ABSTRACT
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide. Schlemm’s canal surgery using an iTrack flexible microcatheter has become popular because of its high quality-of-life issues and the growing demand for less invasive but effective procedures. The unique design of the microcatheter makes it a multimodal tool, which can be used not only in the field of antiglaucoma surgery but also as a drug delivery system to treat various conditions.
Areas covered
This review presents an update on the selected aspects of a drug delivery system using the iTrack microcatheter, including glaucoma gene therapy and posterior-segment diseases, both in animal models and human patients. The authors also report the case of a patient with branch retinal vein occlusion treated with suprachoroidal bevacizumab in the submacular region administered with the iTrack catheter.
Expert opinion
The findings presented in this study may indicate that the application of a microcatheter in open-angle glaucoma gene therapy is reasonable and can be combined with full or partial surgical canaloplasty procedures. Translation of this potential into a treatment modality would require overcoming multiple barriers.
1. Introduction
Glaucoma is the leading cause of irreversible blindness worldwide [Citation1]. The only approach to treat this condition is to reduce the intraocular pressure (IOP). This can be achieved pharmacologically or surgically. Canaloplasty is a natural outflow pathway-based antiglaucoma surgery. Over time, it has been considered one of the most popular surgical methods for the treatment of open-angle glaucoma (OAG). Schlemm’s canal (SC) surgery has become popular because of quality-of-life issues and the growing demand for less invasive but effective procedures.
Although the IOP-lowering potential of canaloplasty is well established, its exact mechanism of action remains unclear. This most likely results from enlarging the lumen of the SC and collector ostia and tensioning the inner wall of the canal with the Prolene suture, thus enabling natural outflow via the conventional pathway [Citation2]. Another possible mechanism for the reduction of IOP is the promotion of drainage into the intrascleral lake, which leads to prolonged drainage of the aqueous humor (AH) in a mechanism similar to that observed in deep sclerectomy [Citation3]. Moreover, the creation of an intrascleral lake offers another drainage route into the intrascleral and suprachoroidal spaces; however, its exact role is still being investigated [Citation4].
Canaloplasty originates from non-penetrating deep sclerectomy and viscocanalostomy, in which high-viscosity sodium hyaluronate is injected into the SC ostia following the preparation of scleral flaps [Citation5,Citation6]. This technique has evolved with the development of the iTrack flexible microcatheter (Nova Eye Inc., Fremont, California, U.S.A.) for 360° cannulation and canal viscodilatation.
The iTrack is a cardiac-surgery-inspired device that was approved by the US Food and Drug Administration in 2008, developing a standard ab externo procedure (see ) [Citation7]. It is a beacon-tipped, 250-µm-thick microcatheter connected to an ophthalmic viscosurgical device (OVD). This enables the threading of the catheter under direct transscleral visualization and simultaneous release of the viscoelastic material during suture placement. Inside the microcatheter, there is a supporting wire and optical fibers that transmit red laser light from the illumination system to the tip. In addition, there is an infusion pathway through which the OVD passes and is released from a syringe. Catheter-based canaloplasty has changed the focus of antiglaucoma surgery from open surgery to less invasive surgery, analogous to cardiac angioplasty. Ophthalmic medications do not reach the posterior segment tissues at the appropriate level during topical treatment because of kinetic problems. Although systemic drug administration is possible, exposure of non-target tissues can cause adverse effects. Consequently, unless effective targeting methods are developed, local ophthalmic drug administration methods are preferred for patients requiring continuous or repetitive treatment [Citation8]. iTrack’s unique design is considered superior to incisional antiglaucoma surgery and becomes a multimodal tool used to access both the anterior and posterior segments to deliver drugs.
In contrast to compliance-dependent pharmacological and risk-carrying surgical treatments for glaucoma, new individualized and long-term approaches are required. The issue of reducing permanent resistance to outflow through gene therapy is attracting increasing attention because of the lack of comprehensive therapy. Canaloplasty with iTrack can be used to direct the transmission of gene vectors into the SC and trabecular meshwork (TM) for OAG gene therapy.
Another important clinical problem is chronic retinal diseases, which require a long-lasting strategies. Many ophthalmic patients require repeated drug administration, mainly in the form of intravitreal injection. This is the source of compliance-related issues and higher risk of complications, among which, endophthalmitis is the most threatening. A sustained-release drug therapy could solve both clinical and socioeconomic problems related to those diseases [Citation9]. Advances in biomaterials, nanotechnology, and ophthalmic surgery have led to a plethora of modalities in ocular drug delivery. Drug delivery systems (DDSs) for the posterior segment can be divided into drug-releasing biomaterials (implants, microspheres, gels, oils), injection devices (microneedles, microcatheters), or refillable devices [Citation10].
This article presented an update on the selected aspects of a DDS using an iTrack microcatheter. Special emphasis is placed on gene therapy in OAG, with a focus on new aspects. We also presented the case of a patient with branch retinal vein occlusion (BRVO) in which iTrack was suprachoroidally used as a DDS. To the best of our knowledge, there are no other reviews on this subject in the recent literature.
2. Case report
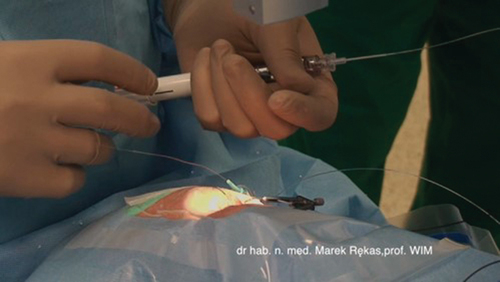
This case report presented the case of a 56-year-old Caucasian man with BRVO and macular edema. The patient’s baseline visual acuity (VA) was logMAR 1.0, and optical coherence tomography (OCT) revealed a macular thickening of 410 µm. Given the rapid progression of symptoms and significant deterioration of the best-corrected visual acuity, submacular drug delivery was recommended to the patient as a procedure with documented efficacy and safety (see ) [Citation11–13].
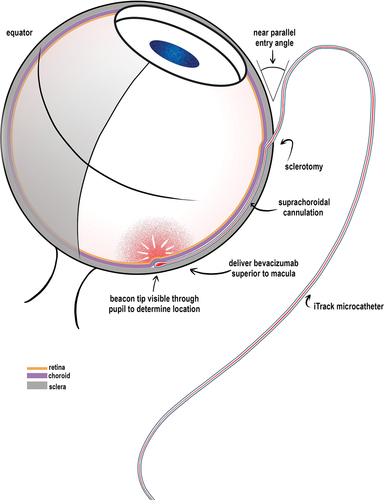
The patient received suprachoroidal bevacizumab in the submacular region using an iTrack catheter. Surgery was performed by one of the authors (MR) using the following technique: Under retrobulbar anesthesia, the eye was rotated to expose the superior temporal quadrant. Conjunctival peritomy was performed, followed by a full-thickness scleral incision with a stab knife 4 mm parallel to the limbus (Supplementary Figure 3 [see movie]). The edges of the sclerotomy sites were carefully dissected to expose the choroid. A small amount of viscoelastic material was injected to elevate the sclera. Subsequently, a microcatheter was introduced into the suprachoroidal space (SCS), and a 1-mL syringe containing 4 mg of bevacizumab was attached to it. The catheter was pushed forward toward the macula, and its path was observed using a microscope and a vitrectomy contact lens using the illuminated tip. When the tip reached the macula, the drug was injected beneath it.
One month postsurgically, the patient’s VA significantly improved to logMAR 0.4, and his central retinal thickness, which was measured by OCT, decreased to 318 µm. Six months postoperatively, the patient’s VA remained unchanged, and no adverse events were noted.
The present study conformed to the ethical principles outlined in the 2013 Declaration of Helsinki and was approved by the Local Bioethics Committee. All patient data were anonymized at the time of collection, and the patient provided written informed consent for the proposed treatment and publication of reports, images, and surgical video.
3. Discussion
3.1. iTrack as a drug delivery system for the posterior segment
There is a need to search for new targeted treatments for the posterior segment other than repeated intravitreal injections. Moreover, local delivery minimizes drug exposure and side effects and thus has advantages over systemic therapy.
3.1.1. Animal studies
There is strong evidence of the applicability of iTrack in animals as a suprachoroidal DDS [Citation8,Citation14,Citation15]. Peden et al. conducted a study in rabbits in which the iTrack microcatheter was used to deliver an adeno-associated virus (AAV) into the SCS and was posteriorly advanced toward the optic disc in the treatment of Leber’s congenital amaurosis [Citation16]. They successfully delivered the AAV vector to the SCS and did not observe any adverse effects caused by either the vector or procedure itself. The authors also demonstrated that the vector successfully transferred the gene product into an injection-induced bleb within the retina and choroid. In another study, Olsen et al. used a microcatheter as a posterior delivery system to inject triamcinolone acetonide (TA) into the porcine SCS. The authors demonstrated that TA remained in the macular region and the optic nerve tissue for >120 days [Citation13]. iTrack was also used by de Smet et al. to repeatedly catheterize the subretinal space in miniature pigs, which was conducted without severe trauma [Citation17]. One of the most promising reports on the use of iTrack as a DDS was by Kang et al. in a rabbit model of uveal melanoma. Their results provide hope to the use of this type of delivery system as a therapeutic agent against uveal melanoma and to reduce complications associated with brachytherapy [Citation18].
3.1.2. Human studies
In addition to experimental data from animal models, there is literature on clinical attempts at suprachoroidal drug administration in patients with retinal vasculopathy. Tetz et al. reported that suprachoroidal injection of a combination of 4.0 mg triamcinolone with 4.0 mg bevacizumab was safe, according to the results obtained in 21 patients with neovascular age-related macular degeneration [Citation12]. A small series of patients, similar to the case presented in this article, was described by Rizzo et al., who administered 1.25 mg of bevacizumab in combination with 4 mg TA into the SCS using iTrack 400 with good results. Of the six patients with macular edema and hard exudates, four gained two lines and the other remained stable [Citation11]. In the case presented in this review, bevacizumab was only administered into SCS. In the authors’ opinion, the surgical technique, even though previously described, is challenging and worth reporting. The methods of choice in the treatment of retinal vein occlusion are intravitreal anti-VEGF injections and laser treatment. The authors’ aim was to share their experience with other therapeutic options.
Except from pharmaceuticals, microcatheter was used as a subretinal delivery system for human umbilical tissue-derived cells. However, despite visual improvement in some patients with geographic atrophy, retinal detachment, and tears were frequent complications [Citation19].
3.1.3. Route of administration
The microcatheter can be used as both a subretinal and suprachoroidal space DDS. The delivery of subretinal substances requires a pars plana vitrectomy. After the vitrectomy is completed, macular retinotomy is performed using a small cannula gauge. The macula is separated by injecting a balanced salt solution (BSS), followed by vector injection into the preformed bleb. The use of the subretinal approach for delivery vector therapy is well recognized and has several advantages. This ensures the transduction of the outer retinal layers through the direct transmission of vectors. However, the surgical technique is a serious drawback of this method. Frequent complications include cataract formation, IOP spikes, retinal tears, and subretinal injection-related complications, such as macular holes. Although the subretinal path has some biases, the suprachoroidal path has been studied to overcome these limitations. The suprachoroidal route is less invasive than the subretinal route. It is also less immunogenic and generates lower levels of antibodies. Moreover, it transports vectors to the external retina, which is a major obstacle for intravitreous injection.
The study by Yiu et al. compared the difference in antibody production between the two pathways of AAV delivery, subretinal versus suprachoroidal space, considering that suprachoroidal delivery was associated with widespread transduction of retinal pigment epithelium (RPE) cells and resulted in diffuse, peripheral expression in the RPE, whereas subretinal delivery, in which the transduction was localized in the macular bleb, led to focal gene expression [Citation20]. In another study by Olsen et al., intravitreal injections of bevacizumab (Avastin; Genentech) had a more sustained effect than suprachoroidal microcannula injection. The two ways of delivery also differed in terms of drug distribution: bevacizumab intravitreally injected collected within the neurosensory retina, whereas the drug was injected suprachoroidally in the retinal outer segments, RPE, and choroid [Citation21]. The authors’ results are consistent with those obtained by Mori et al., who demonstrated that after the injection of AAV-chicken β-actin-green fluorescent protein (GFP) vector, the route-dependent concentrations of vectors were observed [Citation22]. After subretinal injection, prominent transduction occurred in the RPE cells and photoreceptors in the subretinal space; however, no detectable GFP particles appeared in the retinal ganglion cells. After intravitreous injection, prominent transduction occurred in the retinal ganglion cells (and possibly in the displaced amacrine cells), but no GFP was detected in the photoreceptors or RPE.
3.1.4. Safety of the procedure
The surgical technique of suprachoroidal delivery via microcatheter requires a certain amount of dexterity and familiarity with microcatheter. The suprachoroidal space (SCS) is a potential space between sclera and choroid, which means that under normal conditions it is collapsed due to the IOP and fiber connections between both layers [Citation23]. It is only 35 µm thick, but it allows for a slight movement of choroid against sclera on accomodation [Citation24]. It is also a natural outflow pathway for aqueous humor, which is used in glaucoma surgery. Both Cypass (Transcend [Citation25]) and iStent (Glaukos [Citation26]) are drainage devices placed in the SCS via an an ab-interno approach. Any procedure performed in this area has a risk of rupturing the choroid and causing a suprachoroidal hemorrhage – a sight-threatening condition that requires prompt action. Upon injection, the SCS expands and collects fluid at one site. With the use of the microcatheter, it can be slowly distributed in a more diffused way, so that the volume does not extend the SCS excessively. What is more, the iTrack is equipped with an illuminated beacon tip that facilitates transscleral guidance, which is not possible in transscleral injections even with the help of a special injector. However, the triamcinolone acetonide for suprachoroidal injection has been approved safe by the FDA (with no cases of suprachoroidal hemorrhage in the trial [Citation27]) and is already commercially available. Both procedures require a setting of operating room and an experienced surgeon and both carry a risk of suprachoroidal hemorrhage. Other complications described with both methods in humans [Citation11, Citation12, Citation27], i.e. IOP elevation, conjunctival hemorrhage, cataract, or uveitis, are manageable.
3.2. Glaucoma gene therapy
Over the past decades, understanding the molecular pathogenesis of glaucoma has led to the introduction of gene therapy techniques in the armamentarium of antiglaucoma treatments. Potential gene therapy strategies can aim to increase the natural outflow pathway and uveoscleral outflow or decrease IOP production.
The most probable etiology of increased IOP in primary open-angle glaucoma (POAG) is increased resistance in the outflow pathway, more precisely localized in the juxtacanalicular TM (JCT) and/or the inner wall of the SC [Citation28,Citation29]. The JCT is an amorphous layer of cells interspersed within the extracellular matrix (ECM). The natural outflow pathway can be enhanced by modulating JCT contractility [Citation30]. Cellular stiffness, which is increased by intracellular proteins, such as actin stress fibers, actomyosin fiber bundles, and cross-linked actin networks, is responsible for this resistance [Citation31]. Disruption of the actin cytoskeleton also leads to the ease of aqueous outflow in live animals [Citation32]. Various agents can decrease the external resistance. Ethacrynic acid disrupts the actin networks and inhibits cytoskeleton formation [Citation33]. Other compounds are latrunculins, which reduce IOP by shrinking and retracting the TM [Citation34]. Rho and Rho kinases play a special role in regulating intracellular changes and cell – matrix interactions, mainly by affecting actin dynamics [Citation31,Citation35,Citation36]. With their inhibition, TM cells relax significantly, which increases the outflow and decreases IOP [Citation36,Citation37]. This discovery led to the development of a novel group of antiglaucoma medications. Gene therapy to express the inhibitors of the Rho cascade or dominant-negative mutants of actin effectors has been hypothesized to enhance aqueous outflow. The Rho cascade is also a target of bacterial toxins from Clostridium botulinum species. This species produces the exoenzyme C3, which blocks Rho via ADP-ribosyltransferase [Citation38]. C3 expression mediated by an adenoviral vector disrupts the structure of cultured human TM cells and increases the outflow in monkey anterior segments by 90% [Citation39]. Another actin-binding protein is caldesmon. Overexpression of caldesmon disrupts the connections between human TM cells [Citation40] and increases outflow in human and monkey anterior segments [Citation41].
Another mechanism of action for potential antiglaucoma agents is ECM. A number of proteins influence the ECM structure and its interaction with TM cells, such as secreted protein acidic and rich in cysteine (SPARC) and thrombospondin-1 and thrombospondin-2 [Citation42,Citation43]. The aforementioned compounds seem to affect collagen fiber formation, which builds the ECM around the JCT. The IOP is also modulated by the amount of ECM; it increases with ECM production and decreases with ECM degradation (catalyzed by certain metalloproteinases). When SPARC is expressed in the human eye, the levels of metalloproteinases decrease and those of their inhibitors increase [Citation44]. SPARC also appears to regulate segmental outflow. Its lower level positively correlates with the amount of area involved in outflow and thus decreases IOP [Citation42]. Outflow resistance can also be affected by degradation of ECM proteins by matrix metalloproteinases (MMPs). Genes encoding these enzymes have been successfully transferred into the TM of human donors and rat eyes, increasing outflow facilities and decreasing IOP [Citation45,Citation46].
Various vectors have been used to transfer genes, including viral (herpes simplex viruses, adenoviruses) and non-viral delivery systems (DNA, RNA). Intracameral injection is a popular method for introducing these vectors into eye tissues. This injection has been shown to induce gene expression in the TM of animal models [Citation47].
3.3. Canaloplasty in glaucoma gene therapy
The circumferential shape of the SC has the potential to deliver genes to the TM at 360°, which can be more effective and allows changes in gene expression in the whole JCT region. SC-targeted gene transfer spares the corneal and iris tissues and probably requires a lower amount of viral vectors, thus lowering the toxicity of the entire procedure [Citation48]. This model was proven to be efficient in human donor eyes when the MMP-carrying vector delivered to the SC during viscocanalostomy caused high transgene expression in the TM and SC endothelium [Citation46]. Thus, canaloplasty is suspected to provide an even stronger response owing to its ability to access the entire circumference of the canal during catheterization and viscodilatation. However, there may be limitations to the application of canaloplasty, such as the complexity of the surgical technique and the contraindication to repeat it frequently. Natural AH flow from the anterior chamber toward the SC is also counter-directed to gene delivery. A solution to this problem might be depressing the IOP through paracentesis, as confirmed by Grieshaber et al., who measured fluorescein suffusion from the SC into the AC at a reverse gradient [Citation49]. Nevertheless, it is unpredictable whether JCT block transgene/vector particles similar to corneoscleral and uveal TM after intracameral injection [Citation50]. The IOP can return to normal after paracentesis, which may suggest that the TM will be exposed to the transgene/vector again with backflow of aqueous solution from the AC to the SC and stay at the TM for longer periods of time. However, these hypotheses are based on experiments performed on tissues perfused with BSS and are not viscoelastic, as in traditional canaloplasty performed in patients with OAG. Viscoelastic material may remain in TM tissues for up to 2 months [Citation51], which is longer than that required to cause gene expression in target tissues. This may advocate the use of nonviral vectors, which normally transfect genetic material less efficiently, but are less immunogenic. Another matter that remains unclear is how exactly the medium is transferred from the SC to the TM. Except for the natural flow through the pores, additional ruptures of the inner wall of the SC caused by the canaloplasty microcatheter and viscodilatation may play a role [Citation48]. The presence of endothelial defects in both walls of the SC and homogenous viscoelastic-like material in the JCT after viscocanalostomy was observed in monkey eyes [Citation51]. The existence of normal pores in the inner wall of the SC during retroperfusion (from the SC toward the TM) was confirmed by Ethier et al. in enucleated human eyes [Citation52]. For the first time, canaloplasty was performed in live cynomolgus and rhesus monkeys using a miniaturized 175-µm catheter by Aktas et al. The authors of this experiment observed trypan blue diffusion from one site to the entire SC; thus, they concluded that full catheterization of the entire SC may not be necessary to transfer genes [Citation53]. These findings indicate that the application of a microcatheter in POAG gene therapy is reasonable and can be combined with full or partial surgical canaloplasty procedures. A variant of canaloplasty belonging to microinvasive glaucoma surgery (MIGS), is ab-interno canaloplasty (ABiC) [Citation54]. This surgery is performed without sclerectomy, with only clear corneal incision and under gonioscopic view. The microcatheter is inserted into SC via the TM and advanced circumferentially and the OVD is injected simultaneously while it is withdrawn. ABiC has an excellent safety profile and leaves the tissues completely spared, which also makes it an repetitive procedure [Citation55]. The gene vector could be possibly delivered also during this procedure. However, placing the iTrack into SC in ab-interno manner requires good surgical skills and experience in MIGS procedures. ABiC has also lower IOP-lowering potential and is thus reserved for early glaucoma cases. The authors of this paper suggest that other modifications of canaloplasty could be considered for use in POAG gene therapy – namely, mini-canaloplasty [Citation56]. Rekas et al. invented this minimally invasive variant of canaloplasty, where both scleral flaps are smaller in size and serve as access to the SC. In authors’ opinion, the scleral lake may not be necessary not only to lower the IOP [Citation4] but also to deliver gene therapeutics. The variants of canaloplasty could be used standalone or combined with gene therapy depending on the stage of glaucoma, previous history, and anatomical conditions of the eye.
4. Conclusion
In this review, we present the possible point-of-action mechanism for glaucoma gene therapy advancements with the possible use of the iTrack catheter. In contrast to compliance-dependent pharmacological and surgical treatments, which carry a risk of failure, gene therapy may be a permanent solution. However, the safety and efficacy of gene therapy targeting TM needs to be further established. Studies using subretinal syringes in humans [Citation57] or microneedles in primates [Citation20] as DDS have shown the successful transmission of genes; however, they are localized only to areas around the injection sites. The results reported here suggest that microcatheter-based techniques can deliver viruses via SCS to multiple locations through a single incision port. This can result in a larger overall transduction area, which is beneficial for diseases involving the entire retina. Moreover, prolonged intraocular transgene expression was achieved using AAV vectors. Permanent intraocular production of anti-angiogenic proteins is necessary to counter the chronic production of angiogenic stimuli, which can never be achieved with repeated intravitreal injections. Collectively, these findings suggest that the successful delivery of gene replacement and other therapeutic agents to the eye using iTrack devices may become a significant cure for several retinal disorders and a potential therapeutic option for POAG.
5. Expert opinion
Advances in less invasive antiglaucoma surgery give new perspectives on antiglaucoma gene therapy. Procedures such as ab-externo canaloplasty or minicanaloplasty could offer a new route of administration for vectors modifying gene expression in eye’s tissues. However, when it comes to discussion on implementing these developments in clinical practice, the real concern is the availability of gene therapy in managing glaucoma. This type of treatment is not widely recognized because of small population of patients fitting in only few gene treatments approved. Another reason for this is the lack of awareness of such modalities among ophthalmologists. Up to this date, gene therapies for glaucoma are only possible in clinical trials. There is small amount of clinical trials in glaucoma, and their ability is limited to the United States and a few other countries. The only currently ongoing study is led by Stanford University and it refers to the ciliary neurotrophic factor (CNTF), which serves as retinal neuroprotector [Citation58]. TM-targeted transgene delivery methods could result in permanent outflow resistance decrease, however they still require development. Direct delivery of transgenes to SC or TB is similar to the topical antiglaucoma drugs point of action and leads to IOP reduction – by far the only modifiable glaucoma progression factor. Despite being an easily accessible tissue for gene transfer, there are still no clinical trials for TM-targeted gene therapy. The literature reports are limited to studies on human cultured cells/eyes or living animal eyes. Viral vectors delivered in to the eye by intracameral injections may not be easily targeted at the JCT TM and inner wall of SC, mainly because of interfering with uveal and corneoscleral TM, which are not the origin of the main outflow resistance [Citation50]. This is where the potential for canaloplasty lies. This technique allows for a direct access to SC. However, direct attempts have not been performed so far and a precise formulation and solvent for vectors should be specified. Another issue is the cost and difficulty of the procedure, which is more costful than standard incisional surgery and requires more skills than simple intracameral injection.
In general, the number of trials dedicated to ophthalmic diseases is quite low compared to systemic conditions – 1,8% [Citation59]. Surprisingly, even fewer are related to glaucoma. However, the level of applicable licensed gene therapy was finally achieved by human clinical trials on Leber congenital amaurosis and Luxturna. This would be the potential end-point for research on glaucoma gene therapy. The problem with glaucoma, is that in fact it is a number of various conditions with differing underlying pathology, often secondary to other ocular pathologies. Each of them would require selection of an adequate gene to modify. Next steps would be choosing a target tissue and a suitable vector. The new therapy should be then proved in a relevant animal model and validated in the laboratories in terms of its efficacy and reproducibility. Last but not least, it has to undergo a regulatory path like any conventional drug [Citation59].
Gene therapy is in the field of interest of glaucoma specialists. It would eliminate the necessity of regular applications and ocular surface complications. So far, this kind of therapy has been tested in preclinical and clinical trials. Hopefully, further research will help to break the barrier of availability.
Article highlights
Glaucoma is the leading cause of irreversible blindness worldwide.
Microcatheter application in open-angle glaucoma gene therapy is reasonable.
Using iTrack devices may become a significant cure for several retinal disorders.
Using iTrack devices may be a potential therapeutic option for POAG.
The gene therapy for POAG can be delivered by an iTrack device combined with any of the variants of canaloplasty.
Declarations of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download Zip (37.9 MB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17425247.2023.2256657.
Additional information
Funding
References
- Kang JM, Tanna AP. Glaucoma. Med Clin North Am. 2021;105(3):493–510. doi: 10.1016/j.mcna.2021.01.004
- Khaimi M. Canaloplasty using iTrack 250 microcatheter with suture tensioning on Schlemm′s canal. Middle East Afr J Ophthalmol. 2009;16(3):127. doi: 10.4103/0974-9233.56224
- Byszewska A, Konopińska J, Kicińska AK, et al. Canaloplasty in the treatment of primary open-angle glaucoma: patient selection and perspectives. Clin Ophthalmol. 2019; Dec 31;13:2617–2629. doi: 10.2147/OPTH.S155057
- Kicińska AK, Danielewska ME, Rękas M. Safety and efficacy of three variants of canaloplasty with phacoemulsification to treat open-angle glaucoma and cataract: 12-month follow-up. J Clin Med. 2022;11(21):6501. doi: 10.3390/jcm11216501
- Carassa RG, Bettin P, Fiori M, et al. Viscocanalostomy: a pilot study. Acta Ophthalmol Scand Suppl. 1998;227:51–52. doi: 10.1111/j.1600-0420.1998.tb00886.x
- Stegmann R, Pienaar A, Miller D. Viscocanalostomy for open-angle glaucoma in black African patients. J Cataract Refract Surg. 1999;25(3):316–322. doi: 10.1016/S0886-3350(99)80078-9
- Cameron B, Field M, Ball S, et al. Circumferential viscodilation of Schlemm’s canal with a flexible microcannula during non-penetrating glaucoma surgery. Digit J Ophthalmol. 2006;12(1). https://www.djo.harvard.edu/site.php?url=/physicians/oa/929.
- Ranta VP, Urtti A. Transscleral drug delivery to the posterior eye: prospects of pharmacokinetic modeling. Adv Drug Deliv Rev. 2006;58(11):1164–1181. doi: 10.1016/j.addr.2006.07.025
- Kang-Mieler JJ, Osswald CR, Mieler WF. Advances in ocular drug delivery: emphasis on the posterior segment. Expert Opin Drug Deliv. 2014;11(10):1647–1660. doi: 10.1517/17425247.2014.935338
- Kayla JJK, Wenqiang MR, William L. Advances in ocular drug delivery systems. Eye. 2020;34(8):1371–1379. doi: 10.1038/s41433-020-0809-0
- Rizzo S, Ebert FG, Di Bartolo E, et al. Suprachoroidal drug infusion for the treatment of severe subfoveal hard exudates. Retina. 2012;32(4):776–784. doi: 10.1097/IAE.0b013e3182278b0e
- Tetz M, Rizzo S, Augustin AJ. Safety of submacular suprachoroidal drug administration via a microcatheter: retrospective analysis of European treatment results. Ophthalmologica. 2012;227(4):183–189. doi: 10.1159/000336045
- Olsen TW, Feng X, Wabner K, et al. Cannulation of the suprachoroidal space: a novel drug delivery methodology to the posterior segment. Am J Ophthalmol. 2006;142(5):777–787. doi: 10.1016/j.ajo.2006.05.045
- Chen M, Li X, Liu J, et al. Safety and pharmacodynamics of suprachoroidal injection of triamcinolone acetonide as a controlled ocular drug release model. J Control Release. 2015;10(203):109–117. doi: 10.1016/j.jconrel.2015.02.021
- Robinson MR, Lee SS, Kim H, et al. A rabbit model for assessing the ocular barriers to the transscleral delivery of triamcinolone acetonide. Exp Eye Res. 2006;82(3):479–487. doi: 10.1016/j.exer.2005.08.007
- Peden MC, Min J, Meyers C, et al. Ab-externo AAV-mediated gene delivery to the suprachoroidal space using a 250 micron flexible microcatheter. PLoS One. 2011;6(2):17140. doi: 10.1371/journal.pone.0017140
- de Smet MD, Wyse S, Vezina M, et al. Repeated ab-externo catheterization of the sub-retinal space using a microcatheter for targeted delivery of a cell therapy product in a pig model. Invest Ophthalmol Vis Sci. 2012;53(14):5844.
- Kang SJ, Patel SR, Berezovsky DE, et al. Suprachoroidal injection of microspheres with microcatheter in a rabbit model of uveal melanoma. Invest Ophthalmol Vis Sci. 2011;52(14):1459.
- Ho AC, Chang TS, Samuel M, et al. Experience with a subretinal cell-based therapy in patients with geographic atrophy secondary to age-related macular degeneration. Am J Ophthalmol. 2017;179:67–80. doi: 10.1016/j.ajo.2017.04.006
- Yiu G, Chung SH, Mollhoff IN, et al. Suprachoroidal and subretinal injections of AAV using transscleral microneedles for retinal gene delivery in nonhuman primates. Mol Ther - Methods Clin Dev. 2020;16:179–191. doi: 10.1016/j.omtm.2020.01.002
- Olsen TW, Feng X, Wabner K, et al. Pharmacokinetics of pars plana intravitreal injections versus microcannula suprachoroidal injections of bevacizumab in a porcine model. Investig Ophthalmol Vis Sci. 2011;52(7):4749–4756. doi: 10.1167/iovs.10-6291
- Mori K, Gehlbach P, Yamamoto S, et al. AAV-mediated gene transfer of pigment epithelium-derived factor inhibits choroidal neovascularization. Investig Ophthalmol Vis Sci. 2002;43(6):1994–2000.
- Emi K, Pederson JE, Toris CB. Hydrostatic pressure of the suprachoroidal space. Investig Ophthalmol Vis Sci. 1989;30(2):233–238.
- Krohn J, Bertelsen T. Corrosion casts of the suprachoroidal space and uveoscleral drainage routes in the pig eye. Acta Ophthalmol Scand. 1997;75(1):28–31. doi: 10.1111/j.1600-0420.1997.tb00244.x
- Höh H, Grisanti S, Rau M, et al. Two-year clinical experience with the CyPass micro-stent: safety and surgical outcomes of a novel supraciliary micro-stent. Klin Monbl Augenheilkd. 2014;231(4):377–381. doi: 10.1055/s-0034-1368214
- Wellik SR, Dale EA. A review of the iStent ® trabecular micro-bypass stent: safety and efficacy. Clin Ophthalmol. 2015;15(9):677–684. doi: 10.2147/OPTH.S57217
- Yeh S, Khurana RN, Shah M, et al. Efficacy and safety of suprachoroidal CLS-TA for macular edema secondary to noninfectious uveitis: phase 3 randomized trial. Ophthalmology. 2020;127(7):948–955. doi: 10.1016/j.ophtha.2020.01.006
- Maepea O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm ’ s canal in monkeys. Exp Eye Res. 1992;54:879–883. doi: 10.1016/0014-4835(92)90151-h
- Overby DR, Stamer WD, Johnson M. The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall edothelium. Exp Eye Res. 2009;88(4):656–670. doi: 10.1016/j.exer.2008.11.033
- Liu X, Rasmussen CA, Gabelt BAT, et al. Gene therapy targeting glaucoma: where are we? Surv Ophthalmol. 2009;54(4):472–486. doi: 10.1016/j.survophthal.2009.04.003
- Rao PV, Deng P, Sasaki Y, et al. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility *. Exp Eye Res. 2005;80:197–206. doi: 10.1016/j.exer.2004.08.029
- Honjo M, Tanihara H, Inatani M, et al. Effects of Rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Investig Ophthalmol Vis Sci. 2001;42(1):137–144.
- Erickson-Lamy K, Schroeder A, Epstein DL. Ethacrynic acid induces reversible shape and cytoskeletal changes in cultured cells. Investig Ophthalmol Vis Sci. 1992;33(9):2631–2640.
- Peterson JA, Tian B, Geiger B, et al. Effect of latrunculin-B on outflow facility in monkeys. Exp Eye Res. 2000;70(3):307–313. doi: 10.1006/exer.1999.0797
- Rao PV, Deng P, Kumar J, et al. Modulation of aqueous humor outflow facility by the Rho kinase – specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42:1029–1037.
- Rao PV, Deng P, Maddala R, et al. Expression of dominant negative Rho-binding domain of Rho-kinase in organ cultured human eye anterior segments increases aqueous humor outflow. Mol Vis. 2005;11(March):288–297.
- Tian B, Kaufman PL. Effects of the Rho kinase inhibitor Y-27632 and the phosphatase inhibitor calyculin a on outflow facility in monkeys *. Exp Eye Res. 2005;80(2):215–225. doi: 10.1016/j.exer.2004.09.002
- Aktories K, Mohr C, Koch G. Clostridium botulinum C3 ADP-ribosyltransferase. Curr Top Microbiol. 1992;175:115–131.
- Liu X, Hu Y, Filla MS, et al. The effect of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Mol Vis. 2005;11:1112–1121. http://europepmc.org/abstract/MED/16379023
- Grosheva I, Vittitow JL, Goichberg P, et al. Caldesmon effects on the actin cytoskeleton and cell adhesion in cultured HTM cells. Exp Eye Res. 2006;82(6):945–958. doi: 10.1016/j.exer.2006.01.006
- Gabelt BT, Hu Y, Vittitow JL, et al. Caldesmon transgene expression disrupts focal adhesions in HTM cells and increases outflow facility in organ-cultured human and monkey anterior segments. Experimental Eye Research. 2006;82(6):935–944. doi: 10.1016/j.exer.2005.12.002
- Swaminathan SS, Oh DJ, Kang MH, et al. Secreted protein acidic and rich in cysteine (SPARC)-null mice exhibit more uniform outflow. Investig Ophthalmol Vis Sci. 2013;54(3):2035–2047. doi: 10.1167/iovs.12-10950
- Haddadin RI, Oh DJ, Kang MH, et al. Thrombospondin-1 (TSP1)-null and TSP2-null mice exhibit lower intraocular pressures. Investig Ophthalmol Vis Sci. 2012;53(10):6708–6717. doi: 10.1167/iovs.11-9013
- Oh DJ, Kang MH, Ooi YH, et al. Overexpression of SPARC in human trabecular meshwork increases intraocular pressure and alters extracellular matrix. Investig Ophthalmol Vis Sci. 2013;54(5):3309–3319. doi: 10.1167/iovs.12-11362
- Kee C, Sohn S, Hwang J. Stromelysin gene transfer into cultured human trabecular cells and rat trabecular meshwork in vivo and. Invest Ophthalmol Vis Sci. 2001;42(12):2856–2860.
- Hudde T, Apitz J, Bordes-Alonso R, et al. Gene transfer to trabecular meshwork endothelium via direct injection into the Schlemm canal and in vivo toxicity study. Curr Eye Res. 2005;30(12):1051–1059. doi: 10.1080/02713680500323350
- Borrás T, Gabelt BT, Klintworth GK, et al. Non-invasive observation of repeated adenoviral GFP gene delivery to the anterior segment of the monkey eye in vivo. J Gene Med. 2001;3(5):437–449. doi: 10.1002/jgm.210
- Tian B, Kaufman PL. A potential application of canaloplasty in glaucoma gene therapy. Transl Vis Sci Technol. 2013;2(1):2. doi: 10.1167/tvst.2.1.2
- Grieshaber MC, Pienaar A, Olivier J, et al. Clinical evaluation of the aqueous outflow system in primary open-angle glaucoma for canaloplasty. Investig Ophthalmol Vis Sci. 2010;51(3):1498–1504. doi: 10.1167/iovs.09-4327
- Daniel Stamer W, Chan D.W.-H., Ross Ethier, C. Targeted gene transfer to Schlemm’s canal by retroperfusion. Experimental Eye Research. 2008;84(5):843–849. doi: 10.1016/j.exer.2007.01.001
- Tamm ER, Carassa RG, Albert DM, et al. Viscocanalostomy in rhesus monkeys. Arch Ophthalmol. 2004;122(12):1826–1838. doi: 10.1001/archopht.122.12.1826
- Ethier RC, Coloma FM, de Kater AW, et al. Retroperfusion studies of the aqueous outflow system part 2: studies in human eyes. Investig Ophthalmol Vis Sci. 1995;36(12):2466–2475.
- Aktas Z, Tian B, McDonald J, et al. Application of canaloplasty in glaucoma gene therapy. J Ocul Pharmacol Ther. 2014;30(2–3):277–282. doi: 10.1089/jop.2013.0203
- Körber N, Kanaloplastik A, Fallseriestudie G. Ab interno canaloplasty for the treatment of glaucoma: a case series study. Spektrum Augenheilkd. 2018;32(6):223–227. doi: 10.1007/s00717-018-0416-7
- Khaimi MA. Canaloplasty: a minimally invasive and maximally effective glaucoma treatment. Funk J, ed. J Ophthalmol. 2015;2015:485065. doi: 10.1155/2015/485065
- Rękas M, Konopińska J, Byszewska A, et al. Mini-canaloplasty as a modified technique for the surgical treatment of open-angle glaucoma. Sci Rep. 2020;10(1):1–7. doi: 10.1038/s41598-020-69261-y
- Xue K, Groppe M, Salvetti AP, et al. Technique of retinal gene therapy: delivery of viral vector into the subretinal space. Eye. 2017;31(9):1308–1316. doi: 10.1038/eye.2017.158
- Goldberg JL, Beykin G, Satterfield KR, et al. Phase I NT-501 ciliary neurotrophic factor implant trial for primary open angle glaucoma: safety, neuroprotection and neuroenhancement. Ophthalmol Sci. 2023;3(3):100298. doi: 10.1016/j.xops.2023.100298
- Borrás T. The pathway from genes to gene therapy in glaucoma: a review of possibilities for using genes as glaucoma drugs. Asia-Pacific J Ophthalmol. 2017;6(1):80–93. doi: 10.22608/APO.2016126