?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objective
While interest in the use of wearable large-volume injectors for subcutaneous drug delivery is increasing, it remains unclear whether and under what conditions these emerging dosing options are preferred over more frequent but shorter administration of smaller doses using handheld autoinjectors. Therefore, the objective of this study was to examine the characteristics of patients diagnosed with cancer, diabetes, inflammatory and cardiovascular diseases, and treatment attributes that determine device preferences.
Methods
Based on a cross-sectional online choice experiment, 191 participants expressed their preferences without being physically exposed to the devices or performing injections. Logistic hierarchical regression models were used to assess which patient characteristics, and how changes in treatment attributes, drive device preferences.
Results
Participant quality of life reduced the likelihood of preferring wearable large-volume injectors to handheld autoinjectors. Moreover, reducing injection frequency from biweekly to monthly to quarterly injections, and shortening injection duration from 33 to 8 min, significantly increased the likelihood of patients preferring large-volume injectors to autoinjectors (p < 0.001).
Conclusion
The study revealed patient quality of life as predictor of device preference and identified critical inflection points in injection duration and injection frequency, at which patient preferences shift from handheld autoinjectors to wearable large-volume injectors.
1. Introduction
Subcutaneous self-administration of biologics continues to transform global healthcare [Citation1–3]. Shifting from inpatient intravenous infusion to outpatient subcutaneous injection not only improves patient safety, treatment adherence, and quality of life but also reduces the total costs of therapy and healthcare-resource utilization [Citation4–9]. The use of pre-filled handheld autoinjectors has become a de facto standard for self-administration of biologics for a wide range of chronic diseases, such as rheumatoid arthritis, psoriasis, multiple sclerosis, or migraine [Citation10–15]. These spring-driven devices are activated either by the push of a button or by pressure against the injection site, allowing safe and effective administration of a single fixed dose from a prefilled syringe [Citation12,Citation16–18]. In the past, the feasible upper limit for subcutaneous injections was 1.0 mL delivered within less than 10s. However, recent advances in device technologies and formulation science have allowed for this upper boundary to be increased [Citation19–21]. New insights into the usability, efficacy, and safety of large-volume single doses have led to a consensus that handheld autoinjectors may be suitable to deliver fixed doses beyond 2.0 mL with injection durations of up to 30s [Citation22–24]. Moreover, wearable large-volume injectors that enable the administration of even larger single-dose volumes are under development [Citation25–28]. This would further reduce injection frequency, which is a key attribute that drives treatment choices [Citation29–31]. These devices are patched onto the skin using an adhesive and then slowly inject a single large-volume dose well beyond 2.0 mL over several minutes into subcutaneous tissue [Citation25–27].
Wearable large-volume injectors introduce new dosing options that differ from handheld autoinjectors along multiple dimensions, such as device usability, injection frequency, and injection duration [Citation20,Citation32,Citation33]. For example, evolocumab, a monoclonal antibody used to lower low-density lipoprotein cholesterol levels [Citation34], can be self-administered every two weeks within 15s using a prefilled handheld autoinjector or once a month within 5 min using a patient-loaded wearable large-volume injector [Citation35]. However, little is known about which device patients prefer and how treatment attributes such as the injection frequency and injection duration affect preferences. This lack of research is problematic in several ways. First, understanding patient preferences for different dosing options is the basis for informed device and primary packaging container selection, formulation development, and specification of drug delivery profiles. Second, insights into patient preferences for dosing options are needed to support patients and healthcare professionals in making optimal treatment decisions Treatment recommendations must be based on which device presentation and associated dosing option will best meet patients’ expectations and least disrupt their day-to-day routines. In fact, prior research concluded that the availability of the preferred dosing option increases the likelihood of patients adhering to the medication [Citation36]. Empirical work is urgently needed on how patient characteristics and changes in treatment drive patient preferences for handheld autoinjectors or wearable large-volume injector dosing options.
Therefore, the aim of this study was to collect initial empirical evidence on patient preferences for handheld autoinjector and wearable large-volume injector dosing options. The article not only examined which patient characteristics drive preferences between the two device categories, but also how these preferences change as a function of treatment attributes such as injection frequency or injection duration. In particular, the study aimed at understanding the inflection points at which patient preferences shift between the two device categories, thereby providing guidance for future drug-device development and patient-centered clinical decision making.
2. Patients and methods
2.1. Study design and sample
A cross-sectional online survey was conducted among 168 patients diagnosed and treated for at least one of the following chronic diseases for which self-injection treatments are available: arthritis, psoriasis, Crohn’s disease, multiple sclerosis, asthma, osteoporosis, diabetes, cardiovascular diseases, and cancer. In addition, 23 participants without any of the aforementioned diagnoses were recruited as a control group, resulting in a sample with 191 participants. provides an overview of the study participants according to chronic disease diagnosis and self-injection therapy status. Participants were recruited in Germany and had ages of between 18 and 77 years (M = 37.97, SD = 14.66). Of the 111 female and 80 male participants, 104 had self-injection experience either with a prefilled syringe, an autoinjector, or a cartridge-based injection pen. Apart from the screening criteria (i.e. belonging to at least one of the diagnosis groups) and the ability to understand and fill out an online questionnaire in German, no further exclusion criteria were specified. The participants provided informed consent for their data to be used for this study.
Table 1. Overview of study participants (N = 191) by chronic disease diagnosis and self-injection therapy status.
Participants were recruited through an online patient panel, which had previously completed screening questionnaires to identify eligible individuals. Participants were recruited in June 2021 and July 2021 and were compensated for their participation.
2.2. Survey
The survey used in this study was built through a three-step process. The first step included a literature review to identify patient characteristics and treatment attributes that may drive patient preferences for different dosing options. The searches on Google Scholar and in databases such as PubMed, EBSCO, and PubPsych combined words such as ‘injection,’ ‘preference,’ ‘self-medication,’ or the type of injection device used (i.e. ‘autoinjector,’ ‘wearable injector,’ or ‘prefilled syringe’) in the title, abstract, or keywords to identify relevant prior work. The process also included manually examining references and citations in the work identified. Discussions among the authors ensured that no study was overlooked. The first step led to an initial long list of possible patient characteristics and treatment attributes. The second step included eight open-ended qualitative interviews with healthcare professionals to support the development of the survey and validate the constructs that were considered potentially relevant. Seven diabetes nurses and a senior physician specializing in general internal medicine were interviewed about patient perceptions, attitudes, and preferences regarding injection-based treatment options and typical challenges that reduce adherence to self-injection therapy plans. All interviewees had several years of experience in accompanying chronically ill patients who had to perform self-injections. In the third and final step, the coauthors discussed and consolidated the findings from the first two phases to include those patient characteristics and treatment attributes that were deemed most relevant to the survey.
summarizes the 10 patient characteristics and five treatment attributes included in the online survey. Existing instruments and scales were used whenever applicable. If the scales were not available in German, they were translated from the original language into German by one coauthor and reviewed by another coauthor of the study to ensure the accuracy of translation. To reduce the time required to complete the questionnaire, the quality of life was reduced to the most sensible subscale (physical health), and the measurement of visual acuity was shortened and reduced to a single item for each instrument.
Table 2. Instruments used in the online survey to assess (A) patient characteristics and (B) treatment attributes as potential predictor variables of injection device preference.
The online survey started by asking participants about their socio-demographical background and experience with performing self-injections. Handling videos and instructions for the self-injection process using a handheld autoinjector and a wearable large-volume injector were presented to all participants. The patients were then asked to express their general device preference for the following choice task (‘baseline scenario’): a weekly injection with an injection duration of 3 min (including steps before and after injection) that causes mild pain and skin irritation using the handheld autoinjector or a monthly injection with an injection duration of 35 min that causes mild to moderate pain and skin irritation using the wearable large-volume injector (question asked: ‘Which of the two devices would you prefer if both treatments led to same therapeutic success and you had freedom of choice?’).
Participants were then asked to complete pairwise choice tasks to express their preference between handheld autoinjector and wearable large-volume injector dosing options for which various treatment attributes were modified. presents a summary of the 12 pairwise choice tasks and the respective modified treatment attributes that were presented to each participant in the online survey. The presentation of the choice tasks was randomized.
Table 3. Overview of the 12 pairwise choice tasks and respective treatment attributes presented to each participant in the online survey for the handheld autoinjector and wearable large-volume injector dosing options.
2.3. Data analysis
Descriptive statistics and multivariate analyses were performed using the statistical software package R (version 4.1.2, 2021–11-01 Bird Hippie). Logistic regression models were used to analyze the impact of patient characteristics on device preferences. A stepwise forward strategy was used to assess the significance of the impact of different patient characteristics on device preference. The impact of treatment attributes on device preference was analyzed using hierarchical modeling (mixed effects logistic regression) because of the nested data structure of the treatment attributes (i.e. multiple device preference statements per study participant) [Citation42]. Mixed effects modeling is indicated when there is variance in the dependent variable between groups and the assumptions of optimal least square regression modeling are violated (i.e. significant intra class correlation (ICC) value). Such regression models account for the variance within and between groups, thereby considering the dependence of data nested across multiple levels of analyses. A set of null models were run to test whether mixed effects models were appropriate, that is, whether the observations were not independent.
3. Results
3.1. Descriptive statistics
shows descriptive statistics and correlations for the participant characteristics included in the study.
Table 4. Descriptive statistics and correlation coefficients for participant characteristics included in the study.
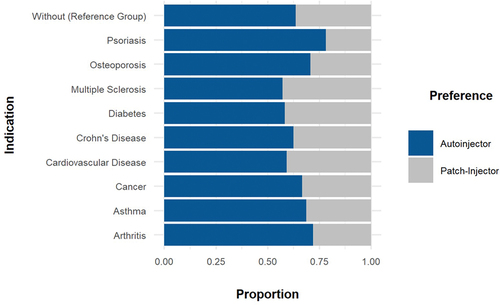
Overall, 31.6% of the participants preferred the large-volume wearable injector over the handheld autoinjector in the baseline scenario. Although there were differences in preference between patient groups, the overall device preference in the baseline scenario remained with the handheld autoinjector. While 36.4% of participants from the control group preferred the wearable large-volume device to the handheld autoinjector, this number varied from 21.9% to 42.9% because patients suffering from psoriasis least preferred the wearable device and patients suffering from multiple sclerosis most preferred the wearable device. illustrates the participant injection device preference per disease area.
3.2. Effects of patient characteristics on device preference
Logistic regression models were used to test the effects of patient characteristics on preference for wearable large-volume injectors or handheld autoinjectors in the baseline scenario. The results are presented in .
Table 5. Summary statistics for the logistic regression model based on patient characteristics with the preference for wearable large-volume injector as the response variable.
The coefficient of quality of life is – 0.23 (p < 0.001), indicating a strong and statistically significant negative relationship between participant quality of life and likelihood of preferring wearable large-volume injectors over handheld autoinjectors. For example, for an average injection-experienced female patient, the probability of preferring the wearable large-volume injector is 24.3%. When the quality of life score decreases to one or two standard deviations below the mean level, the probability that such a patient prefers a wearable large-volume injector increased from 24.3% to 42.9% or 24.3% to 63.8%, respectively. Consequently, an injection-experienced female patient whose quality of life score is two standard deviations lower than the mean level is more than 2.5 times as likely to prefer a wearable large-volume injector over a handheld autoinjector. The probability of preferring a wearable large-volume injector over a handheld autoinjector increased similarly for the other patient groups. When the quality of life scores fell two standard deviations below the respective mean levels, the probability increased from 34.1% to 74.0%, 30.3% to 70.5%, and 41.2% to 79.4% for an average female patient without injection experience, average injection-experienced male patient, and average male patient without injection experience, respectively.
Moreover, the models showed a negative but statistically insignificant effect of skin sensitivity (γ = – 0.01, p > 0.01), age (γ = – 0.01, p > 0.01), presence of osteoporosis (γ = – 0.91, p > 0.01), and self-injection experience (γ = – 0.50, p > 0.01) on wearable large-volume injector preference. Dexterity (γ = 0.01, p > 0.01) and sex (male) (γ = 0.30, p > 0.01) were found to have a positively but statistically insignificantly impact on the preference for wearable large-volume wearable injectors. The stepwise forward strategy to build the regression model did not result in the inclusion of other patient-related characteristics. In particular, all other diagnosed disease states were omitted, even if between-group differences in overall devices preferences were observed for the baseline scenario ().
3.3. Effects of changing treatment attributes on device preference
and provide a summary of how device preference changes in response to changing treatment attributes across the scenarios included. As a key insight, the analysis identifies inflection points, at which the overall preference shifted from handheld autoinjector to wearable large-volume injector dosing options and vice versa. Decreasing injection duration and frequency of wearable large-volume injectors was consistently associated with an increase in the preference for this device category.
Figure 2. Injector preference based on varying wearable large-volume injector injection duration (8 min versus 33 min) and frequency (biweekly, monthly, and quarterly).
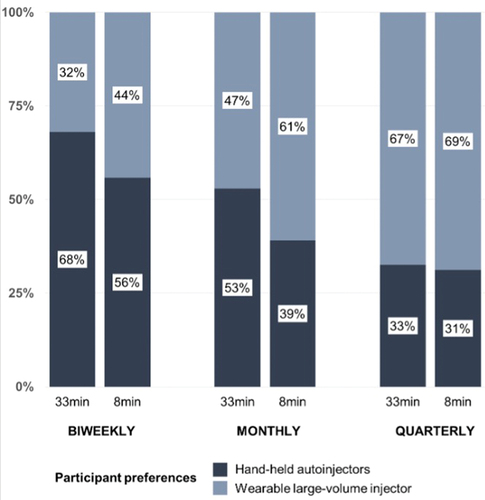
Table 6. Preferences for wearable large-volume injectors (WLVI) over handheld autoinjectors (HHA) for various treatment scenarios as a percentage of all preference statements (Preference statements WLVI/Total preference statements).
While only 32.1% of the participants preferred the wearable large-volume injector (biweekly injections lasting 33 min) to the handheld autoinjector (weekly injection lasting 3 min), 69.1% of the participants preferred this device when the injection duration was reduced from 33 to 8 min and the injection frequency from biweekly to quarterly injections. Regardless of injection duration, the general preference shifted from handheld autoinjectors (weekly injections) to wearable large-volume injectors when the injection frequency was reduced to quarterly injections. Similarly, the reduction in injection duration for wearable large-volume injectors from 33 to 8 min resulted in an overall shift in preference from handheld autoinjector to wearable large-volume injector dosing options (from 49.2% to 58.4%). A shift in preferences was also observed when the patients had to choose between a single dose with a wearable injector and three consecutive injections with an autoinjector to receive the same overall dose (from 46.2% at 33 min to 65.8% at 8 min). Injection duration and frequency virtually masked the effects of pain sensation and skin reaction on device preferences (). Therefore, it can be inferred that injection duration and frequency were the two predominant variables driving patient choices.
Fixed effect logistic regression models (Models 1 to 4) were then used to quantify the effects of changing key treatment attributes on device preference (see ). A model without predictors was first computed to assess the unexplained variance within and between Level 2 (study participant) [Citation42]. For the final models presented in , ICC values and chi-square tests reveal that between 89% (Model 1) and 98% (Model 3) of the variance in device preference statements resided between the study participants. Accordingly, mixed effects models were appropriate to predict device preference based on treatment attributes.
Table 7. Results of the mixed effects logistic regression model predicting preference for wearable large-volume injector based on treatment attributes.
First, Model 1 describes that reducing injection frequency with wearable large-volume injectors from biweekly to monthly injections and from biweekly to quarterly injections significantly increased the likelihood that patients will prefer these devices to handheld autoinjectors (Model 1: γ = 1.70, p < 0.001; Model 1: γ = 2.54, p < 0.001). Specifically, the model highlights the inflection point between monthly and quarterly injections, at which the average patient becomes more likely to prefer wearable large-volume injectors over handheld autoinjectors. Moreover, Model 1 showed a statistically significant interaction term (Model 1: γ = −1.21, p < 0.05), suggesting synergistic effects of the two treatment attributes, that is, injection duration and injection frequency, on device preference. Second, Model 2 shows how increasing the injection duration from 8 to 33 min for a single injection with a wearable injector compared to three consecutive injections with the handheld autoinjector reduces the likelihood that patients prefer the former over the latter (Model 2: γ = −1.92, p < 0.001). Third, Model 3 studies the scenarios in which the perceived pain for rapid drug administration using autoinjectors (moderate pain) differs to that for injection over different injection durations (8 and 33 min) using the wearable large-volume injector (mild pain). Increasing injection duration was associated with lower likelihood that patients will prefer wearable large-volume injectors to handheld autoinjectors (Model 3: γ = −10.51, p < 0.001). Despite the moderate pain sensation for the handheld autoinjector, reducing the injection duration of the wearable large-volume injector from 30 to 5 min did not change the overall preference for the device. The overall preference remained with the handheld autoinjector: 66.3% and 54.3% preferred the handheld autoinjector and wearable large-volume injector, respectively. Fourth, the results show that stronger skin reactions (moderate versus mild) when using wearable large-volume injectors reduce the attractiveness of these devices relative to handheld autoinjectors, but the positive effects of shortening injection duration on the likelihood that patients will prefer wearable large-volume injector over handheld autoinjectors is maintained (Model 4: γ = 1.62, p < 0.001).
4. Discussion
Based on a cross-sectional online survey and choice experiment, this study examined the impact of patient characteristics and treatment attributes on patient preferences for handheld autoinjector and wearable large-volume injector dosing options. This study advances the insight that overall device preferences not only depend on patient characteristics but also shift between the two device types as a function of changing treatment attributes. First, patients’ quality of life negatively impacted their likelihood of preferring wearable large-volume injectors over handheld autoinjectors. Second, a reduction in injection frequency and injection duration and their interaction effects increased the likelihood that patients would prefer wearable large-volume injectors to handheld autoinjectors. These findings have important implications for drug and device development, as they illustrate how decisions during early-stage clinical development may determine patient perceptions and market acceptance of the drug.
The results suggest that patients with high quality of life prefer more frequent but shorter injections using handheld autoinjectors over less frequent but longer drug self-administration using wearable large-volume injectors. While patients’ quality of life has been repeatedly studied as an outcome of chronic disease therapy interventions [Citation43], little attention has been paid to quality of life as the predictor of treatment choices. The current study provides empirical evidence that patient quality of life is negatively related to the likelihood of preferring less frequent but longer injections over more frequent but shorter injections. These findings suggest that despite lower injection frequency, longer injections using wearable large-volume injectors might be perceived as more disruptive by patients with high overall quality of life, that is, patients with better physical health (e.g. low levels of pain and discomfort). Moreover, patients with lower quality of life seemed to appreciate the hands-free operation and additional reassurance offered by wearable injectors with advanced visual and audible feedback elements; consequently, they preferred less frequent injections but longer injection durations. Interestingly, patients’ quality of life appears to mask potential disease-specific effects on treatment preferences. Although shows that preferences for injectors differ according to disease area, these effects are not statistically significant. For example, even if patients with psoriasis seem to prefer least wearable large-volume injectors, overall quality of life appears to be a better predictor of patient preference and thus the underlying factor for therapy choices, regardless of patient diagnosis. These results confirm previous initial findings that patient decision making is complex and influenced by numerous clinical and nonclinical factors, including quality of life [Citation44,Citation45].
This study also provides the key finding that changing injection frequency and duration shifts overall preference between device categories, highlighting inflection points in device preference that are important for drug development and clinical decision making (). This empirical study confirms previous work in which the frequency of injections to drive patient preferences and treatment decisions was identified [Citation30,Citation46]. For instance, switching from daily to weekly injections was the most important predictor of preference for injectable glucagon-like peptide-1 receptor agonist treatment options [Citation29]. Similarly, a discrete choice experiment concluded that participants with growth hormone deficiency would switch to a less frequent injection administration [Citation31]. This study builds on these findings but provides a more nuanced perspective on the inflection points at which general preferences change as a function of injection frequency. As such, while only 32% of participants preferred a biweekly injection with a wearable large-volume injection device over a handheld autoinjector, this proportion increased to 47% for a monthly injection and 67% for a quarterly injection that is administered over 35 min.
Interestingly, the likelihood of patients preferring biweekly self-administration using a wearable large-volume injector to a handheld autoinjector increases by a factor of almost 1.5 when the injection duration of the device is reduced to 8 min. Although the effect of injection duration attenuates for less frequent injections, the results show that the injection duration is a key characteristic influencing patient preferences between dosing options. Given that most biologics marketed to date are self-injected using handheld autoinjectors within approximately 10 to 15s [Citation22], little attention has been paid to injection duration as a predictor of patient choices for subcutaneous dosing options. However, this study shows that reducing the injection duration from 33 min to 8 min increases the likelihood of preferring wearable large-volume injectors over handheld autoinjectors from 49.1% to 58.1%, indicating an inflection point in patient choices regardless of injection frequency. Consequently, formulation and device technology to shorten injection duration may change overall preferences and positively impact commercial uptake of drugs that are subcutaneously self-injected [Citation20,Citation25]. Previous research has made similar observations across disease states, but the results were limited to care settings in which healthcare professionals administered intravenous infusions for hours in the hospital, as opposed to patients who self-administered a single bolus dose subcutaneously within minutes (or seconds) at home. For example, patient preferences for trastuzumab subcutaneous injection over intravenous infusion was primarily because of the reduction of the administration time: a reduction of 68–80% in the time spent on infusion chairs resulted in a strong convenience benefit and positive patient perceptions [Citation4,Citation47]. Similarly, a discrete choice experiment on patients with rheumatoid arthritis concluded that reducing the administration time of rituximab from 3.25 to 2 h was an even stronger predictor of patient preferences than reducing the infusion frequency from quarterly to twice per year [Citation48]. Therefore, the present study echoes previous calls for a combination of wearable large-volume injectors and novel formulation technologies that not only allow less frequent administration of large single bolus doses but also more rapid subcutaneous delivery [Citation20,Citation25]. This study confirms that injection frequency and duration are the strongest predictors of treatment choices and largely mask the effects of other attributes, such as pain and skin reactions.
Although the study identified several patient characteristics and treatment attributes as predictors of device preference, the significant variance at the individual patient level remains unexplained. The reasons why individuals prefer one dosing option over another are multifaceted, complex, and subject to change along the treatment journey [Citation36]. Therefore, the study re-emphasizes the need to commercialize multiple dosing options and drug delivery device presentations in parallel to allow to change individual treatment preferences. Personalized therapy decisions that account for patient preferences are key to ensure long-term treatment adherence and optimal health outcomes [Citation36].
These findings must be interpreted considering the limitations of the study, which highlight important areas for future work. Patients were not exposed to the physical devices, nor did they perform any actual injections. Including actual real-world experiences may impact on user preferences and treatment choices positively. Moreover, the online survey may suffer from a sampling bias. While the goal of the study was to model device preferences to ensure optimal treatment outcomes, patients who typically do not adhere to therapy may not have participated. Thus, the study may provide only limited insights into treatment decisions of the most critical patient sub-population. In addition, preferences may change as patients become more accustomed to self-injection. Rather than examining static choices between therapy options, future work could study preferences for different treatment regimens that include switching between dosing options (e.g. after completion of an initial titration phase). Finally, while understanding patient preferences is an important aspect of medication adherence, future research should also include payer, provider, pharmacist, or physician perspectives to model treatment choices. For example, a physician’s effort for initial patient onboarding and subsequently guiding patients during treatment may shape their preference for dosing options, and less frequent and shorter dosing may not always be preferred.
5. Conclusion
This study examined a-priori patient preferences for subcutaneous self-administration options based on autoinjectors and wearable large-volume injectors. The study was based on a cross-sectional online survey including 191 participants from Germany, without being exposed to the physical devices or performing injections. The findings suggest that both patient characteristics and treatment attributes significantly influence treatment choices and user preferences for dosing options. These results highlight critical inflection points, at which overall preferences shift from handheld autoinjectors to wearable large-volume injectors and vice versa. First, the study not only showed that patients’ quality of life significantly decreases the likelihood of preferring wearable large-volume injectors over handheld autoinjectors, but also identified patient characteristics that were irrelevant to such treatment choices in practice, such as prior self-injection experience, age, and diagnosis. Second, a reduction in injection frequency (from biweekly to monthly to quarterly) and injection duration (from 35 to 8 min) was found to increase the likelihood that patients would prefer wearable large-volume injectors to handheld autoinjectors, with the effect of injection duration seen to be as strong as the effect of injection frequency. These insights underscore the importance of decisions regarding target injection duration and frequency during device and formulation development, as these aspects may determine future market acceptance and treatment success.
Article highlights
Wearable large-volume injectors represent an emerging option for subcutaneous drug delivery, allowing less frequent injections compared to conventional self-administration using handheld autoinjectors.
It remains unclear whether and under which conditions such alternative dosing options using wearable-large volume injectors are preferred by patients over well-established handheld autoinjectors.
Based on an online survey and a choice experiment, this study examines which patient characteristics and treatment attributes determine patients’ preference for handheld autoinjectors over wearable large-volume injectors in hypothetical use scenarios.
The study shows that preferences depend on patient quality of life, injection frequency and injection duration, and identifies inflection points in treatment attributes where preferences shift from handheld autoinjector to wearable large-volume injectors.
The effect of injection frequency on preference for wearable large-volume injectors is consistent with the results of previous studies, but the effect of injection duration on device preference is more pronounced than expected.
Declaration of interest
A Schneider, C Jordi and J Lange are employees of Ypsomed AG. H Kolrep, Hanns-Peter H and S Gierig are employees of HFC Human-Factors-Consult Gmbh. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
A Schneider, C Jordi, J Lange, H Kolrep and Hanns-Peter H prepared the design and set-up of the study. Hanns-Peter H and S Gierig performed the study and initial analysis of the data. Statistical analysis and modelling was performed by H Kolrep. A Schneider drafted the manuscript with input, and critical content review, by all authors. All authors approved the final version for publication and agree to be accountable for all aspects of the work.
Additional information
Funding
References
- Jones GB, Collins DS, Harrison MW, et al. Subcutaneous drug delivery: an evolving enterprise. Sci Transl Med. 2017 Aug 30;9(405).
- Viola M, Sequeira J, Seiça R, et al. Subcutaneous delivery of monoclonal antibodies: how do we get there? J Control Release. 2018 Sep 28;286:301–314.
- Bittner B, Richter W, Schmidt J. Subcutaneous Administration of Biotherapeutics: an overview of current challenges and opportunities [journal article]. BIoDrugs. 2018 Oct 01;32(5):425–440.
- Pivot X, Gligorov J, Müller V, et al. Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013 Sep 01;14(10):962–970.
- Pivot X, Verma S, Fallowfield L, et al. Efficacy and safety of subcutaneous trastuzumab and intravenous trastuzumab as part of adjuvant therapy for HER2-positive early breast cancer: final analysis of the randomised, two-cohort PrefHer study. European journal of cancer. Oxford England: Elsevier; 1990) 2017. Nov Vol. 86:82–90
- Franken M, Kanters T, Coenen J, et al. Hospital-based or home-based administration of oncology drugs? A micro-costing study comparing healthcare and societal costs of hospital-based and home-based subcutaneous administration of trastuzumab. Breast. 2020;52:71–77.
- Stoner KL, Harder H, Fallowfield LJ, et al. Intravenous versus subcutaneous drug administration. Which do patients prefer? A systematic review. Patient-Patient-Centered Outcomes Res. 2015;8(2):145–153.
- Walsh C, Minnock P, Slattery C, et al. Quality of life and economic impact of switching from established infliximab therapy to Adalimumab in patients with rheumatoid arthritis. Rheumatology. 2007;46(7):1148–1152.
- Martin A, Lavoie L, Goetghebeur M, et al. Economic benefits of subcutaneous rapid push versus intravenous immunoglobulin infusion therapy in adult patients with primary immune deficiency. Transfus Med. 2013;23(1):55–60.
- Dou Z, Eshraghi J, Guo T, et al. Performance characterization of spring actuated autoinjector devices for Emgality and Aimovig. Curr Med Res Opin. 2020;36(8):1343–1354. doi:10.1080/03007995.2020.1783219.
- Wray S, Hayward B, Dangond F, et al. Ease of use of two autoinjectors in patients with multiple sclerosis treated with interferon beta-1a subcutaneously three times weekly: results of the randomized, crossover REDEFINE study. Expert Opin Drug Deliv. 2018;15(2):127–135. doi:10.1080/17425247.2018.1407755.
- Kivitz A, Segurado OG. HUMIRA pen: a novel autoinjection device for subcutaneous injection of the fully human monoclonal antibody Adalimumab. Expert Rev Med Devices. 2007 Mar;4(2):109–116.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015 Jun;29(6):1082–1090.
- Schneider A, Kolrep H, Jordi C, et al. How to prevent medication errors: a multidimensional scaling study to investigate the distinguishability between self-injection platform device variants. Expert Opin Drug Deliv. 2019 [2019 Jul 03];16(8):883–894.
- Sigurgeirsson B, Browning J, Tyring S, et al. Secukinumab demonstrates efficacy, safety, and tolerability upon administration by 2 ml autoinjector in adult patients with plaque psoriasis: 52-week results from MATURE, a randomized, placebo-controlled trial. Dermatol Ther. 2022;35(3):e15285.
- Lange J, Richard P, Bradley N. Usability of a new disposable autoinjector platform device: results of a formative study conducted with a broad user population. Med Devices (Auckl). 2015;8:255–264.
- Mead J, Dammerman R, Rasmussen S. Patient Reported Ease-of-Use with a Disposable Autoinjector in Individuals with Migraine. Patient Prefer Adherence. 2020;14:1137–1144.
- Heo Y-A. Mepolizumab prefilled syringe and autoinjector: a profile of their use in severe eosinophilic asthma. Drugs Therapy Perspect. 2020;36(5):1–8. doi:10.1007/s40267-020-00711-3.
- Mathaes R, Koulov A, Joerg S, et al. Subcutaneous Injection Volume of Biopharmaceuticals—Pushing the Boundaries. J Pharm Sci. 2016 [2016 Jul 01];105(8):2255–2259.
- Shi GH, Connor RJ, Collins DS, et al. Subcutaneous Injection Performance in Yucatan Miniature Pigs with and without Human Hyaluronidase and Auto-injector Tolerability in Humans. AAPS PharmSciTech. 2021 Jan 06;22(1):39.
- Jain M, Doughty D, Clawson C, et al. Tralokinumab pharmacokinetics and tolerability when administered by different subcutaneous injection methods and rates. Int J Clin Pharmacol Ther. 2017 Jul;55(7):606–620.
- Schneider A, Mueller P, Jordi C, et al. Hold the device against the skin: the impact of injection duration on user’s force for handheld autoinjectors. Expert Opin Drug Deliv. 2020 [2020 feb 01];17(2):225–236.
- Sigurgeirsson B, Browning J, Tyring S, et al. 27599 Secukinumab administration by a 2 mL autoinjector demonstrates high efficacy with comparable safety and tolerability in adult patients with plaque psoriasis: 16-week results from MATURE. J Am Acad Dermatol. 2021;85(3):AB154.
- Tangen LF, Lundbom JS, Skarsvåg TI, et al. The influence of injection speed on pain during injection of local anaesthetic. J Plast Surg Hand Surg. 2016 [2016 jan 02];50(1):7–9.
- Badkar AV, Gandhi RB, Davis SP, et al. Subcutaneous Delivery of High-Dose/Volume Biologics: current Status and Prospect for Future Advancements. Drug Des Devel Ther. 2021;15:159–170.
- Woodley WD, Yue W, Morel DR, et al. Clinical Evaluation of an Investigational 5ml Wearable Injector in Healthy Human Subjects. Clin Transl Sci. 2021;14(3): 859–869. doi:10.1111/cts.12946.
- Lange J, Schneider A, Jordi C, et al. Formative Study on the Wearability and Usability of a Large-Volume Patch Injector. Med Devices (Auckl). 2021;14:363–377.
- Woodley WD, Morel DR, Sutter DE, et al. Clinical evaluation of large volume subcutaneous injection tissue effects, pain, and acceptability in healthy adults. Clin Transl Sci. 2022;15(1):92–104.
- Nguyen H, Posner J, Kalsekar I, et al. A discrete-choice experiment to quantify patient preferences for frequency of glucagon-like peptide-1 receptor agonist injections in the treatment of type 2 diabetes. Current Med Res and Opinion. 2016;32(2):251–262.
- Blackwood C, Sanga P, Nuamah I, et al. Patients’ Preference for Long-Acting Injectable versus Oral Antipsychotics in Schizophrenia: results from the Patient-Reported Medication Preference Questionnaire. Patient Prefer Adherence. 2020;14:1093–1102.
- McNamara M, Turner-Bowker DM, Westhead H, et al. Factors Driving Patient Preferences for Growth Hormone Deficiency (GHD) Injection Regimen and Injection Device Features: a Discrete Choice Experiment. Patient Prefer Adherence. 2020;14:781.
- Zijlstra E, Jahnke J, Fischer A, et al. Impact of Injection Speed, Volume, and Site on Pain Sensation. J Diabetes Sci Technol. 2018;12(1):163–168.
- Torjman MC, Machnicki R, Lessin J, et al. Evaluation of an investigational wearable injector in healthy human volunteers. Expert Opin Drug Deliv. 2017 Jan;14(1):7–13.
- Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376(18):1713–1722.
- Amgen. Full Prescribing Information: REPATHA (evolocumab) injection, for subcutaneous use. USFaD A, editor. THousand Oaks CA United States: Amgen Inc.; 2021.
- Cowan R, Cohen JM, Rosenman E, et al. Physician and patient preferences for dosing options in migraine prevention. J Headache Pain. 2019 [2019 may 09];20(1):50.
- Whillans J, Nazroo J. Assessment of visual impairment: the relationship between self-reported vision and ‘gold-standard’ measured visual acuity. British Journal of Visual Impairment. 2014;32(3):236–248.
- Conti PC, de Azevedo LR, de Souza NV, et al. Pain measurement in TMD patients: evaluation of precision and sensitivity of different scales. J Oral Rehabil. 2001 Jun;28(6):534–539.
- Sautner J. Assessment von Hand-Osteoarthrosen mithilfe von Patienten-zentrierten Fragebögen [Assessment of Hand Osteoarthritis and Patient Centred Outcome Measures]. Aktuelle Rheumatologie. 2009 Jun 07;34(3):153–156.
- Misery L, Jean-Decoster C, Mery S, et al. A new ten-item questionnaire for assessing sensitive skin: the Sensitive Scale-10. Acta Derm Venereol. 2014 Nov;94(6):635–639.
- World Health Organization. Division of Mental Health. (1996). WHOQOL-BREF: introduction, administration, scoring and generic version of the assessment: field trial version, December 1996.
- Hox JJ, Moerbeek M, Van de Schoot R. Multilevel analysis: techniques and applications. New York and London: Routledge; 2017.
- Megari K. Quality of life in chronic disease patients. Health Psychol Res. 2013;1(3):27.
- Zafar SY, Alexander SC, Weinfurt KP, et al. Decision making and quality of life in the treatment of cancer: a review. Support Care Cancer. 2009 [2009 feb 01];17(2):117–127.
- Hajjaj FM, Salek MS, Basra MK, et al. Non-clinical influences on clinical decision-making: a major challenge to evidence-based practice. J R Soc Med. 2010;103(5):178–187.
- Stewart KD, Johnston JA, Matza LS, et al. Preference for pharmaceutical formulation and treatment process attributes. Patient Prefer Adherence. 2016;10:1385–1399.
- Pivot X, Gligorov J, Müller V, et al. Patients’ preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2-positive early breast cancer: final analysis of 488 patients in the international, randomized, two-cohort PrefHer study. Ann Oncol. 2014;25(10):1979–1987.
- Hauber B, Raimundo K, Zlotnick S, et al. AB1110 Patient Preference for a Shorter Infusion Time of Rituximab in RA. Ann Rheum Dis. 2015;74(Suppl 2):1272.