning that a larger proportion of the population will have an improved skillset to operate 3D printers for more complex tasks, such as within the pharmaceutical industry.
Article highlights
Additive Manufacturing (AM) and Microfluidics (MFs) are both able to provide environmentally friendly and economically viable methods for manufacturing.
Reducing the amount of materials used, especially solvents, is an attractive property for these emerging technologies.
Increasing operator expertise will drastically improve the sustainability of AM and MFs.
Sustainability drawbacks for AM include legal concerns over digital intellectual property, and drawbacks for MFs include scalability issues.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Masson-Delmotte V, Zhai P, and Roberts D, et al. Global warming of 1.5 C. IPCC Spec Rep Impacts Global Warming. 2018;1(5):49–91.
- Mountford H, Waskow D, and Gonzalez L, et al. COP26: key outcomes from the un climate talks in glasgow. World Resources Institute. 2021.
- Belkhir L, Elmeligi A. Carbon footprint of the global pharmaceutical industry and relative impact of its major players. J Clean Prod. 2019;214:185–194.
- Malik A, Lenzen M, McAlister S, et al. The carbon footprint of Australian health care. Lancet Planet Health. 2018;2(1):e27–e35.
- Jose J, Philip L. Continuous flow pulsed power plasma reactor for the treatment of aqueous solution containing volatile organic compounds and real pharmaceutical wastewater. J Environ Manage. 2021;286:112202.
- Fallah Z, Zare EN, and Ghomi M, et al. Toxicity and remediation of pharmaceuticals and pesticides using metal oxides and carbon nanomaterials. Vol. 275. Elsevier: Chemosphere; 2021. p. 130055.
- Lin J, Zhou Q, Zhu W, et al. Formation of formaldehyde as an artifact peak in head space GC analysis resulting from decomposition of sample diluent DMSO: a GC-MS investigation with deuterated DMSO. J Pharm Biomed Anal. 2020;188:113361.
- Yaseen G, Ahmad M, and Zafar M, et al. Green sustainable process for chemical and environmental engineering and science. In: Current status of solvents used in the pharmaceutical industry. ScienceDirect: Elsevier; 2021.
- Alshemari A, Breen L, Quinn G, et al. Can we create a circular pharmaceutical supply chain (CPSC) to reduce medicines waste? Pharmacy. 2020;8(4):221.
- Charoo NA, Barakh Ali SF, Mohamed EM, et al. Selective laser sintering 3D printing–an overview of the technology and pharmaceutical applications. Drug Dev Ind Pharm. 2020;46(6):869–877.
- Cadman B, Wright D, Bale A, et al. Pharmacist provided medicines reconciliation within 24 hours of admission and on discharge: a randomised controlled pilot study. BMJ Open. 2017;7(3):e013647.
- McCoubrey LE, Elbadawi M, Basit AW. Current clinical translation of microbiome medicines. Trends Pharmacol Sci. 2022;43(4):281–292.
- Lee S. Miniaturized passive hydrogel check valves for the treatment of hydrocephalic fluid retention. Arizona State University. 2020. p. 153.
- Liang K, Brambilla D, Leroux JC. Is 3D printing of pharmaceuticals a disruptor or enabler? Adv Mater. 2019;31(5):1805680.
- Francesko A, Cardoso VF, Lanceros-Méndez S. Chapter 1 - Lab-on-a-chip technology and microfluidics. In: Santos HA, Liu D, Zhang H, editors. Microfluidics for pharmaceutical applications. USA: William Andrew Publishing; 2019. p. 3–36.
- Nielsen J, Kaldor J, Irwin A, et al. Bespoke regulation for bespoke medicine? A comparative analysis of bioprinting regulation in Europe, the USA and Australia. J 3D Print Med. 2021;5(3):155–167.
- Elvira KS. Microfluidic technologies for drug discovery and development: friend or foe? Trends Pharmacol Sci. 2021;42(7):518–526.
- Wang T, Yu C, Xie X. Microfluidics for environmental applications. Singapore: Springer; 2020.
- Devi A, Mathiyazhagan K, Kumar H. Additive manufacturing in supply chain management: a systematic review. Singapore: Springer; 2021.
- Ma F, Zhang H, Hon KKB, et al. An optimization approach of selective laser sintering considering energy consumption and material cost. J Clean Prod. 2018;199:529–537.
- Ngo TD, Kashani A, and Imbalzano G, et al. Additive manufacturing (3D printing): a review of materials, methods, applications and challenges. Composites Part B Eng. 2018;143:172–196.
- Peng T, Kellens K, and Tang R, et al. Sustainability of additive manufacturing: an overview on its energy demand and environmental impact. Vol. 21. Netherlands: Additive Manufacturing; 2018. p. 694–704.
- Gardan J. Additive manufacturing technologies: state of the art and trends. Addit Manuf Handb. 2017: 149–168
- Niaki MK, Torabi SA, Nonino F. Why manufacturers adopt additive manufacturing technologies: the role of sustainability. J Clean Prod. 2019;222:381–392.
- Cicala G, Latteri A, Del Curto B, et al. Engineering thermoplastics for additive manufacturing: a critical perspective with experimental evidence to support functional applications. J Appl Biomater Funct Mater. 2017;15(1):10–18.
- Booth JW, Alperovich J, Chawla P, et al. The design for additive manufacturing worksheet. J Mech Des. 2017;139(10):100904.
- Colorado HA, Velásquez EIG, Monteiro SN. Sustainability of additive manufacturing: the circular economy of materials and environmental perspectives. J Mater Res Technol. 2020;9(4):8221–8234.
- Zontek TL, Ogle BR, Jankovic JT, et al. An exposure assessment of desktop 3D printing. J Che Health & Saf. 2017;24(2):15–25.
- Awad A, Fina F, Goyanes A, et al. 3D printing: principles and pharmaceutical applications of selective laser sintering. Int J Pharm. 2020;586:119594.
- Jamróz W, Szafraniec J, Kurek M, et al. 3D printing in pharmaceutical and medical applications–recent achievements and challenges. Pharm Res. 2018;35(9):1–22.
- Yang Y, Li L, Pan Y, et al. Energy consumption modeling of stereolithography‐based additive manufacturing toward environmental sustainability. J Ind Ecol. 2017;21(S1):S168–S178.
- Kaza A. Medical applications of stereolithography: an overview; 2018.
- Mathew E, Pitzanti G, Larrañeta E, et al. 3D printing of pharmaceuticals and drug delivery devices. Pharmaceutics. 2020;12(3):266.
- Mathew E, Domínguez-Robles J, Stewart SA, et al. Fused deposition modeling as an effective tool for anti-infective dialysis catheter fabrication. ACS Biomater Sci Eng. 2019;5(11):6300–6310.
- Caminero M, Chacón JM, and García-Moreno I, et al. Interlaminar bonding performance of 3D printed continuous fibre reinforced thermoplastic composites using fused deposition modelling. Vol. 68. ScienceDirect: Polymer Testing; 2018. p. 415–423.
- Enemuoh EU, Menta VG, Abutunis A, et al. Energy and eco-impact evaluation of fused deposition modeling and injection molding of polylactic acid. Sustainability. 2021;13(4):1875.
- Skowyra J, Pietrzak K, Alhnan MA. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur J Pharm Sci. 2015;68:11–17.
- Baran E, Erbil H. Surface modification of 3d printed pla objects by fused deposition modeling: a review. Colloids and Interfaces. 2019;3(2):43
- Zhang W-N, Wang L, and Feng Z, et al. Research progress on selective laser melting (SLM) of magnesium alloys: a review. Vol. 207. Germany: Optik; 2020. p. 163842.
- Zhang J, Li F, and Zhu Q, et al. Preparation of silicon carbide reinforced aluminium matrix composites (SiC/Al) by selective laser melting. IOP Conference Series: Materials Science and Engineering; 2019. UK: IOP Publishing.
- Yap CY, Chua CK, Dong ZL, et al. Review of selective laser melting: materials and applications. Appl Phys Rev. 2015;2(4):041101.
- Wu Y-F, Kazmi SMS, Munir MJ, et al. Effect of compression casting method on the compressive strength, elastic modulus and microstructure of rubber concrete. J Clean Prod. 2020;264:121746.
- Meagher P, O’Cearbhaill ED, Byrne JH, et al. Bulk metallic glasses for implantable medical devices and surgical tools. Adv Mater. 2016;28(27):5755–5762.
- Heaney DF. Handbook of metal injection molding. UK: Woodhead Publishing; 2018.
- Mader M, Schlatter O, Heck B, et al. High-throughput injection molding of transparent fused silica glass. Science. 2021;372(6538):182–186.
- Sarkis J. Supply chain sustainability: learning from the COVID-19 pandemic. Int J Oper Prod Manage. 2020;41(1):63–73.
- Huang Y-S, Fang -C-C, Lin Y-A. Inventory management in supply chains with consideration of Logistics, green investment and different carbon emissions policies. Vol. 139. Elsevier: Computers & Industrial Engineering; 2020.
- Sauerwein M, Doubrovski E, Balkenende R, et al. Exploring the potential of additive manufacturing for product design in a circular economy. J Clean Prod. 2019;226:1138–1149.
- Stoof D, Pickering K. Sustainable composite fused deposition modelling filament using recycled pre-consumer polypropylene. Composites Part B Eng. 2018;135:110–118.
- Zhu C, Li T, Mohideen MM, et al., Realization of circular economy of 3D printed plastics: a review. Polymers. 13(5): 744. 2021.
- Uddin M, Williams D, Blencowe A. Recycling of selective laser sintering waste nylon powders into fused filament fabrication parts reinforced with Mg particles. Polymers. 2021;13(13):2046.
- Arrizubieta JI, Ukar O, Ostolaza M, et al. Study of the environmental implications of using metal powder in additive manufacturing and its handling. Metals. 2020;10(2):261.
- Dadbakhsh S, Verbelen L, Verkinderen O, et al. Effect of PA12 powder reuse on coalescence behaviour and microstructure of SLS parts. Eur Polym J. 2017;92:250–262.
- Del Re F, Contaldi V, Astarita A, et al. Statistical approach for assessing the effect of powder reuse on the final quality of AlSi10Mg parts produced by laser powder bed fusion additive manufacturing. Int J Adv Manuf Technol. 2018;97(5–8):2231–2240.
- Kellens K, Baumers M, Gutowski TG, et al. Environmental dimensions of additive manufacturing: mapping application domains and their environmental implications. J Ind Ecol. 2017;21(S1):S49–S68.
- Diment LE, Thompson MS, Bergmann JHM. Clinical efficacy and effectiveness of 3D printing: a systematic review. BMJ Open. 2017;7(12):e016891.
- Nadagouda MN, Rastogi V, Ginn M. A review on 3D printing techniques for medical applications. Curr Opin Chem Eng. 2020;28:152–157.
- Beer N, Hegger I, Kaae S, et al. Scenarios for 3D printing of personalized medicines - A case study. Explor Res Clin Social Pharm. 2021; 4: 100073.
- Krauel L, Valls-Esteve A, and Tejo-Otero A, et al. 3D-Printing in surgery: beyond bone structures. A review. Ann 3D Printed Med. 2021;4:100039.
- Tejo-Otero A, Buj-Corral I, Fenollosa-Artés F. 3D printing in medicine for preoperative surgical planning: a review. Ann Biomed Eng. 2020;48(2):536–555.
- Trauner KB. The emerging role of 3D printing in arthroplasty and orthopedics. J Arthroplasty. 2018;33(8):2352–2354.
- Kurpjuweit S, Schmidt CG, Klöckner M, et al. Blockchain in additive manufacturing and its impact on supply chains. J Bus Logist. 2021;42(1):46–70.
- Ghobakhloo M. Industry 4.0, digitization, and opportunities for sustainability. J Clean Prod. 2020;252:119869.
- Abideen AZ, Sundram VPK, Pyeman J, et al. Digital twin integrated reinforced learning in supply chain and logistics. Logistics. 2021;5(4):84.
- Goh GD, Sing SL, Yeong WY. A review on machine learning in 3D printing: applications, potential, and challenges. Artif Intell Rev. 2021;54(1):63–94.
- Sun J, Zhou W, Huang D, et al. An overview of 3d printing technologies for food fabrication. Food Bioprocess Technol. 2015;8(8):1605–1615.
- Nachal N, Moses JA, Karthik P, et al. Applications of 3D printing in food processing. Food Eng Rev. 2019;11(3):123–141.
- Rogers H, Srivastava M. Emerging sustainable supply chain models for 3D food printing. Sustainability. 2021;13(21):12085.
- Severini C, Azzollini D, and Albenzio M, et al. On printability, quality and nutritional properties of 3D printed cereal based snacks enriched with edible insects. Vol. 106. UK: Food Research International; 2018. p. 666–676.
- Naranjo E, Merfa MV, and Santra S, et al. Zinkicide is a ZnO-based nanoformulation with bactericidal activity against Liberibacter crescens in batch cultures and in microfluidic chambers simulating plant vascular systems. Appl Environ Microbiol. 2020;86(16):20–00788.
- Vicente FA, Plazl I, Ventura SPM, et al. Separation and purification of biomacromolecules based on microfluidics. Green Chem. 2020;22(14):4391–4410.
- Wlodarczyk KL, Hand DP, Maroto-Valer MM. Maskless, rapid manufacturing of glass microfluidic devices using a picosecond pulsed laser. Sci Rep. 2019;9(1):20215.
- Jasińska L, Malecha K. Microfluidic modules integrated with microwave components—overview of applications from the perspective of different manufacturing technologies. Sensors. 2021;21(5):1710.
- Gharib R, Auezova L, Charcosset C, et al. Effect of a series of essential oil molecules on DPPC membrane fluidity: a biophysical study. J Iran Chem Soc. 2018;15(1):75–84.
- Weaver E, O’Connor E, Cole DK, et al. Microfluidic-mediated self-assembly of phospholipids for the delivery of biologic molecules. Int J Pharm. 2022;611:121347.
- Zhang J, Wang K, Teixeira AR, et al. Design and scaling up of microchemical systems: a review. Annu Rev Chem Biomol Eng. 2017;8(1):285–305.
- Pena-Pereira F, Kloskowski A, Namieśnik J. Perspectives on the replacement of harmful organic solvents in analytical methodologies: a framework toward the implementation of a generation of eco-friendly alternatives. Green Chem. 2015;17(7):3687–3705.
- Gilbertson LM, Zimmerman JB, Plata DL, et al., Designing nanomaterials to maximize performance and minimize undesirable implications guided by the principles of green chemistry. Chem Soc Rev. 44(16): 5758–5777. 2015.
- Tong R, Zhang L, Yang X, et al. Emission characteristics and probabilistic health risk of volatile organic compounds from solvents in wooden furniture manufacturing. J Clean Prod. 2019;208:1096–1108.
- Zhang K, Li L, and Huang L, et al. The impact of volatile organic compounds on ozone formation in the suburban area of Shanghai. Vol. 232. Elsevier: Atmospheric Environment; 2020. p. 117511.
- Bian F, Sun L, Cai L, et al. Colloidal crystals from microfluidics. Small. 2020;16(9):1903931.
- Ahn J, Ko J, and Lee S, et al. Microfluidics in nanoparticle drug delivery; From synthesis to pre-clinical screening. Adv Drug Deliv Rev. 2018;128:29–53.
- Bhattacharya S, Kumar S, Agarwal AK, 2019. Paper microfluidics. Singapore: Springer.
- Andar A, Hasan M-S, Srinivasan V, et al. Wood microfluidics. Anal Chem. 2019;91(17):11004–11012.
- Wang C, Yu C. Analytical characterization using surface-enhanced Raman scattering (SERS) and microfluidic sampling. Nanotechnology. 2015;26(9):092001.
- Yew M, Ren Y, Koh KS, et al. A review of state-of-the-art microfluidic technologies for environmental applications: detection and remediation. Global Challenges. 2019;3(1):1800060.
- García Alonso D, Yu M, Qu H, et al. Advances in microfluidics‐based technologies for single cell culture. Adv Biosyst. 2019;3(11):1900003.
- Kelley SO, Mirkin CA, Walt DR, et al. Advancing the speed, sensitivity and accuracy of biomolecular detection using multi-length-scale engineering. Nat Nanotechnol. 2014;9(12):969–980.
- Yao B, Zhang J, Kou T, et al. Paper‐based electrodes for flexible energy storage devices. Adv Sci. 2017;4(7):1700107.
- Mianehro A. Electrospun bioscaffold based on cellulose acetate and dendrimer-modified cellulose nanocrystals for controlled drug release. Carbohydr Polym Technol Appl. 2022;3:100187.
- Liu Y, Li T, Han Y, et al. Recent development of electrospun wound dressing. Curr Opin Biomed Eng. 2021;17:100247.
- Truong TH, Musilová L, and Kašpárková V, et al. New approach to prepare cytocompatible 3D scaffolds via the combination of sodium hyaluronate and colloidal particles of conductive polymers. Sci Rep. 2022;12(1):1–16.
- Agarwal S, Greiner A. On the way to clean and safe electrospinning-green electrospinning: emulsion and suspension electrospinning. ?Polym Adv Technol. 2011;22(3):372–378.
- Deng Y, Lu T, and Cui J, et al. Bio-based electrospun nanofiber as building blocks for a novel eco-friendly air filtration membrane: a review. Vol. 277. Netherlands: Separation and Purification Technology; 2021. p. 119623.
- Dou Y, Zhang W, Kaiser A. Electrospinning of metal–organic frameworks for energy and environmental applications. Adv Sci. 2020;7(3):1902590.