ABSTRACT
Introduction
Injected mRNA vaccines have been proven effective and safe in the SARS-CoV-2 pandemic. Using the machinery of the cell, mRNA vaccines translate into an antigen, which triggers an adaptive immune response. The effectiveness of intramuscular administered mRNA vaccines wanes in the months post-vaccination, which makes frequent booster administrations necessary. To make booster administration easier and increase efficacy, pulmonary administration could be investigated. The aim of this literature study was therefore to review the published preclinical (animal) studies on the safety and efficacy of pulmonary administered mRNA vaccines.
Areas covered
We first provide background information on mRNA vaccines and immunological mechanisms of vaccination. Thereafter, we provide an evaluation of published animal studies, in which mRNA vaccines (or mRNA containing nanoparticles) were delivered into the lungs. We covered the following areas: biodistribution, cellular uptake, immune response, protection, and safety. All relevant papers were found using PubMed/MEDLINE database.
Expert opinion
In our opinion, head-to-head comparison studies examining the safety and efficacy of intramuscular injected and pulmonary administered liquid mRNA vaccines should be performed first. When pulmonary delivered mRNA vaccines are shown to be effective and safe, inhalable dry powder formulations should be engineered. Finally, the tolerability of patients with respiratory diseases should be considered.
1. Introduction
mRNA vaccines are of increased interest in the scientific world, predominantly caused by recent developments in the SARS-CoV-2 pandemic. This pandemic has transformed the once-dismissed concept of mRNA vaccines into a powerful platform for eliciting potent immune responses. Unlike traditional vaccines, such as live-attenuated and inactivated organisms, or purified products derived from them, mRNA vaccines provide our cells with instructions on how to synthesize a specific protein – or in some cases a small part of a protein – to trigger an adaptive immune response [Citation1–3]. To facilitate uptake by cells, mRNA is often formulated into ionizable lipid nanoparticles (LNPs). This technology has been successfully implemented by BioNTech/Pfizer and Moderna, resulting in the well tolerated and highly efficacious mRNA vaccines BNT162b2 and mRNA-1273, respectively [Citation1,Citation2]. These vaccines have been shown to reduce hospitalization rates by more than 90% upon infection with SARS-CoV-2 (B.1.1.7 variant) in people who received two doses via intramuscular injection [Citation4–6]. The protection conferred by these vaccines, however, has been shown to wane in the months following vaccination, even in individuals that received three doses [Citation7–9]. The waning immunity is caused by a reduced immune response or by the fact that the SARS-CoV-2 variants of concern (VoC) display significantly different antigens than the Wuhan-Hu-1 strain that is used for the mRNA vaccines, but most likely both reasons play a role [Citation10–12]. The need for frequent booster injections is a burden to the health system and individual patient. In addition, the transmissibility of the SARS-CoV-2 virus remains fairly high [Citation13,Citation14]. These findings highlight the importance of further considering strategies for improving the efficacy of mRNA vaccines, for instance, by looking into alternative administration routes (e.g. pulmonary, intramucosal, intradermal).
To improve the vaccine’s efficacy, it could be beneficial to use the same route of administration for the vaccine as the natural route of infection. Administering mRNA vaccines against airborne transmitted viruses via the pulmonary route, for instance, would eliminate the use of needles as well as the risk of needle-stick injuries and requirements for trained healthcare personnel [Citation15]. Moreover, it is a lower burden to the vaccinee. The most important advantage of pulmonary administration, however, lies in the fact that this route not only produces a systemic immune response (e.g. IgG production), as is the case after intramuscular injection, but also a local mucosal immune response (e.g. IgA production) [Citation15–17]. Intramuscular administration of SARS-CoV-2 mRNA vaccines has shown to increase serum IgA levels as well, yet serum IgA peak levels were lower and declined more rapidly than serum IgG levels [Citation18–20]. Furthermore, it has been shown that after SARS-CoV-2 infection secretory IgA levels in the mucosa persist longer and are more potent than serum IgA levels [Citation18,Citation20]. IgA responses are of particular interest since it has been shown that IgA leads to cross-protection after influenza infection or pulmonary vaccination with an influenza vaccine in both mice and human [Citation21–26]. Therefore, the mucosal response has been postulated to improve the efficacy of vaccines [Citation27–29]. The value of administering vaccines via the pulmonary route has been elegantly demonstrated by Tomar et al. [Citation30]. In this study, the authors compared intramuscular injection to pulmonary administration in BALB/c mice and found the former approach yielded only IgG titers, whereas administration via the pulmonary route generated IgG and IgA titers [Citation30]. This effect was observed for both influenza and hepatitis B vaccines, meaning immune responses are orchestrated not only against airborne infectious diseases but also those spread via the blood or body fluids. Administering mRNA vaccines via the pulmonary route therefore seems highly attractive.
Already more than 50 years ago, the efficacy of pulmonary administered influenza whole inactivated virus vaccine was demonstrated in humans [Citation31]. In the past decade, several studies have been published in which pulmonary administration of mRNA-containing nanoparticles was evaluated in laboratory animals [Citation32–53]. Some of those were focused on characterizing cell uptake and biodistribution, whereas others focused on analyzing immunological effects and safety. Only few studies examined mRNA used as a vaccine. We therefore decided to include studies focusing on mRNA vaccines, mRNA nanoparticles to increase protein levels or nanoparticles containing model mRNA as well. At this point, it is not entirely clear whether the pulmonary delivery route is suitable for the administration of mRNA vaccines, as the current state of knowledge has not been adequately reviewed yet. The aim of this literature study was therefore to investigate whether administration of mRNA vaccines via the pulmonary route is safe and effective based on published animal studies. In this review, we first provide background information on mRNA vaccines as well as immunological mechanisms of vaccination. We then discuss the published animal studies in terms of biodistribution, cell uptake, immunological effects, and safety. The review will end with our expert opinion, describing our interpretation of the data and vision for the future: a future that looks beyond intramuscular injection as a go-to method for administering mRNA vaccines.
2. mRNA vaccines
mRNA vaccines contain mRNA strands, encoding a disease-specific antigen, formulated into nanoparticles [Citation54]. Constructing the mRNA sequence represents the first step in vaccine development and requires special consideration. The mRNA is constructed using in vitro transcription (IVT). IVT is a process where the promoter of synthesized DNA is recognized by a polymerase that produces multiple copies of the mRNA. The synthesized DNA is optimized by flanking the open reading frame (ORF) with untranslated regions (UTRs) and adding a poly-A tail at the 3’ end to allow for efficient translation and stabilization [Citation3,Citation54–60]. Following transcription, the mRNA is purified and additional modifications are added, e.g. a 5’ cap [Citation55,Citation56]. Other modifications are made to enhance the potency of mRNA vaccines, e.g. the substitution of base pairs reduces the activation of endosomal RNA-sensing proteins, such as toll-like receptor (TLR) 7/8 [Citation56,Citation58,Citation59,Citation61]. Uridine, for example, is often replaced by 1-methylpsuedouridine (m1Ψ), as the latter avoids activation of the antiviral immune response that leads to translational suppression in cells and, by extension, strongly reduced cellular and humoral immunity [Citation54,Citation59,Citation61–63]. These mRNA modifications enable efficient antigen expression.
The internalization of mRNA into cells requires nanoparticles [Citation64–66]. Using nanoparticles is crucial, as mRNA cannot readily pass cell membranes due to its relatively large size and negative charge [Citation54,Citation64]. On top of that, nanoparticles protect the single-stranded mRNA molecules from degradation by circulating nucleases. In the past decades, a myriad of (platform) technologies have been developed, often exploiting the unique properties of polymers, peptides, or lipids [Citation17,Citation67,Citation68]. The use of polymers, which may be synthetic (e.g. polyethylenimine (PEI)) or originate from natural sources (e.g. chitosan), was immensely popular in the past, but has slowly started giving way to lipid-based nanoparticles. This shift was caused by the increased availability of lipid nanoparticle technologies that have more desirable safety profiles. Due to these advantages and the fact that LNPs are used in the FDA-approved vaccines, they will be discussed in more detail [Citation69]. Details about polymer-based and peptide-based nanoparticles are described elsewhere [Citation60,Citation67,Citation68,Citation70]. LNPs represent the most advanced, clinically available technology. Formerly, positively charged LNPs were used, while recently neutral LNPs are more common [Citation71]. LNPs are composed of various types of phospholipids, such as ionizable lipids, helper lipids, cholesterol, and polyethylene glycol (PEG) lipids [Citation54,Citation60,Citation64,Citation69,Citation72]. Each of these lipids serves an important role. In physiological conditions, LNPs are neutral, but upon uptake into endosomes, the ionizable lipids on the surface become charged, leading to endosomal disruption and release of mRNA into the cytosol [Citation64]. The other lipids are used for stabilization purposes and for controlling the size of nanoparticles. LNPs have also been shown to be exceptionally safe since low inflammation levels and a good tolerability have been shown in rats and monkeys and a low reactogenicity has been shown in human after intramuscular injection of LNPs [Citation1,Citation2,Citation73,Citation74].
Upon intramuscular injection, mRNA-containing nanoparticles are taken up by cells located near the injection site and by cells located in distal tissues (e.g. lymph nodes and liver) [Citation75,Citation76]. Cells capable of internalizing mRNA vaccines include not only antigen presenting cells (APCs), such as dendritic cells and macrophages, but also other nucleated cells, such as endothelial and epithelial cells [Citation3]. Following endocytosis, endosomal escape of the mRNA into the cytosol is facilitated by the nanoparticle [Citation3,Citation61]. The mRNA is subsequently translated by ribosomes, resulting in the production of antigen [Citation3,Citation61]. BNT162b2 and mRNA-1273, for example, produce full-length spike proteins of SARS-CoV-2 and BNT162b1 encodes for the SARS-CoV-2 receptor-binding domain (RBD) [Citation1,Citation2,Citation77]. Antigens in the cytosol are then degraded into smaller fragments by means of proteasomal degradation, after which the fragments are presented on the surface of cells by major histocompatibility complex (MHC)-1 proteins [Citation3,Citation77]. This route is available to APCs and all other nucleated cells. The antigen can also be excreted by, e.g., APCs to be consequently taken up again by APCs. In this case, the antigen is degraded into fragments through lysosomal degradation, and the resulting fragments are expressed on the surface of APCs by MHC-2 [Citation3]. Since the mRNA is genetically modified to make it more stable, the mRNA can stay in the cytosol and produce the antigen for a few days before it is degraded [Citation38].
3. Immunological mechanisms of vaccination
Once APCs, in particular, dendritic cells, have finished processing antigens and are expressing peptide fragments on their surface via MHC proteins, they migrate toward the draining lymph nodes via the afferent lymph vessels [Citation3]. In the lymph nodes, APCs interact with naive lymphocytes that bear receptors capable of recognizing specific peptide fragment, a process called clonal selection. Only a small fraction of these lymphocytes have receptors highly specific for the antigen. These cells then proliferate and differentiate, resulting in the production of effector cells. This step is called clonal expansion [Citation3]. This process gives rise to CD8+ cytotoxic T cells, which are able to kill infected cells, and CD4+ T helper cells, which help B cells to become antibody-producing plasma cells [Citation78]. This represents the cellular component of the adaptive immune response [Citation78]. The humoral component involves the production of virus-neutralizing antibodies by plasma cells. Selected lymphocytes are also transported to ‘infected’ tissue via efferent lymph nodes and blood. Some of the lymphocytes persist in the body, providing long-term immunological memory of pathogens or parts of a pathogen [Citation3,Citation61]. The length of protection varies; in case of SARS-CoV-2, it may be fairly short-lived (<6 months) especially when new VoCs emerge [Citation7,Citation12,Citation79].
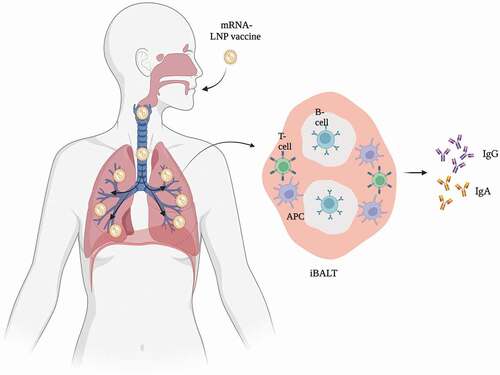
The lymphoid system plays an important role in orchestrating adaptive immune responses to pathogens. It is therefore not surprising that the route of administration for vaccines affects the extent to which such responses develop. Unlike intramuscular injection, pulmonary administration of vaccines against airborne transmitted viruses leads to the formation of inducible bronchus-associated lymphoid tissue (iBALT) [Citation16,Citation80–82]. iBALT represents a tertiary lymphoid structure, containing B cells and plasma cells as well as T cells and APCs, and is an effective priming site for mucosal immune responses [Citation16,Citation80–82]. The immune responses initiated in iBALT are slower than those in the lymph nodes, as iBALT formation takes some time. Still, iBALT is able to process antigens, neutralize pathogens by producing IgG and IgA antibodies, and react quickly upon secondary infections () [Citation16,Citation80,Citation81]. This system also contributes to a broader humoral immune response upon infection or vaccination compared to the systemic immune response. Where the systemic immune response mainly triggers the production of IgG and to a lesser extent IgA, the mucosal immune system triggers the production of high levels of IgG as well as (local) IgA [Citation16]. IgA, produced by the mucosal tissue, serves an important function because it neutralizes pathogens and prevents them from binding to mucosal tissue [Citation16,Citation83–86].
Once infections have been cleared, or when the primary adaptive immune response has passed, effector lymphocytes and antibodies have accumulated at the infected site, or the site of injection for vaccines [Citation63,Citation78]. This offers protection against the same pathogen in the short term. Long-term protection is offered by memory B and T lymphocytes, which persist to afford protective immunity upon re-encountering a specific pathogen, or a small part of it [Citation61,Citation63,Citation78,Citation83]. In case of re-exposure, a secondary adaptive immune response is initiated [Citation63,Citation83]. This response is considerably stronger and faster than the primary response. In fact, successive exposure to a particular antigen leads to even more pronounced responses [Citation63,Citation83]. The resulting antibodies typically have an improved binding affinity for the respective targets [Citation63]. BNT162b2 and mRNA-1273, for instance, require two doses to generate sufficient cellular and humoral immunity against SARS-CoV-2 and for some individuals three doses are recommended [Citation1,Citation2,Citation9,Citation87,Citation88]. Memory cells are therefore essential. The maintenance of these cell populations, be it within the lymphoid system or mucosal surfaces, does not require the presence of the original antigen; memory cells persist as long as they are exposed to pro-survival cytokines, such as IL-7 and IL-15 [Citation89]. Accordingly, pulmonary vaccination seems promising.
4. Animal studies
We studied whether pulmonary administration of mRNA vaccines is a safe and efficacious alternative to intramuscular injection. To do so, we reviewed published animal studies, as clinical data is lacking. Studies were identified using the PubMed/MEDLINE database. We identified 22 publications, 6 of which explored immunological effects, 12 characterized the effects of model mRNA, and 8 focused on using mRNA-containing nanoparticles for restoring protein levels (). In all studies, the mRNA has been pulmonary administered in liquid formulations, except for one study, where mRNA was administered as dry powder formulation [Citation49]. Even if there is no specific focus on vaccination and protection, studies in which mRNA nanoparticles have been administered pulmonary can still provide valuable information, e.g. when it comes to biodistribution, cellular uptake, immunogenicity, protein expression, and safety (). Although the number of publications about pulmonary administered mRNA is not high yet, the interest has risen over the years (). The characteristics of each study are summarized in .
Figure 2. Publication trends. a. The number of publications examining pulmonary administered mRNA as a vaccine, model mRNA or as a medicine. b. Total amount of publications examining the parameters discussed in this review. c. Cumulative publications since the first publication.
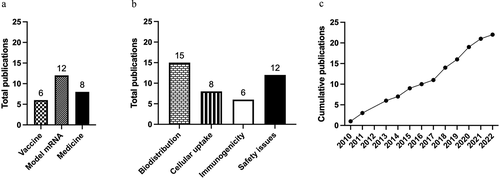
Table 1. Overview of publications on preclinical research on the efficacy and safety of pulmonary administered mRNA.
4.1. Biodistribution
The first step of pulmonary vaccination is the administration of the formulation to the airways. We therefore first explored biodistribution profiles, as the deposition site has been shown to affect adaptive immune responses [Citation30]. In one of the earlier reports, Su et al. [Citation41] showed that intranasally administered mRNA-containing nanoparticles stay in the nasal cavity until 12 h after administration (). Lung deposition, however, remained limited. A few years later, Phau et al. and Li et al. reported similar findings: high expression levels of luciferase were detected in the nasal cavity only [Citation34,Citation51]. Limited lung deposition was mostly observed due to the fact that the authors administered too low volumes (i.e. one dose of 15–20 µL per nostril). Larger volumes and repeated intranasal administrations (e.g. two doses of 20 µL) do lead to a fairly uniform deposition of nanoparticles containing mRNA throughout the lungs, as was shown by Robinson et al. [Citation46].
Figure 3. Biodistribution. a. Study by Su et al. [Citation41] showing the mRNA expression in the nasal cavity. Adapted with permission from Su et al. [Citation41]. Copyright 2022 American Chemical Society. b. Study by Patel et al. [Citation36] showing biodistribution of pulmonary administered mRNA in each lobe. c. The five lobes of a mice lung. d. Study by Tiwari et al. [Citation40] showing the immunohistochemistry staining.
![Figure 3. Biodistribution. a. Study by Su et al. [Citation41] showing the mRNA expression in the nasal cavity. Adapted with permission from Su et al. [Citation41]. Copyright 2022 American Chemical Society. b. Study by Patel et al. [Citation36] showing biodistribution of pulmonary administered mRNA in each lobe. c. The five lobes of a mice lung. d. Study by Tiwari et al. [Citation40] showing the immunohistochemistry staining.](/cms/asset/83a3a13d-a61b-42b0-9f74-30b783ee4970/iedd_a_2131767_f0003_oc.jpg)
Besides the volume, the device used to deliver the nanoparticles containing mRNA to the lungs is relevant. With microsprayers and nebulizers, a large volume can be inhaled (50–100 µL), which ensures that the mRNA-containing nanoparticles reach the peripheral airways [Citation33,Citation36,Citation38,Citation40,Citation47]. Patel et al. [Citation36], for example, used a nebulizer to administer the mRNA nanoparticles pulmonary and showed that mRNA is distributed throughout every lobe (). Immunofluorescent staining showed that mRNA-containing nanoparticles administered pulmonary using a microsprayer or nebulizer are evenly distributed throughout the lungs, as was shown by Tiwari et al. [Citation40] and Lokugamage et al. [Citation47] (). Two publications described that they delivered the mRNA nanoparticles intratracheally, via instillation or spraying [Citation45,Citation48]. Using these techniques, the mRNA-containing nanoparticles reached the deeper lungs as well [Citation45,Citation48]. The deposition site of pulmonary delivered mRNA vaccines should be examined in detail because it is known that the ideal deposition site of a vaccine differs per disease [Citation30]. Since lung tissue is vulnerable, a delicate, but potent immune response is desired minimizing adverse effects.
The mRNA nanoparticles should have a sufficient residence time in the nasal cavity or lungs since the mRNA needs time to be taken up by the cells and produce the antigen. The residence time is influenced by the type of nanoparticle. For example, Li et al. [Citation34] showed that the fluorescent signal of mRNA-containing PEI nanoparticles in the nasal cavity decays within 1 h and the signal was significantly lower than that of cyclodextrin-PEI (CP2k) nanoparticles. Besides the residence time, the transfection efficiency is determined by the type of nanoparticle as well. Patel et al. [Citation36] showed that mRNA encapsulated in a PEI nanoparticle is translated into significantly less protein than mRNA-containing hDD90-118 nanoparticles. Lipid nanoparticles are suggested to have an optimal protein production, as was shown by Van Hoecke et al. [Citation48] and Pardi et al. [Citation38]. They both showed a high protein concentration that peaked 24 h after pulmonary administration and after 48 h, no protein was measured in the lungs anymore. Taken together, these studies highlight the importance of using adequate techniques for achieving lung deposition of mRNA vaccines and the need to use deposition profiles or formulations that have a sufficiently long residence time in the airways to be taken up by the cells and produce a sufficient amount of antigen.
4.2. Cellular uptake
Once mRNA-containing nanoparticles are delivered into the airways, they should be internalized by cells in order to initiate protein production. It is not fully known, however, by which mechanism and by which cells nanoparticles are internalized. The proposed uptake mechanism of nanoparticles is endocytosis and pinocytosis, as shown in cell cultures [Citation90,Citation91]. We expect that these uptake mechanisms ensure cellular uptake of mRNA nanoparticles following pulmonary administration as well, although this has not been investigated yet. We therefore analyzed studies to collect more information about this issue. The first study to assess cell uptake was conducted by Lorenzi et al. [Citation32], who prepared naked mRNA encoding for Hsp65 in an attempt to protect mice from tuberculosis. Using flow cytometry, the authors found that the nanoparticles were taken up mostly by dendritic cells, while uptake by macrophages and B cells was marginal. Two years later, Andries et al. [Citation35] reported that mRNA-containing GL67/DOPE/DMPE-PEG5000 nanoparticles were mainly taken up by APCs in mice via phagocytosis, as the authors observed increased expression levels of IL-12, which is produced by APCs in response to antigenic stimulation. Interestingly, Van Hoecke et al. [Citation48] showed that DOPAP/cholesterol nanoparticles containing mRNA were predominantly taken up by alveolar macrophages, and to a lesser extent by dendritic cells. These observations were corroborated by Hajam et al. [Citation44], who found that macrophages were responsible for the uptake of empty chitosan nanoparticles in chickens. Unfortunately, the authors did not examine the uptake of nanoparticles containing mRNA.
Although it seems as if only APCs take up nanoparticles, evidence suggests that they can also be internalized by nonimmune cells. Patel et al. [Citation36], for example, used flow cytometry to reveal that epithelial as well as endothelial cells took up poly(beta-aminoester) (PBAE) nanoparticles containing luciferase mRNA. This might be related to differences in the materials that the particles are made of. APCs probably take up nanoparticles in a relatively nonspecific manner, whereas for other cell types it may depend on the material. Similarly, Li et al. showed that mRNA encapsulated in CP2k nanoparticles were taken up by nasal epithelial cells and nasal associated lymphoid tissue (NALT) [Citation34]. However, it is not clear whether these mRNA-CP2k nanoparticles ended up in the lungs as well [Citation34]. Extensive uptake by a large number of cell types was demonstrated by Mays et al. [Citation43], who administered naked mRNA encoding for Foxp3 in an attempt to treat allergic asthma. The authors found that the mRNA was taken up by eosinophils, CD4+ T cells, alveolar macrophages, and alveolar type II cells as well as neutrophils and lymphocytes, albeit to a lesser extent [Citation43]. These findings are remarkable, considering the fact that the uptake of naked mRNA was shown to be negligible in vitro, owing to its large size and negative charge as well as susceptibility to nucleases [Citation54,Citation64]. Collectively, however, these studies do indicate that nanoparticles, when administered via the pulmonary route, are mostly internalized by APCs, which is not surprising as they are known to have a tremendous phagocytotic capacity [Citation92].
4.3. Immunogenicity
Cellular uptake of mRNA vaccines should be followed by protein expression inducing a potent immune response. We therefore took a closer look at studies that characterized humoral and cellular responses as well as the extent of protection conferred by pulmonary vaccination. Six published animal studies presented data on humoral and/or cellular responses, of which three also conducted challenge experiments (). In each study, mRNA vaccines were delivered to either the nasal cavity or the lung via intranasal administration, using sufficient volumes, or intratracheal instillation.
Table 2. Pulmonary mRNA vaccination studies.
4.3.1. Humoral immune response
The humoral, or antibody, immune response is essential for neutralizing foreign material, preventing the attachment of pathogens to host cells and marking the pathogen for destruction [Citation3,Citation93]. The first study that reported data on IgA and IgG titers was carried out by Li et al. [Citation34]. In this study, intranasal administration of mRNA vaccines encoding for gp120 of human immunodeficiency virus (HIV) in mice was shown to increase IgG and IgA titers in the cervix and nasal tissue. The authors also confirmed the importance of incorporating mRNA into a nanoparticle, as this contributed to significantly higher levels of IgG and IgA than naked mRNA. Moreover, the type of nanoparticle affected IgA and, to a lesser extent, IgG levels. Nanoparticles prepared from PEI yielded lower antibody titers than those made of CP2k. The cause of this difference is not fully understood, but it could be caused by intrinsic toxicity of PEI, leading to the death of cells that take up nanoparticles (e.g. APCs) or variations on uptake efficiency between the different nanoparticles [Citation36,Citation94]. Unfortunately, Li et al. [Citation34] did not challenge mice with HIV after vaccination, and it is therefore not known whether protection was conferred. Follow-up work by Li et al. described similar results [Citation53]. In this case, the authors treated mice with a mRNA vaccine encoding for ovalbumin. The nanoparticles were made from PEI or CP2k, the latter of which proved to produce superior levels of vaginal IgA and IgG. It remains unclear, however, whether antibody levels in the pulmonary mucosa follow the same pattern. As the authors used mRNA encoding ovalbumin, protection was not considered.
Later, Hajam et al. [Citation44] studied IgA production in lung mucosa of chickens after pulmonary vaccination using either empty chitosan nanoparticles, chitosan nanoparticles with influenza HA2 and M2E proteins, or chitosan nanoparticles with influenza HA2 and M2E proteins and mRNA encoding for these proteins. The addition of mRNA to the nanoparticles resulted in higher IgA levels than those containing only protein. This effect was not observed for IgG titers. These authors also challenged the vaccinated chickens with live influenza virus. Chickens that received both protein and mRNA-containing nanoparticles had significantly higher levels of virus-neutralizing titers than those receiving only protein-containing nanoparticles. Unfortunately, head-to-head comparisons between intramuscular injections and pulmonary administration are very limited. In fact, the only study known to have done so was carried out by Anderluzzi et al. [Citation50], who examined pulmonary delivered mRNA encoding for rabies virus glycoprotein (RVG) encapsulated in four different lipid nanoparticles in mice (). While the four types of lipid nanoparticles only slightly differed in their lipid composition, their anti-RVG IgG responses differed significantly when administered intramuscularly. No humoral response was shown after pulmonary vaccination since the mRNA vaccine was swallowed and did not reach the lungs. It is suggested that throat deposition occurred upon intranasal administration of the vaccine, followed by mucociliary clearance, resulting in swallowing of the mRNA nanoparticles. In conclusion, these studies showed that pulmonary administration leads to increased IgA and IgG titers. However, it remains to be seen to what extent such levels provide protection relative to the protection achieved after intramuscular administration.
4.3.2. Cellular immune response
Several studies examined whether pulmonary administered mRNA vaccines induced a cellular immune response (). The first study examining the T-cell response after pulmonary mRNA vaccination was conducted by Phau et al. [Citation52]. These researchers studied whether mRNA vaccines induced an anti-tumor immune response. After intranasal vaccination, CD8 + T-cells were found in the spleen of mice vaccinated with mRNA nanoparticles, but this was not the case for naked mRNA. This suggests that the produced antigen is only able to trigger a CD8 + T-cell anti-tumor response when the mRNA is encapsulated in a nanoparticle, ensuring efficient transfection. It was not studied, however, whether memory cells were formed. In two follow-up studies by Li et al. [Citation34,Citation53] it was shown that the cellular immune response depends on the type of nanoparticle used. The CD8+ and CD4+ T-cell responses after vaccination with a CP2k nanoparticle were significantly higher than after vaccination with a PEI nanoparticle, CP600 nanoparticle, and naked mRNA [Citation34,Citation53]. Mai et al. [Citation49] also showed that the carrier influences the cellular immune response. In this study, the authors administered an mRNA vaccine encoding for CK19, using cationic liposome/protamine complexes. These particles showed higher levels of CD8+ and CD4+ T-cells than nanoparticles made from either solely liposomes or protamine. In the same year, Hajam et al. [Citation44] showed that pulmonary administration of chitosan nanoparticles with influenza HA2 and M2E protein and mRNA encoding for these proteins led to an increase of CD4+ T-cells and a moderate increase of CD8 + T-cells.
None of these studies, however, made direct comparisons between pulmonary administration and intramuscular injection. To determine whether lung delivery of mRNA results in a different immune response than intramuscular injection, both administration routes should be considered. So far, only Anderluzzi et al. [Citation50] examined the cellular immune response after either intramuscular or pulmonary administration of a mRNA vaccine to mice. Similar to the humoral immune response, the lipid composition of the nanoparticles determined the cellular immune response after intramuscular administration. The cellular immune response after pulmonary administration could not be determined since the vaccine was swallowed and did not reach the lungs. In conclusion, these studies showed that pulmonary administration of mRNA vaccines leads to increased levels of CD8+ and CD4+ T-cells. However, it remains unclear how these levels compare to the levels obtained after intramuscular administration.
4.3.3. Protection
The purpose of an mRNA vaccine is to provide protection. Hajam et al. [Citation44] showed that chickens pulmonary vaccinated with chitosan nanoparticles with influenza HA2 and M2E protein and mRNA or nanoparticles containing solely the HA2 and M2E proteins had a low viral load and their lung histology showed no signs of inflammation after influenza challenge. The chickens vaccinated with empty chitosan nanoparticles showed a high viral load and inflammation in their lungs after influenza challenge. Protection has also been seen for pulmonary delivered naked mRNA that does not act as a vaccine but encodes for an anti-pathogen protein. An increased life span and a normal lung histology were shown when mice were challenged with a disease post-mRNA treatment, while mice not receiving the mRNA treatment experienced a shorter life span and severe inflammation in their lungs after disease challenge [Citation32,Citation42,Citation43]. mRNA-containing nanoparticles can also produce anti-pathogen proteins. Lokugamage et al. [Citation47] and Van Hoecke et al. [Citation48] showed that mice pulmonary receiving nanoparticles containing mRNA encoding for proteins important during influenza infection (i.e. FcγRIV VHH-M2e VHH and aFI6) first lost weight after a challenge with live H1N1 and H3N2 influenza virus, but they recovered within a few days. Mice not receiving the mRNA nanoparticles lost too much weight and reached a humane endpoint. All in all, lung delivered mRNA vaccines and nanoparticles containing mRNA producing anti-pathogen proteins provide protection. However, again it is unclear how these protective effects relate to the protection after intramuscular injection.
4.4. Safety
Apart from evaluating efficacy, we also investigated whether pulmonary administration of mRNA-containing nanoparticles is safe. Several components of mRNA vaccines could cause safety issues, among others the mRNA, the produced antigen, the nanoparticle, and the dose. The safety of pulmonary administered mRNA vaccines is among others dependent on the components and the structure and physical properties of the nanoparticle used. Li et al. [Citation53] showed that mRNA encapsulated in CP2k and CP600 nanoparticles did not cause substantial changes in tissue morphology, whereas PEI showed elevated levels of IL-6, which is a known pro-inflammatory cytokine. Similar results were shown by Patel et al. [Citation36]. Using H&E staining, they showed that lung delivery of mRNA-containing hDD90-118 nanoparticles resulted in normal lung histology and weight loss was not observed. However, mRNA encapsulated in PEI resulted in weight loss, although inflammation of the lungs was not observed (). This result was confirmed by Tiwari et al. [Citation40], who also showed that lung delivery of mRNA encapsulated in PEI nanoparticles led to weight reduction in mice. These studies showed that PEI nanoparticles are toxic and should not be used for pulmonary delivery of mRNA [Citation94]. Besides PEI, lipids can have toxic effects as well. Andries et al. [Citation35] showed that mRNA-containing nanoparticles build up from GL67, DOPE, and DMPE-PEG5000 lipids led to an increased production of pro-inflammatory cytokines (TNFα and IL-6), representing a potential safety concern of these charged and surface-active molecules. On the other hand, amphotericin B liposomes, containing distearoyl phosphatidylglycerol sodium salt, has been used by inhalation already in the clinic [Citation95,Citation96].
Figure 4. Histology of the lung after pulmonary mRNA administration. A. Study of Patel et al. [Citation36]. Histology of lungs of mice after inhaling three doses of bPEI and hDD90-118 nanoparticles containing mRNA at day 8. The architecture of the alveoli is maintained in lungs of mice receiving either bPEI or hDD90-118 nanoparticles. Lungs exposed to bPEI do show red blood cells. Alveolar macrophages are present in the lungs and the bronchiolar architecture is maintained after receiving either bPEI or hDD90-118 nanoparticles. B. Study of Qiu et al. [Citation33]. The lungs of untreated mice appeared healthy. Lungs of mice intratracheally treated with 10 µg lipopolysaccharide (LPS) showed an irregular distribution of the air spaces and infiltration of inflammatory cells into the alveolar and interstitial spaces was observed. Lungs of mice treated with mRNA PEG12KL4 nanoparticles did not show inflammation.
![Figure 4. Histology of the lung after pulmonary mRNA administration. A. Study of Patel et al. [Citation36]. Histology of lungs of mice after inhaling three doses of bPEI and hDD90-118 nanoparticles containing mRNA at day 8. The architecture of the alveoli is maintained in lungs of mice receiving either bPEI or hDD90-118 nanoparticles. Lungs exposed to bPEI do show red blood cells. Alveolar macrophages are present in the lungs and the bronchiolar architecture is maintained after receiving either bPEI or hDD90-118 nanoparticles. B. Study of Qiu et al. [Citation33]. The lungs of untreated mice appeared healthy. Lungs of mice intratracheally treated with 10 µg lipopolysaccharide (LPS) showed an irregular distribution of the air spaces and infiltration of inflammatory cells into the alveolar and interstitial spaces was observed. Lungs of mice treated with mRNA PEG12KL4 nanoparticles did not show inflammation.](/cms/asset/63ac97fb-4744-4102-975f-4db704bbfd11/iedd_a_2131767_f0004_oc.jpg)
Besides nanoparticles, naked mRNA raises safety concerns as well. Lorenzi et al. [Citation32] showed that pulmonary administration of naked mRNA in mice resulted in increased TNFα levels, a pro-inflammatory cytokine, which could be caused by the absence of m1Ψ. Remarkably, opposite results were shown by Legere et al. [Citation37]. These researchers showed that transfection of naked mRNA in the bronchi of horses did not result in inflammation. This is interesting, since naked mRNA has been shown to lead to the activation of TLR 7/8 which plays a role in the innate inflammatory response [Citation97]. It is therefore suggested that this inflammatory response might be species dependent. Besides the role of the nanoparticle in inflammation, the dose is important as well. Qiu et al. [Citation33] showed that pulmonary administration of 5 µg mRNA-containing nanoparticles to mice does not lead to inflammation in the lungs, nor was weight loss observed (). However, lung delivery of 10 µg of the same type of nanoparticles did lead to an increase in pro-inflammatory cytokines and caused a weight loss of 6%. These findings were confirmed by the study of Van Hoecke et al. [Citation48]. These researchers also found minor levels of pro-inflammatory cytokines after pulmonary administration of 5 µg mRNA-containing nanoparticles to mice. Fortunately, most animal studies showed no increase in pro-inflammatory cytokines after pulmonary administration of both naked and mRNA-containing nanoparticles [Citation33,Citation40,Citation45,Citation48,Citation50,Citation98–101]. All in all, the safety of lung delivered mRNA-containing nanoparticles is mainly determined by the composition of the nanoparticle and the dose.
5. Conclusion
The aim of this literature study was to determine whether pulmonary administration of mRNA vaccines represents a suitable alternative to intramuscular injection. To that end, we carefully reviewed animal studies. These studies clearly showed that a uniform distribution of mRNA over the lung can be achieved when mRNA containing vaccines are properly pulmonary administered. Upon lung delivery, the mRNA is taken up mostly by APCs, owing to their phagocytotic capacity. In turn, APCs were shown to successfully express and present the antigens, resulting in the initiation of potent adaptive immune responses, both on a humoral and cellular level. Some studies also demonstrated that this approach protected animals from disease during challenges. With respect to safety, we were able to identify some issues related to the materials that nanoparticles are prepared from; PEI, for example, caused acute inflammation. In future studies, comparisons should be made between pulmonary administration and intramuscular injection. This will improve our understanding of whether pulmonary administration of mRNA vaccines could lead to dose-sparing and better protection, for instance.
6. Expert opinion
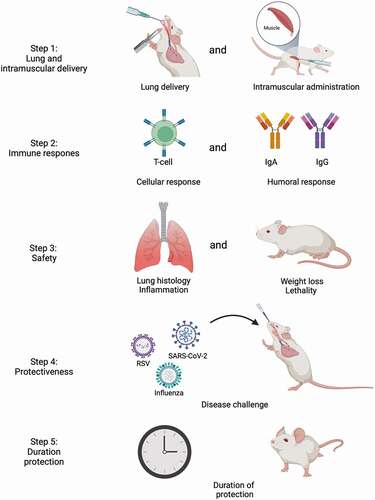
We identified several omissions and challenges when examining the publications investigating pulmonary delivered mRNA. For example, fundamental data about the uptake mechanism of the mRNA containing nanoparticles by cells, quantitative data about the amount of protein that is produced by the mRNA, and a connection between the amount of protein produced and the extent of the immune response initiated are lacking. Moreover, the reviewed publications did not take intramuscular injection of mRNA vaccines into account as a control when investigating pulmonary delivery of mRNA vaccines. In the future, studies should investigate differences in humoral and cellular responses in a head-to-head comparison between intramuscular injection and pulmonary administration of mRNA vaccines, in particular concerning IgA titers. IgA plays a crucial role in the humoral mucosal immune response fighting respiratory infections and it leads to cross-protection [Citation21–26]. In line with this, it has been shown that intrapulmonary and intranasal delivered vaccines against SARS-CoV in rodents and influenza in humans showed a better protection against infection than intramuscular injection, due to increased IgA levels after pulmonary vaccine delivery [Citation21,Citation102–105]. Moreover, future studies should investigate whether the immune response results in protection and how long immunity through pulmonary vaccination lasts ().
Figure 5. Lung or intramuscular delivered mRNA vaccine development roadmap. The steps that need to be undertaken to examine whether pulmonary delivery of mRNA vaccines is safe and effective. Step 1: a group of mice is pulmonary vaccinated with the mRNA vaccine and a group is vaccinated intramusculary. Step 2: the cellular and humoral response are measured in both groups of mice. Step 3: the safety of the mRNA vaccines is assessed by measuring lung histology, inflammation, weight loss and lethality. Step 4: the mice are challenged with the disease of interest to assess whether the vaccine shows protection. Step 5: the duration of protection is measured. Image created with BioRender.com.
Besides the induction of a protective immune response, a pulmonary delivered mRNA vaccine should be proven safe. It was described that pulmonary administration of polymer-based nanoparticles engineered from PEI caused increased levels of pro-inflammatory cytokines and the animals lost weight, indicating that PEI is too toxic [Citation36,Citation40,Citation53]. Lipid nanoparticles carrying mRNA administered pulmonary have been considered safe according to the discussed animal studies in this review. Furthermore, clinical data of the intramuscular delivered mRNA vaccines against SARS-CoV-2 show that lipid nanoparticles are safe and participants to clinical phase I and II trials where intramuscular delivered mRNA lipid nanoparticles are used to treat different types of cancer tolerate the lipid nanoparticles very well [Citation1,Citation2,Citation4,Citation5,Citation106]. However, detailed information about the safety of lipid nanoparticle following pulmonary delivery is not yet available. Several techniques are available to determine the toxicity of pulmonary administered nanoparticles in the lung: H&E staining, deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL), and KI67. With these techniques, inflammation, cell death, and cell proliferation, respectively, can be examined [Citation107–109]. A relatively new technique to examine toxicity is human lung slices, a technique where precision-cut slices of the lungs are exposed to the compound of interest [Citation110]. Tissue slices are especially interesting for short-term toxicity studies. However, we suggest using a carefully chosen animal model to study safety, immune responses, and protection of the mRNA vaccines in a living system (e.g. non-human primates) [Citation111].
When sufficient information is available showing that pulmonary administration of mRNA vaccines is safe and effective, a suitable inhalable formulation should be developed. Various inhalation devices exist, such as soft mist inhalers, nebulizers, metered-dose inhalers, and dry powder inhalers (DPIs) [Citation107,Citation112,Citation113]. Of these devices, DPIs are of particular interest since they are distributed easily and the administration is simple [Citation114–116]. Pulmonary vaccination with DPIs has several advantages over liquid formulations, as discussed in a review by Tonnis et al. [Citation15]. mRNA vaccine powder suitable for inhalation could be engineered by spray-drying or spray-freeze-drying in combination with stabilizing excipients, as discussed in the study of Saluja et al. [Citation117]. We expect that sugars are essential to ensure a good stability of mRNA nanoparticles, since mRNA vaccines are inherently unstable, requiring a cold-chain [Citation73]. Stabilizing excipients like sucrose seems promising since it effectively protects the mRNA-containing nanoparticles from freezing damage [Citation73,Citation118]. Yet, a translation must be made to spray-drying and spray-freeze-drying [Citation17]. We therefore recommend examining different sugars and different conditions for spray-drying and spray-freeze-drying (e.g. flow rate, freezing-rate) in order to engineer a mRNA-containing nanoparticle formulation with a long-term stability, preferably at ambient conditions.
Whilst formulating mRNA vaccines into inhalable dry powders, special care should be taken to investigate whether they are well-tolerated by patients suffering from respiratory diseases, especially those of a chronic nature (e.g. chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis, and asthma). In these patients, the lung architecture may be severely remodeled, affecting not only their ability to inhale but also to elicit effective immune responses [Citation119]. This could affect the efficacy and safety of pulmonary delivered mRNA vaccines. A risk of mRNA delivered pulmonary is that an inflammatory response could be induced. This risk could be reduced by modifying the mRNA, e.g. by replacing uridine by m1Ψ [Citation54,Citation59,Citation61–63]. It is suggested that nanoparticles are safe to inhale for patients with respiratory diseases, since several types of inhalable nanoparticles have already been used in medication for asthma, COPD, and lung cancer, providing positive treatment effects [Citation120]. These data indicate that also mRNA containing nanoparticles can be safely administered to patients suffering from lung diseases via the respiratory route. However, the safety and efficacy aspects of lung delivered mRNA have not yet been examined extensively. We suggest that first the safety and efficacy of different forms of mRNA and mRNA-containing formulations (e.g. nanoparticles) including the effect of composition, structure, and physical properties delivered into the lung should be examined in animal models with respiratory diseases. Consequently, these pulmonary administered mRNA vaccines could be investigated in clinical studies.
In 5 years from now, we expect that more information regarding the efficacy and safety of pulmonary delivered, and intramuscular injected mRNA vaccines will be available. First, the short-term safety of mRNA lipid nanoparticles using lung slices, and in particular human lung slices, will be examined. When the short-term safety is clear, and a suitable lipid nanoparticle is formulated, a preclinical animal study should be performed. Within 5 years more insights into the efficacy and safety of both pulmonary and intramuscularly administered mRNA vaccines, preferably in a head-to-head comparison, will be gained. When the safety and efficacy are clear, we can work toward the development of dry and stable inhalable formulations, which are also tolerated by patients suffering respiratory diseases. The results so far are convincing, indicating that more research regarding lung delivered mRNA vaccines is worthwhile.
Article highlights
mRNA vaccines have been successfully used to tackle the SARS-CoV-2 pandemic.
Current SARS-CoV-2 mRNA vaccines require frequent administration of booster injections, which may be easier when given via the pulmonary route.
To further improve the protection against respiratory infections, the vaccines could be delivered at the entry portal of the pathogen: the lung mucosa.
Reviewed studies have shown that mRNA vaccines administered via the pulmonary route elicit humoral and cellular immune responses.
Future studies should further characterize immunological responses and safety aspects, and work toward inhalable formulations that are well tolerated also by patients suffering from lung diseases.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615.
- Kim J, Eygeris Y, Gupta M, et al. Self-assembled mRNA vaccines. Adv Drug Deliv Rev. 2021;170:83–112.
- Ramanathan K, Antognini D, Combes A, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829.
- Tang P, Hasan MR, Chemaitelly H, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021;27(12):2136–2143.
- Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27(9):1614–1621.
- Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385(24):e83.
- Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-Dose and 3-Dose effectiveness of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance — VISION network, 10 states, august 2021-January 2022. MMWR Recomm Rep. 2022;71(7):255–263.
- Levine-Tiefenbrun M, Yelin I, Alapi H, et al. Waning of SARS-CoV-2 booster viral-load reduction effectiveness. Nat Commun. 2022;13(1):1–4.
- Goel RR, Painter MM, Apostolidis SA, et al. mRNA vaccination induces durable immune memory to SARS-CoV-2 with continued evolution to variants of concern. Science. 2021;374(6572):abm0829.
- Harvey WT, Carabelli AM, Jackson B, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409–424.
- Jung C, Kmiec D, Koepke L, et al. Omicron: what makes the latest SARS-CoV-2 variant of concern so concerning? J Virol. 2022;96(6):e0207721.
- Franco-Paredes C. Transmissibility of SARS-CoV-2 among fully vaccinated individuals. Lancet Infect Dis. 2022;22(1):16.
- Liu Y, Rocklöv J. The effective reproductive number of the Omicron variant of SARS-CoV-2 is several times relative to Delta. J Travel Med. 2022;29(3):taac037.
- Tonnis WF, Lexmond AJ, Frijlink HW, et al. Devices and formulations for pulmonary vaccination. Expert Opin Drug Deliv. 2013;10(10):1383–1397.
- Heida R, Hinrichs WLJ, Frijlink HW. Inhaled vaccine delivery in the combat against respiratory viruses: a 2021 overview of recent developments and implications for COVID-19. Expert Rev Vaccines. 2022;21(7):957–974.
- Mabrouk MT, Huang WC, Martinez-Sobrido L, et al. Advanced materials for SARS-CoV-2 Vaccines. Adv Mater. 2022;34(12):1–17.
- Wisnewski AV, Luna JC, Redlich CA, et al. IgA responses to COVID-19 mRNA vaccines. PLoS One. 2021;16(6):1–7.
- Salvagno GL, Henry BM, Di Piazza G, et al. Anti-spike s1 iga, anti-spike trimeric igg, and anti-spike rbd igg response after bnt162b2 covid-19 mRNA vaccination in healthcare workers. J Med Biochem. 2021;40(4):327–334.
- Azzi L, Gasperina DD, Veronesi G, et al. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. eBioMedicine. 2022;75:1–10.
- Asahi-Ozaki Y, Yoshikawa T, Iwakura Y, et al. Secretory IgA antibodies provide cross-protection against infection with different strains of influenza B virus. J Med Virol. 2004;74(2):235–328.
- Van Riet E, Ainai A, Suzuki T, et al. Mucosal IgA responses in influenza virus infections; thoughts for vaccine design. Vaccine. 2012;30(40):5893–5900.
- Qi M, Zhang XE, Sun X, et al. Intranasal nanovaccine confers homo- and hetero-subtypic influenza protection. Small. 2018;14(13):1–9.
- Kim YH, Bang YJ, Park HJ, et al. Inactivated influenza vaccine formulated with single-stranded RNA-based adjuvant confers mucosal immunity and cross-protection against influenza virus infection. Vaccine. 2020;38(39):6141–6152.
- Nakajima R, Supnet M, Jasinskas A, et al. Protein microarray analysis of the specificity and cross-reactivity of influenza virus hemagglutinin-specific antibodies. mSphere. 2018;3(6):1–15.
- Overton ET, Goepfert PA, Cunningham P, et al. Intranasal seasonal influenza vaccine and a TLR-3 agonist, rintatolimod, induced cross-reactive IgA antibody formation against avian H5N1 and H7N9 influenza HA in humans. Vaccine. 2014;32(42):5490–5495.
- Mato YL. Nasal route for vaccine and drug delivery: features and current opportunities. Int J Pharm. 2019;572:118813.
- Mudgal R, Nehul S, Tomar S. Prospects for mucosal vaccine: shutting the door on SARS-CoV-2. Hum Vaccines Immunother. 2020;16(12):2921–2931.
- Alu A, Chen L, Lei H, et al. Intranasal COVID-19 vaccines: from bench to bed. eBioMedicine. 2022;76:103841.
- Tomar J, Tonnis WF, Patil HP, et al. Pulmonary immunization: deposition site is of minor relevance for influenza vaccination but deep lung deposition is crucial for hepatitis B vaccination. Acta Pharm Sin B. 2019;9(6):1231–1240.
- Waldman RH, Mann JJ, Small PA. Immunization against influenza: prevention of illness in man by aerosolized inactivated vaccine. J Am Med Assoc. 1969;207(3):520–524.
- Lorenzi JCC, Trombone APF, Rocha CD, et al. Intranasal vaccination with messenger RNA as a new approach in gene therapy: use against tuberculosis. BMC Biotechnol. 2010;10(1):1–11.
- Qiu Y, Man RCH, Liao Q, et al. Effective mRNA pulmonary delivery by dry powder formulation of PEGylated synthetic KL4 peptide. J Control Release. 2019;314:102–115.
- Li M, Zhao M, Fu Y, et al. Enhanced intranasal delivery of mRNA vaccine by overcoming the nasal epithelial barrier via intra-and paracellular pathways. J Control Release. 2016;228:9–19.
- Andries O, De Filette M, De Smedt SC, et al. Innate immune response and programmed cell death following carrier-mediated delivery of unmodified mRNA to respiratory cells. J Control Release. 2013;167(2):157–166.
- Patel AK, Kaczmarek JC, Bose S, et al. Inhaled nanoformulated mRNA polyplexes for protein production in lung epithelium. Adv Mater. 2019;31(8):1–7.
- Legere RM, Cohen ND, Poveda C, et al. Safe and effective aerosolization of in vitro transcribed mRNA to the respiratory tract epithelium of horses without a transfection agent. Sci Rep. 2021;11(1):1–12.
- Pardi N, Tuyishime S, Muramatsu H, et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release. 2015;217:345–351.
- Kormann MSD, Hasenpusch G, Aneja MK, et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat Biotechnol. 2011;29(2):154–159.
- Tiwari PM, Vanover D, Lindsay KE, et al. Engineered mRNA-expressed antibodies prevent respiratory syncytial virus infection. Nat Commun. 2018;9(1):3999.
- Su X, Fricke J, Kavanagh DG, et al. In vitro and in vivo mRNA delivery using lipid-enveloped pH-responsive polymer nanoparticles. Mol Pharm. 2011;8(3):774–787.
- Mahiny AJ, Dewerth A, Mays LE, et al. In vivo genome editing using nuclease-encoding mRNA corrects SP-B deficiency. Nat Biotechnol. 2015;33(6):584–586.
- Mays LE, Ammon-Treiber S, Mothes B, et al. Modified Foxp3 mRNA protects against asthma through an IL-10-dependent mechanism. J Clin Invest. 2013;123(3):1216–1228.
- Hajam IA, Senevirathne A, Hewawaduge C, et al. Intranasally administered protein coated chitosan nanoparticles encapsulating influenza H9N2 HA2 and M2e mRNA molecules elicit protective immunity against avian influenza viruses in chickens. Vet Res. 2020;51(1):1–17.
- Haque AKMA, Dewerth A, Antony JS, et al. Chemically modified hCFTR mRNAs recuperate lung function in a mouse model of cystic fibrosis. Sci Rep. 2018;8(1):1–14.
- Robinson E, MacDonald KD, Slaughter K, et al. Lipid nanoparticle-delivered chemically modified mRNA restores chloride secretion in cystic fibrosis. Mol Ther. 2018;26(8):2034–2046.
- Lokugamage MP, Vanover D, Beyersdorf J, et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat Biomed Eng. 2021;5(9):1059–1068.
- Van Hoecke L, Verbeke R, De Vlieger D, et al. mRNA encoding a bispecific single domain antibody construct protects against influenza A virus infection in mice. Mol Ther Nucleic Acids. 2020;20:777–787.
- Mai Y, Guo J, Zhao Y, et al. Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cell Immunol. 2020;354:104143.
- Anderluzzi G, Lou G, Woods S, et al. The role of nanoparticle format and route of administration on self-amplifying mRNA vaccine potency. J Control Release. 2022;342:388–399.
- Phua KKL, Leong KW, Nair SK. Transfection efficiency and transgene expression kinetics of mRNA delivered in naked and nanoparticle format. J Control Release. 2013;166(3):227–233.
- Phua KKL, Staats HF, Leong KW, et al. Intranasal mRNA nanoparticle vaccination induces prophylactic and therapeutic anti-tumor immunity. Sci Rep. 2014;4:4–10.
- Li M, Li Y, Peng K, et al. Engineering intranasal mRNA vaccines to enhance lymph node trafficking and immune responses. Acta Biomater. 2017;64:237–248.
- Schoenmaker L, Witzigmann D, Kulkarni JA, et al. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int J Pharm. 2021;601:120586.
- Weissman D. mRNA transcript therapy. Expert Rev Vaccines. 2014;14(2):265–281.
- Schlake T, Thess A, Fotin-Mleczek M, et al. Developing mRNA-vaccine technologies. RNA Biol. 2012;9(11):1319–1330.
- Verbeke R, Lentacker I, De Smedt SC, et al. The Dawn of mRNA vaccines: the COVID-19 case. Journal of Controlled Release: Official Journal of the Controlled Release Society. 2021;333:511–520.
- Iavarone C, O’hagan DT, Yu D, et al. Mechanism of action of mRNA-based vaccines. Expert Rev Vaccines. 2017;16(9):871–881.
- Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines-a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279.
- Ho W, Gao M, Li F, et al. Next-Generation vaccines: nanoparticle-mediated DNA and mRNA delivery. Adv Healthc Mater. 2021;10(8):1–17.
- Szabó GT, Mahiny AJ, Vlatkovic ICOVID-19. mRNA vaccines: platforms and current developments. Mol Ther. 2022;30(5):1850–1868.
- Thompson MG, Sacco MT, Horner SM. How RNA modifications regulate the antiviral response. Immunol Rev. 2021;304(1):169–180.
- Chiu C, Openshaw PJ. Antiviral B cell and T cell immunity in the lungs. Nat Immunol. 2015;16(1):18–26.
- Evers MJW, Kulkarni JA, Van Der Meel R, et al. State-of-the-art design and rapid-mixing production techniques of lipid nanoparticles for nucleic acid delivery. Small Methods. 2018;2(9):1700375.
- Ulmer JB, Mason PW, Geall A, et al. RNA-based vaccines. Vaccine. 2012;30(30):4414–4418.
- Tan L, Zheng T, Li M, et al. Optimization of an mRNA vaccine assisted with cyclodextrin-polyethyleneimine conjugates. Drug Deliv Transl Res. 2020;10(3):678–689.
- Chow MYT, Qiu Y, Lam JKW. Inhaled RNA therapy: from promise to reality. Trends Pharmacol Sci. 2020;41(10):715–729.
- Rauch S, Jasny E, Schmidt KE, et al. New vaccine technologies to combat outbreak situations. Front Immunol. 2018;9(1963):1–24.
- Weng Y, Huang Y. Advances of mRNA vaccines for COVID-19: a new prophylactic revolution begins. Asian J Pharm Sci. 2021;16(3):263–264.
- Mitchell MJ, Billingsley MM, Haley RM, et al. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124.
- Hinrichs WLJ, Sanders NN, De Smedt SC, et al. Inulin is a promising cryo- and lyoprotectant for PEGylated lipoplexes. J Control Release. 2005;103(2):465–479.
- Van Lysebetten D, Malfanti A, Deswarte K, et al. Lipid-Polyglutamate nanoparticle vaccine platform. ACS Appl Mater Interfaces. 2021;13(5):6011–6022.
- Crommelin DJA, Anchordoquy TJ, Volkin DB, et al. Addressing the cold reality of mRNA vaccine stability. J Pharm Sci. 2021;110(3):997–1001.
- Sedic M, Senn JJ, Lynn A, et al. Safety evaluation of lipid nanoparticle–formulated modified mRNA in the Sprague-Dawley rat and cynomolgus monkey. Vet Pathol. 2018;55(2):341–354.
- Stokes A, Pion J, Binazon O, et al. Nonclinical safety assessment of repeated administration and biodistribution of a novel rabies self-amplifying mRNA vaccine in rats: toxicity and biodistribution of rabies SAM vaccine. Regul Toxicol Pharmacol. 2020;113:104648.
- Landesman-Milo D, Peer D. Altering the immune response with lipid-based nanoparticles. J Control Release. 2012;161(2):600–608.
- Heine A, Juranek S, Brossart P. Clinical and immunological effects of mRNA vaccines in malignant diseases. Mol Cancer. 2021;20(1):1–20.
- Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Ann Oncol. 2021;184:861–880.
- Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924–944.
- Randall TD. Structure, Organization, and Development of the Mucosal Immune System of the Respiratory Tract. 4th Ed. Vol. 1. 2015.Amsterdam: Elsevier ;p. 43–61.
- Randall TD. Bronchus-Associated Lymphoid Tissue (BALT) structure and function. Adv Immunol. 2010;107:187–241.
- Silva-Sanches A, Randall TD. Role of iBALT in respiratory immunity. Curr Top Microbiol Immunol. 2020;426:21–43.
- Nothelfer K, Sansonetti PJ, Phalipon A. Pathogen manipulation of B cells: the best defence is a good offence. Nat Rev Microbiol. 2015;13(3):173–184.
- Kato A, Hulse K,E, Tan BK, et al. B lymphocyte lineage cells and the respiratory system. Bone. 2013;131(4):933–957.
- Mantis NJ, Forbes SJCP. Secretory IgA: arresting microbial pathogens at epithelial borders. Bone. 2010;29(4–5):383–406.
- Jin Z, Gao S, Cui X, et al. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int J Pharm. 2019;572(118731):1–16.
- Barda N, Daga N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;198(10316):2093–2100.
- Moreira ED, Kitchin N, Xu X, et al. Safety and efficacy of a third dose of BNT162b2 Covid-19 vaccine. N Engl J Med. 2022;386(20):1910–1921.
- Cieri N, Camisa B, Cocchiarella F, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121(4):573–584.
- Zhang S, Gao H, Bao G. Physical principles of nanoparticle cellular endocytosis. ACS Nano. 2015;9(9):8655–8671.
- Gilleron J, Querbes W, Zeigerer A, et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol. 2013;31(7):638–646.
- Geissmann F, Manz MG, Jung S, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–661.
- Joller N, Weber SS. Antibody OA. - Fc receptor interactions in protection against intracellular pathogens. Eur J Immunol. 2011;41(4):889–997.
- Beyerle A, Irmler M, Beckers J, et al. Toxicity pathway focused gene expression profiling of PEI-based polymers for pulmonary applications. Mol Pharm. 2010;7(3):727–737.
- Van Ackerbroeck S, Rutsaert L, Roelant E, et al. Inhaled liposomal amphotericin-B as a prophylactic treatment for COVID-19-associated pulmonary aspergillosis/aspergillus tracheobronchitis. Crit Care. 2021;25(1):1–4.
- Kuiper L, Ruijgrok EJ. A review on the clinical use of inhaled amphotericin B. J Aerosol Med Pulm Drug Deliv. 2009;22(3):213–227.
- Tanimura N, Miyake K. Toll like receptors (TLRs) and Innate Immunity. Vol. 183. Berlin/Heidelberg: Springer; 2015. p. 1–240.
- Abbas AK, Lichtman AH, Pillai S. Cellular and molecular immunology. 8th Ed. Amsterdam: Elsevier. 2015. p. 1–533.
- Tanaka T, Narazaki M, Kishimoto T. IL-6 in Inflammation, immunity, and disease. Cold Spring Harbor Perspectives in Biology. 2014;6(10):1–16.
- Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunological Reviews. 2018;281(1):8–27.
- Idriss HT, Naismith JH. TNFα and the TNF receptor superfamily: structure-function relationship(s). Microsc Res Tech. 2000;50(3):184–195.
- Quinti I, Mortari EP, Fernandez Salinas A, et al. IgA antibodies and IgA deficiency in SARS-CoV-2 infection. Front Cell Infect Microbiol. 2021;11:1–5.
- See RH, Zakhartchouk AN, Petric M, et al. Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus. J Gen Virol. 2006;87(3):641–650.
- Hassan AO, Kafai NM, Dmitriev IP, et al. A single-dose intranasal Chad vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183(1): 169–184.
- Ainai A, Tamura SI, Suzuki T, et al. Intranasal vaccination with an inactivated whole influenza virus vaccine induces strong antibody responses in serum and nasal mucus of healthy adults. Hum Vaccines Immunother. 2013;9(9):1962–1970.
- Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20(1):1–23.
- Zhou QT, Tang P, Leung SSY, et al. Emerging inhalation aerosol devices and strategies: where are we headed? Adv Drug Deliv Rev. 2014;75:3–17.
- Majtnerová P, Roušar T. An overview of apoptosis assays detecting DNA fragmentation. Mol Biol Rep. 2018;45(5):1469–1478.
- Yang C, Zhang J, Ding M, et al. Ki67 targeted strategies for cancer therapy. Clin Transl Oncol. 2018;20(5):570–575.
- Neuhaus V, Danov O, Konzok S, et al. Assessment of the cytotoxic and immunomodulatory effects of substances in human precision-cut lung slices. J Vis Exp. 2018;2018(135):1–14.
- Cryan SA, Sivadas N, Garcia-Contreras L. In vivo animal models for drug delivery across the lung mucosal barrier. Adv Drug Deliv Rev. 2007;59(11):1133–1151.
- Chandel A, Goyal AK, Ghosh G, et al. Recent advances in aerosolised drug delivery. Biomed Pharmacother. 2019;112:108601.
- Tiddens HAWM, Bos AC, Mouton JW, et al. Inhaled antibiotics: dry or wet? Eur Respir J. 2014;44(5):1308–1318.
- De Boer AH, Hagedoorn P, Hoppentocht M, et al. Dry powder inhalation: past, present and future. Expert Opin Drug Deliv. 2017;14(4):499–512.
- Sibum I, Hagedoorn P, De Boer AH, et al. Challenges for pulmonary delivery of high powder doses. Int J Pharm. 2018;548(1):325–336.
- AboulFotouh K, Cui Z, Williams RO. Next-generation COVID-19 vaccines should take efficiency of distribution into consideration. AAPS PharmSciTech. 2021;22(3):126.
- Saluja V, Amorij JP, Kapteyn JC, et al. A comparison between spray drying and spray freeze drying to produce an influenza subunit vaccine powder for inhalation. J Control Release. 2010;144(2):127–133.
- Ball RL, Bajaj P, Whitehead KA. Achieving long-term stability of lipid nanoparticles: examining the effect of pH, temperature, and lyophilization. Int J Nanomedicine. 2017;12:305–315.
- Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–1351.
- Rabiei M, Kashanian S, Samavati SS, et al. Characteristics of SARS-CoV2 that may be useful for nanoparticle pulmonary drug delivery. J Drug Target. 2021;30(3):1–11.