?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Introduction
The urgency to replace the propellants currently in use with the new sustainable ones has given rise to the need for investigation and reformulation of pMDIs.
Areas covered
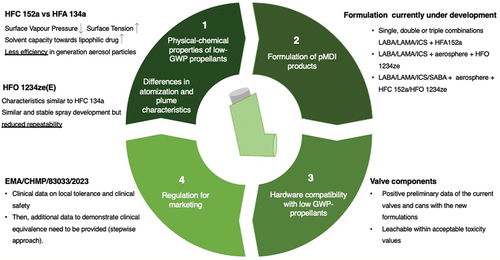
The reformulation requires in-depth knowledge of the physico-chemical characteristics of the new propellants, which impact the atomization capacity and the plume geometry. Among the investigated propellants, HFA 152a, due to its lower vapor pressure and higher surface tension compared to HFA 134a, deliver larger particles and has a higher solvent capacity toward lipophilic drugs. On the other hand, HFO 1234ze has properties more similar to HFA 134a, but showed lower reproducibility of the generated spray, indicating a possible high susceptibility to variation in the consistency of the dose delivered. In addition, the device components currently in use are compatible with the new propellants. This allowed promising preliminary results in the re-formulation of pMDIs by academia and pharma companies. However, there is little information about the clinical studies required to allow the marketing of these new products.
Expert opinion
Overall, studies conducted so far show that the transition is technically possible, and the main obstacle will be represented by the investment required to put the product on the market.
1. Introduction
In 1955, a little girl’s request to turn her asthma medicine into a spray, like those for hair, sparked a revolution in inhaler devices [Citation1]. Shortly thereafter, Riker Laboratories introduced the first pressurized metered dose inhaler (pMDI) for the treatment of asthma and chronic obstructive pulmonary disease (COPD), a technology which rapidly became the most widely applied for inhalation devices for the treatment of pulmonary diseases [Citation2]. In fact, most treatment options are available in this delivery systems which are also portable and user-friendly [Citation3]. The pMDI consists of an aluminum canister filled with a formulation, either a solution or suspension of micronized drug particles and other excipients, in a propellant, clamped with a metering valve and inserted into a plastic actuator. Currently, pMDI products contain as propellant norflurane (HFA 134a) or, to a lesser extent (about 13% in the US [Citation4]), apaflurane (HFA 227ea) which are two greenhouse gases, with a global warming potential (GWP) of 1300 and 3350, respectively.
Despite emissions from pMDI medicinal products represent less than 0.1% of the total emission of greenhouse gases [Citation5], recent amendments on the phase down of propellants with high global warming potential have led nations to take steps to switch to more environmentally sustainable inhaler devices. The total greenhouse gas emissions depend on the market share that pMDIs have in that country. reports the five most impacting markets of inhalation products in terms of sales in the United Kingdom (UK) and European Union (EU). For the five analyzed markets, 90% of the total CO2 equivalent emissions from inhalers are related to the use of pMDIs. Among all, the UK recorded the highest value of CO2 equivalent emitted related to the fact that about 70% of the inhalers sold were pMDIs [Citation6]. As a consequence, UK National Health Service has started to encourage the prescription of equivalent products in the form of dry powder inhalers (DPI) or soft mist inhalers as an alternative to pMDIs [Citation7]. However, this switching is not as smooth as it might seem. In fact, pMDIs and DPIs require preparation of the device and inhalation techniques during dose intake drastically different from each other: i.e. slow and prolonged breathing with the need for coordination for the pMDIs and a quickest possible inspiration for the DPIs. As a result, switching from one to the other without proper patient education could lead to adverse consequences for the patient’s health [Citation8]. Indeed, it was found that patients who continued using pMDI for the treatment of asthma had a better outcome than those whose inhaler was replaced with a DPI [Citation9]. Moreover, the deterioration of the patient’s health conditions would lead to significantly increased healthcare costs due to more frequent consultations for primary care, emergency visits and access to hospitals[Citation8]. From an economic point of view, it is very cost effective to supply a patient with a reliever medication in the form of a pMDI. For example, despite the presence of a DPI alternative, the salbutamol sulfate (SS) reliever pMDI accounts for 90% of the total sales; this is because a single dose costs just $0.06 [Citation10].
Table 1. Data calculated of total greenhouse gases emissions (as kilotonnes of CO2 equivalent) from pharmaceutical inhaled products in different countries in 2019 (modified from ref [Citation6]).
Therefore, rather than replacing the use of pMDIs with less effective alternatives, the pharmaceutical world, also pushed by the regulations discussed in the next section, has turned toward the identification of alternative propellants with less impact on the environment in terms of carbon footprint. Two compounds with a low GWP such as 1,1-difluoroethane (HFA 152a) and 1,3,3,3-tetrafluoropropene (HFO 1234ze(E), hereinafter referred to as HFO 1234ze) have been identified. The impact on global warming of these two propellants is approximately 100 or 1000 times lower than those currently in use for HFA 152a and HFO 1234ze, respectively [Citation11]. A study to predict CO2 emissions 10 years from now, showed that by switching to pMDIs containing low-GWP propellants it is possible to achieve an 89% reduction in total CO2 emissions from inhalers [Citation6].
The physico-chemical, safety and flammability characteristics of these low-GWP propellants are still under investigation even if the first patents on their use date back to 2006 [Citation12]. During the transition to non-chlorofluorocarbon propellants, the use of HFA 152a was also proposed, but was quickly abandoned as it is an extremely flammable gas, with a lower explosive limit of just 3.9 volume percent in air [Citation13]. Despite that, studies have shown that its use could represent a relative risk for the patient, and even though of low level, when HFA 152a is associated with other flammable co-solvents such as ethanol [Citation14]. On the other hand, the flammability of the propellant leads to a huge investment from pharmaceutical companies to make their production sites compliant to rules for production with a potentially explosive atmosphere (ATEX). This involves the location of industrial plants away from population centers and their structural adaptation according to the relevant regulations depending on the country in which they are located. Additionally, the facility’s insurance company may require further safety conditions beyond those required by state law. Usually, the insurer’s requirements are more stringent than those of the national standard. On the other hand, HFO 1234ze is a nonflammable gas, identified as safe when handled under normal service conditions and, in presence of a flame, only a very narrow flammable range was experimentally detected [Citation15]. Finally, both the new low-GWP propellants showed similar antimicrobial properties to the ones currently in use, thus facilitating the reformulation of new pMDIs [Citation16].
This manuscript will cover the critical aspects to be faced in the transition toward new propellant-based pMDIs which are depicted in . In detail, after an overview of the physico-chemical properties of the new gases and their impact on atomization, the main results collected to date by the academic and industrial world operating in this direction will be illustrated. Next, the pMDI products subject to the intellectual property (IP) filed will be described, followed by the studies reporting the compatibility of the cans/valves with the new gases and concluding with considerations relating to the regulatory aspects and description of the clinical studies currently underway.
2. Emerging criteria governing the use of fluorinated gases
The Montreal Protocol, signed in 1987, resulted in a ban on substances that deplete the ozone layer, including chlorofluorocarbon (CFC) propellants used at that time for the formulation of pMDIs [Citation1]. While the ban still allowed the sale of CFC-based inhaler products until viable alternatives were put on the market, the transition was not straightforward. In fact, it was necessary to develop products like the existing ones in terms of dose emitted and aerodynamic particle size distribution, but this was challenging considering the different chemical-physical characteristics of the HFA propellants. To be able to adapt to the new propellants and also improve the products on the market with new technologies, not only equivalent products but also products defined ‘the same only better’ have been studied [Citation17]. The Protocol made it possible to globally reduce the use of ozone depleting substances resulting in the recovery of the ozone layer that will be completed by 2066.
In EU, a regulation on Fluorinated gases (F-gases) came into force in January 2015, incorporating bans and quotas on the use of these compounds, including HFA 134a and HFA 227ea, with the aim of gradually reducing their use to a fifth of 2014 sales by 2030 [Citation18]. In 2016, the Kigali amendment to the Montreal protocol marked, starting from 2019, the beginning of a worldwide phase-down of these gases [Citation19]. However, the countries which signed the agreement were divided in different groups with different baseline years and phase down schedules. As shows, in the developing countries (Article 5) a later and milder phase down, starting from 2029 to 2032, is expected as opposite for the developed countries, for which the phase down started already in 2019.
Figure 2. Phase-down for the different Parties following the Kigali amendment and EU F-gases regulation indicated as reduction % of the baseline.
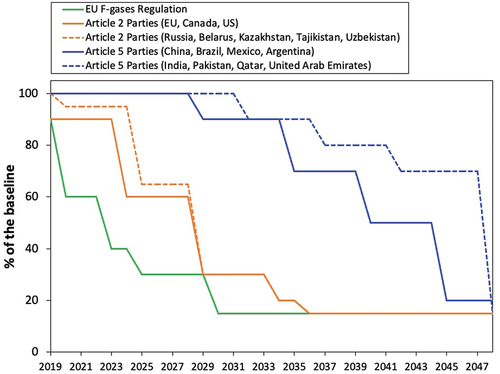
Even if the propellants for inhalation products have a longer phase-down time compared to the gases used in other sectors [Citation18], there is an urgency to complete the development of the replacement products. This is because the supply source and cost of the pharmaceutical propellants currently in use will become increasingly less favorable during the phase-down [Citation11]. In addition, the phase out for 2030 means that the new products will need to be approved within 7 years, which is an extremely short length of time in relation to that required to develop, clinically test, and register the replacement products.
Another aspect to be considered with the phase out is the control of greenhouse gases-based pMDI products imports in Europe. This is because it would be counterproductive in reducing carbon footprint to encourage and reward countries that are reluctant to phase down, while penalizing EU and UK companies that have invested and risked much to comply to a forced transition.
In addition to the phase-down of F-gases, the European Chemical Agency’s (ECHA) is currently drafting a proposal to ban the use and production of per- and polyfluoroalkyl substances (PFASs) [Citation20]. Although HFO 1234ze has the lowest global warming potential of the proposed replacement propellants, it has been included among the substances put under observation by the new proposal on PFASs. It is suggested that, following atmospheric degradation, the propellant releases into the environment trifluoroacetic acid which is known to be a very persistent chemical with acute toxicity to humans. The six-month open consultation period of the proposal began on 22nd March 2023. During the six months open consultation time, scientific data highlighting the importance and environmental advantages of utilizing HFO 1234ze as a propellant may be submitted by the industries. ECHA’s scientific committees’ evaluation and proposal approval is anticipated to be during 2025. In addition to HFO 1234ze, ECHA’s includes fluoropolymers as a chemical under observation, which are used for the coating of some pMDI canisters.
In the event that the use of HFA 152a and HFO 1234ze were also banned, all patients would be forced to use mainly DPIs. This exchange of product types is very risky not only because many treatments that are available as pMDIs are not as DPIs but also because, as mentioned above, the latter require a totally different inhalation procedure from the former and the patient would have to adapt with difficulty [Citation3].
For the use of HFA 152a as a replacement pMDI propellant, a raw material cost equivalent to that of current propellants can be expected. Within the product, the cost of the propellant represents approximately 1% of the overall cost of the pMDI [Citation21].
3. Impact of the physico-chemical characteristics of the new propellants on initial droplets that form upon atomization
Since the liquefied propellant during its expansion is the energy source for the formation of the aerosol droplet dispersion, its physico-chemical characteristics () strongly impact the quality of the generated atomized aerosol. Before tackling formulation development, it is important to understand the atomization process and spray plume characteristics of pMDIs using the new gases. In fact, studies conducted on this topic indicate that there are some differences in plume characteristics for HFA 152a and HFO 1234ze pMDIs compared to currently used propellants that could impact patient acceptance and consequently total lung dose [Citation22]. Here, factors such as droplet size distribution and droplet evaporation rate are decisive as well as the plume velocity and plume force, spray duration, plume temperature and plume geometry.
Table 2. Physico-chemical characteristics of propellants used for the formulation of pMDIs.
Going back to basics, the pMDI aerosol generation process consists of two phases: droplet formation (Phase I) and aerosol maturation (Phase II). Stein and Myrdal have extensively described the relative influence of the two phases on the delivery efficiency of a pMDI [Citation23].
The key process during the ‘droplet formation phase’ is the atomization of the formulation into small droplets [Citation23]. At the start of the atomization event, formulation exiting the valve orifice flows into the expansion chamber sump that is at ambient pressure. Multiphase flow is predominantly atomized before exiting the nozzle [Citation24] where droplets can be produced by shear due to a velocity difference at the liquid–vapor interfaces or by the breaking of bubbles and liquid as they enter the nozzle [Citation25,Citation26]. Critical factors influencing the size distribution of the initial droplets include the vapor pressure and surface tension of the formulation as well as the valve size and actuator orifice diameter [Citation23].
In the ‘aerosol maturation phase’ the evaporation of the volatile and semi-volatile materials occurs. Once the droplets are atomized, the high volatility propellant and semi-volatile cosolvent rapidly evaporate leaving the residual aerosol. The size of residual particles is influenced both by the formulation composition but also by the size of the initial droplets. To successfully deposit in the lung, atomized droplets must rapidly evaporate to a size which does not deposit in oropharynx region but reaches the patient’s lung.
The dynamic plume characteristics of the aerosols generated with the new propellants have been recently investigated and compared with those of the propellants currently in use, with and without the addition of ethanol [Citation22]. Here, previously validated theoretical approaches have been applied together with experimental measurements. During atomization, pressure and temperature in expansion chamber tend to change over time: as the propellant enters the sump, there is a rapid pressure rise and a brief temperature drop but both tend to decrease as the metering valve empties [Citation26]. It was found that the pressure of the plume follows the rank order of the propellant vapor pressure (HFA 134a > HFA 152a > HFO 1234ze > HFA 227ea) with a plume duration decreasing slightly with increased peak sump pressure. The addition of ethanol determined a decrease of the peak sump pressures due to the reduction of the saturated vapor pressure (SVP) of the mixtures.
The results of the plume force confirmed that it is related to the SVP of the propellant as described by the model used for its prediction [Citation27]. In fact, HFA 227ea which is the one with the lowest SVP showed the lowest plume force generated (11.4 mN) [Citation22]. On the other hand, the force generated by HFA 134a and HFA 152a propellants appeared to be very similar in magnitude and about 20 mN. Since plume force is related to the amount of deposition in the oropharynx, similar HFA 152a throat deposition to HFA 134a can be expected [Citation27]. The inclusion of ethanol, decreasing the SVP of the formulations, resulted in a consistent reduction of the plume force, as expected.
Overall, there was an excellent agreement between measured and theoretical parameters evaluated except for the sump pressure and plume force values of HFO 1234ze/ethanol mixture due to the limitation of using Raoult’s law for SVP estimation.
Regarding the plume temperature measured at the position representing the tongue surface in a mouth-throat model, HFA 227ea was found the warmest, HFO 1234ze and HFA 134a colder and HFA 152a the coldest. With the latter, a temperature of −27°C was recorded, corresponding to the boiling point of the propellant, indicating that the droplets containing the propellant deposited in this region, causing the cooling after its evaporation. In this case, the addition of ethanol reduced the differences between the minimum temperatures measured but maintaining the same rank order. This aspect should not be underestimated and will have to be further studied in clinical since the acceptability of the patient depends on what they feel upon inhalation and that the products currently in use generate temperatures that reach at least −12°C [Citation22].
Finally, the size distribution of the initial droplets is affected by the vapor pressure of the propellant. It is known that the lower the vapor pressure of the formulation, the lower the energy to atomize the formulation [Citation23]. The particle size of the droplets that form upon atomization is then directly related to the mass median aerodynamic diameter (MMAD) of the aerosol that forms during the aerosol maturation phase [Citation23]. This had already been highlighted during the transition to non-CFC propellants since, maintaining the same formulation composition, HFA-based pMDIs reached a respirable particle fraction (i.e. percentage of particles <5 µm) of more than 50%, against a maximum of 30% reached by the phased-out CFC-containing pMDIs [Citation28].
When ethanol is added as co-solvent, formulation properties such as vapor pressure, viscosity, and surface tension are altered impairing the ability of the formulation to be atomized into fine droplets (i.e. effect on Phase I) [Citation29]. In a recent study [Citation30], SVP variation at different temperatures was evaluated in both HFA 152a and HFO 1234ze ethanol binary mixtures. It was found that the addition of the ethanol reduced the SVP of each mixture; a behavior previously observed for mixtures of HFA 134a or HFA 227ea with ethanol [Citation31].
In relation to the size distribution of the initial atomized droplets, this can be calculated by measuring the MMAD value of the residual particles and using the following equation described by Stein and Myrdal [Citation29]:
where ρdroplet is the initial droplet density, CNV is the weight fraction of nonvolatiles in the formulation and ρresidual is the residual particle density.
The initial droplet calculation was performed with formulations where only the propellant changed and a 0.5% w/w PEG300 was included in order to generate measurable residual particles [Citation23]. The highest initial droplet mass median diameter (MMD) of 12.0 µm was obtained from aerosols produced by HFA 152a without excipients, followed by the droplets generated by HFO 1234ze (10.4 µm) and finally by HFA 134a and HFA 227ea (9.7–9.3 µm). The results are inversely related to propellant densities as predicted by the equation, and the addition of ethanol, which causes a decrease in the formulation density, resulted in an increased droplet size. Moreover, a theoretical framework recently developed by Gavtash [Citation32] was applied and comparison with experimental MMD measurements gave good correlation except for HFA 134a and HFA 227ea formulations containing ethanol.
Gavtash et al. correlated propellant characteristics, such as SVP and density, with the velocity of the spray generated [Citation27]. Since HFA 152a was the lowest density propellant, the pressure of the propellant is used to accelerate a lighter mass resulting in a higher velocity of the spray generated. On the other hand, Shur et al. reported observations that droplet velocities at 20 mm from a standard generic actuator mouthpiece (orifice diameter (OD) 0.48 mm/jet length (JL) 1.5 mm) were fastest for HFA 227ea; whilst HFA 134a and HFO 1234ze had similar but slightly lower velocities; and HFA 152a gave the slowest droplet velocity [Citation33]. The two measurements however focused on very different moments of the spray development, the latter set of data reported the velocity at early times (few milliseconds) immediately after the emission from the orifice, while the former measures observed the velocity of the spray plume up to the exhaustion of the propulsive phase (150–300 ms). Moreover, the slower velocities observed for HFA 152a in the early time experiments could be due also to larger mean droplet diameter observed for this propellant (1.6 vs. ca. 1.3 µm).
Investigations relating to the stability and reproducibility of the spray have recently been performed using high-speed imaging by Rao et al. [Citation34]. Spray stability is the fluctuation of the spray in its temporal development whereas spray reproducibility is the variation between different sprays. Both the cloud features strongly impact the pMDI efficacy. In fact, while the stability influences the inhalation timing and the delivered dose amount, the reproducibility influences the dose-to-dose consistency. The study investigated the spray development of HFA 152a and HFO 1234ze in comparison to HFA 134a. It was reported that, due to the lower vapor pressure and density of the HFA 152a compared to the reference propellant, HFA 134a, the spray profile was wider and denser, leading to a temporal spray development less stable but repeatable. On the other hand, the HFO 1234ze showed more similarity to the reference, with a stable spray development but with a reduced repeatability [Citation34]. The reduced stability and reproducibility of new propellants compared to the HFA 134a could indicate a high susceptibility to variation in the consistency of the dose delivered, with the need for further formulation optimization of the pMDI. The inclusion of beclomethasone dipropionate (BDP) as a solution with 8% w/w ethanol did not modify the way in which the spray developed for each of the different propellants [Citation35]. The inclusion of ethanol reduced the differences observed between each of the propellants. When a suspension of SS was observed (without ethanol) in each of the propellants, the HFO 1234ze formulation was reported to have a more repeatable plume. However, the narrower plume formation led to an insufficient air entrainment, poor mixing, and thus larger particles. After establishing the impact that the propellant has on the spray pattern development and aerodynamic particle size distribution (APSD), it is possible to optimize the product by modifying the device components. By using a shorter orifice length, in fact, it is possible to widen the HFO 1234ze spray, and replicate the plume geometry emitted by an HFA 134a pMDI [Citation35].
4. Current formulation development based on HFA 152a and HFO 1234ze
In the light of what has been described, it is understandable that effort is required to create combinations of formulations with compatible devices and components, such that a match of APSD is achieved when transitioning from current products to products containing new propellants.
4.1. Influence of propellant density on sedimentation
Propellant density has an influence upon the accuracy of the dose delivered from a suspension pMDI resulting from the gravitational stability of the formulation, and therefore for the phenomena of creaming and settling. Indeed, according to Stoke’s law, the extent of sedimentation or surfacing depends on the difference in density between the drug particle and the suspending medium [Citation36]. A slow sedimentation favors the maintenance of the homogeneity of the drug in the formulation and therefore of the correctness of the dosage in the case of a delay between product shaking and actuation. In this regard, it has recently been demonstrated that both emitted dose (ED) and fine particle dose (FPD) were significantly higher than the labeled values for suspensions in HFA 134a where the drug crystals settle when they were not shaken before use or when activated with a delay of more than 60s after the shaking [Citation37,Citation38]. Conversely, when the suspensions were set up in HFA 227ea, the drug particles tended to cream and therefore if the product was not dispensed immediately after shaking, the doses were undermeasured compared to the control values [Citation37,Citation38].
Considering the very low density of HFA 152a compared to other propellants, this could suggest difficulties in matching the density of drug particles, which usually have a density greater than 1 g/mL. However, studies have shown that the high viscosity of the propellant is able to mitigate this phenomenon. The sedimentation time of SS in a suspension of HFA 152a was higher (2.0 min) than that in pure HFA 134a (0.5 min) or even when in this latter case oleic acid was added to the formulation to further increase the viscosity of the medium [Citation39].
4.2. Solvent capacity of the new propellants
In the case of suspension systems, the solubility level of a drug in the propellant has an impact the stability of the particle size of the droplet generated. During the transition from CFCs to HFAs it was noted that there was an increase in the solubility of the lipophilic drug in the latter propellant [Citation40], the same behavior can be seen when switching from HFA 134a to HFA 152a. In fact, the solubility of BDP in HFA 152a was reported to be 300 µg/mL, twice the concentration observed in HFA 227ea and three times the concentration observed in HFA 134a and HFO 1234ze. This behavior may be associated with the higher dipole moment (2.30 debye) of the HFA 152a compared to those of the other propellants (1.44–2.06 debye). This higher solubility of the drug in HFA 152a could allow to lower the co-solvent during the reformulation of these products [Citation33]. Contrary to this, salbutamol sulfate, a hydrophilic drug, is reported to have no measurable solubility in the propellants investigated. Thus, reformulation as a suspension, as it was for HFA 134a, may allow similar particle size distribution and deposition within the lungs, ultimately eliciting the same clinical response.
Moreover, if the stabilization of the suspension requires the use of excipients, such as surfactants, it was demonstrated that the HFA 152a is able to solubilize higher quantities of oleic acid (2.5%) or Span 80 (0.22%) than the HFA 134a [Citation39].
4.3. Modulation of aerodynamic profile generated by new propellant pMDIs
By changing the propellant, the evaporation rate of the droplets toward their residual particle sizes (i.e., Phase II effects) is modified, influencing their aerodynamic distribution. Formulation composition plays a role on the maturated particle solid state and morphology as well [Citation41].
The impact of the propellant characteristics on the aerodynamic distribution of particles generated was investigated using a model solution-based formulation (target delivered dose of 500 µg) containing 2 mg/mL of dissolved drug and 8% w/w of ethanol. The in vitro aerosol deposition revealed that regardless of the propellant used, i.e. HFA 134a, HFA 152a and HFO 1234ze, the fine particle dose was similar for all the products (167–177 µg). On the other hand, the MMAD of HFA 152a differed significantly from those of HFA 134a and HFO 1234ze (1.5 µm versus 0.8–0.9 µm, respectively) [Citation42]. Therefore, even if the amount of drug mass associated with droplets less than 5 µm aerodynamic diameter was observed to be similar for each propellant investigated, other aspects in addition to SVP (such as higher surface tension and viscosity) have an influence upon the generation of fine particles from a pMDI. Moreover, this result can also be justified by the large size distribution of the initial droplets that formed upon HFA 152a formulation atomization [Citation22].
Interestingly, it has been demonstrated that formulations of BDP 250 µg/shot prepared with HFA 134a, HFA 227ea or HFA 152a, can be designed to have the same aerodynamic profile [Citation43]. It has been demonstrated that modulation can be achieved by addition of a nonvolatile component (e.g. glycerol) and considered choice of metering volume and the actuator orifice diameter. If in a previous case the similarity of two BDP-based products in HFA 134 and HFA 227ea had been achieved by fine-tuning all three of these factors [Citation44], this time, with HFA 152a, the matching of the aerodynamic parameters was simpler. In detail, the preparation of an HFA 152a-based pMDI with 15% w/w ethanol and 0.33 mm actuator orifice diameter resulted in a lower MMAD (1.6 µm) than that of the corresponding products containing the old propellants (1.9 µm). Then, just the addition of a small quantity of non-volatile component, such as glycerol, made it possible to obtain the equivalence of the three products in vitro. The equation used for the calculation, which relates the MMAD values of two products to their respective non-volatile components (NVC) values is the following [Citation45]:
Where, MMADA = The observed MMAD of formulation A, (µm)
MMAD1 = The desired MMAD of formulation 1, (µm)
NVCA = Total nonvolatile content of solution formulation A, (%w/w)
NVC1 = Total nonvolatile content of solution formulation 1 (%w/w)
Subsequently, the effect of inspiratory airflow and the use of a spacer on aerodynamic performance was evaluated on these three formulations. The spacer was used only in the case of the 30 L/min airflow. The FPD < 5 µm of each formulation sampled with the AeroChamber Plus was not significantly different (p > 0.05) than the one observed without the inhalation chamber at 60 or 90 L/min (). This study indicates that both the formulation and the pMDI hardware can have an impact on the aerosol characteristics, thus on the therapeutic outcome. When used, the spacer removes larger particles which would impact in the oropharynx reducing the delivered dose (88–108 µg) but increasing the fine particle fraction (FPF) (87–91%) [Citation46].
Figure 3. Fine particle dose of BDP (µg) for ethanol-based solution with HFA 152a, HFA 134a or HFA 227ea at experimental conditions: airflow rate of 30 L/min, 60 L/min and 90 L/min or at 30 L/min with AeroChamber (adapted from ref [Citation46]).
![Figure 3. Fine particle dose of BDP (µg) for ethanol-based solution with HFA 152a, HFA 134a or HFA 227ea at experimental conditions: airflow rate of 30 L/min, 60 L/min and 90 L/min or at 30 L/min with AeroChamber (adapted from ref [Citation46]).](/cms/asset/5091c70f-68b4-47ab-89fc-cce334b98e7b/iedd_a_2264184_f0003_oc.jpg)
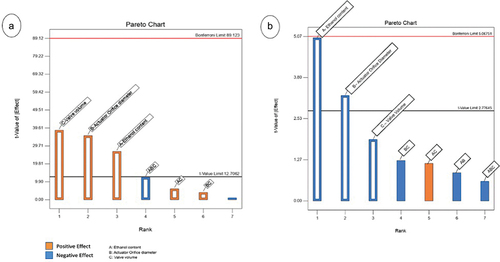
An exploratory study [Citation47] was conducted to investigate how ethanol content, valve volume and actuator orifice diameter may impact output dose accuracy and FPF in a BDP solution using HFA 152a. The study showed how decreasing the valve volume, the actuator orifice diameter and the ethanol content, it was possible to increase the precision of the doses emitted from the product, obtaining a coefficient of variation (CV) of 2.5%. In addition, as reported by the Pareto chart (), when ethanol content and the actuator orifice diameter were reduced to 8%w/w and to 0.25 mm, respectively, a 50 µl metered dose generated a highly respirable dose (FPF of 57.0%) and a MMAD of 1.1 µm. These results are in line with what has already been demonstrated by the previous findings: FPF is directly proportional to propellant concentration and inversely related to valve volume and orifice diameter.
Figure 4. Pareto Chart illustrating the results of the design of experiments used to formulate a BDP (100 µg/actuation) in HFA 152a. A) impact of the factors on the coefficient of variation (CV%) of the emitted dose; B) impact of the factors on the FPF%.
Alongside corticosteroids, reformulation of SS has been addressed as it represents a gold standard product. As anticipated, it is insoluble in both new propellants proposed as well as in ethanol, and therefore it will be reformulated as a suspension trying to match the performance of the current HFA 134 suspension.
From a formulation point of view, it is necessary to decide whether to set up the product without ethanol or whether to include it. In the former case, the typical pMDI manufacturing process involves a one-step filling of a homogeneous drug-propellant mixture maintained under pressure and under recirculation. As an alternative to facilitate the process, dosing of the SS powder directly into a plasma-treated 19 mL canisters followed by the addition of propellant HFA 152a has been proposed [Citation48]. The results showed that the ED was consistent both within the product life and after 12 months of storage. Adopting the same process production approach, Kay et al. introduced into plain aluminum canisters a novel propellant dispersible tablet (Respitab) composed by micronized SS, menthol and lactose followed by the addition of HFA 152a. The product demonstrated efficient drug content uniformity and unmodified APSD (with FPF of 49%) after 6 months’ storage 40°C/75% relative humidity [Citation49].
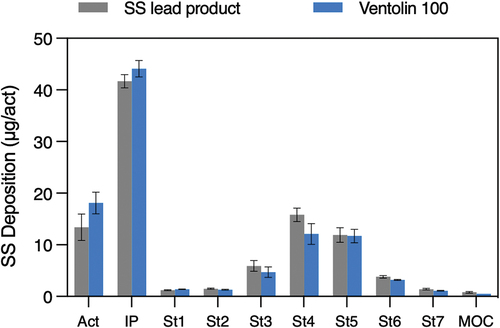
In the case of ethanol containing SS suspensions, a two-step filling is carried out. First the canister is filled with a concentrated suspension of SS in ethanol followed by the crimping of the metering valve and only then the propellant is added. Recently, adopting this process, we observed that the ethanol addition (in the range 1–12% w/w) improved the SS emitted dose. At higher ethanol content levels (between 12% and 15%) the effect was not significant. Similarly, if the actuator OD increased, this modification led to a rise in ED. On the other hand, the FPF was maximized when both factors decreased. Hence, in the construction of the product it is necessary to find a compromise that maintains high lung deposition without impairing the ED. The study led to the identification of the best performing product characterized by a 50 µl valve (RxPack, IT), 0.3 mm actuator OD and 6% (w/w) ethanol content. With this configuration the MMAD was 2.55 µm and the FPF was 45.5%. illustrates the aerodynamic deposition of this product in the NGI compared to that of Ventolin (GSK), although our purpose was not to make a copy product.
5. IP landscape of formulations based on new propellants
Apart from the academic works reported above, intellectual property (IP) generated by different pharmaceutical companies is already associated with formulation comprising HFA 152a and HFO 1234ze as propellant vehicles ().
Table 3. PCT deposited regarding the use of the low-GWP propellants and patent rights will derive from the listed WO applications.
Browsing said IP landscape, Koura (previously named Mexichem) and Honeywell appear among the first pioneer applicants. In a Patent Cooperation Treaty (PCT) application of the latter company published in 2006, medicinal compositions comprising a fluoroolefin propellant and at least one medicinally active compound were disclosed [Citation12]. The HFO should be a medicinally acceptable carrier and have at least two but less than seven carbon atoms. From this group of compounds HFO 1234 has emerged as the best candidate for its physico-chemical and non-flammability characteristics.
In a PCT application, published in 2012, Mexichem claimed solution-based formulations of HFA 152a containing at least fluticasone propionate (FP) or BDP [Citation50]. The product was claimed to be a solution with ethanol as cosolvent. A product based on FP (50 µg/shot) prepared with 5% ethanol provided an FPD equal to 26.6 µg and the aerosol with an MMAD of 3.2 µm. Similarly, the performance of BDP (250 µg/shot) prepared with 2% ethanol provided an extremely fine aerosol (MMAD of 1.5 µm) and an FPD of 116 µg.
Subsequently, in 2018 the same applicant filed a PCT application claiming the invention of a pharmaceutical suspension or a solution of BDP and formoterol fumarate (FF) based on HFA 152a optionally with the addition of glycerol to modulate the aerosol particle size [Citation51]. The examples illustrate that a solution of the two drugs in 10% ethanol and HFA 152a in strength 250 + 6 µg (BDP + FF) provided particles with MMAD around 1.54 µm and an FPF of 58%. The same composition formulated in HFA 134a gave rise to a finer aerosol and this result is due to the different properties of the two propellants listed above. With HFA 134a the MMAD was around 1.4 µm for both drugs and the FPF was 64%.
In the same year, Mexichem filed a twin application directed to pMDI composition of indacaterol and HFA 152a as suspension or solution [Citation52]. The data reported include a suspension prepared by directly weighing the active pharmaceutical ingredient (API) (100 µg/shot) into an uncoated canister and adding the propellant. The product had a stable drug content after 3-months of storage under the accelerated conditions investigated. The APSD was also reported to be robust over the observation time with a delivered dose of around 72 µg, a FPD of 30 µg, and the MMAD of 4.6 µm. Interestingly, the indacaterol in HFA 152a had slower settling times than the corresponding product in HFA 134a (1.89 min versus 1.06 min).
Mexichem also filed parallel PCT applications concerning the rights for a formulation containing tiotropium eventually with a long-acting beta-agonist (LABA) or/and an inhaled corticosteroid (ICS) solubilized or suspended in HFA152a [Citation53]. In a 10 µg tiotropium bromide suspension, the FPD of 2.5 µg matched the value obtained in HFA 134a. In addition, the evaluation of the FPD after stress storage conditions (50°C/75%) showed that the formulation in HFA 152a increased the FPD to 5.5 µg after 15 days, unlike the formulations in HFA 134a and in HFA 227ea which drastically worsened aerodynamic performances.
In 2019 Chiesi filed an international Patent for a triple combination therapy delivering a anticholinergic, a corticosteroid, and a long-acting beta-2 agonist formulated as a solution. Example formulations included glycopyrronium bromide (GP), FF in HFA propellant (HFA 134a, HFA 152a or HFA 227ea) contained within a canister made of stainless steel [Citation54]. Subsequently, three more applications were filed by Chiesi relating to unexpectantly finding that coated canisters improved the stability of corticosteroid and a long-acting beta-2 agonist formulations [Citation55]. Stability was exemplified with data comprising FF and BDP in solution with HCl and 12% w/w ethanol in HFA 152a packaged in fluorinated ethylene propylene (FEP) coated canisters; pH and FF content were maintained at 4.2 and 96% residual respectively, up to 6-months storage at 25°C. Similarly, the use of the coated can with a series of different polymers was claimed for the triple combination product including an ICS, a LABA and a long-acting muscarinic agent (LAMA) in HFA or HFO propellant [Citation56]. The proprietary formulation is a solution comprising a mineral or organic acid for the pH regulation or a cosolvent. The document demonstrated that, when FF was formulated in HFA 152a, the use of a FEP coating guaranteed a stabilization of the pH of 4.6 of for a prolonged period of time (6 months). On the contrary, by using an uncoated can, the pH substantially increases with respect to the measure at time zero (up to 5.7), leading to a potential decrease of the FF content.
Further, in 2022 Chiesi filed a patent application directed to a triple combination with at least two inorganic acids for the pH adjustment [Citation57]. In detail, the invention comprised LABA, LAMA and optionally with the addition of an ICS. The formulation is prepared as solution with a co-solvent and with HCl and H3PO4 for the stabilization of the pH leading to a maintenance of FF drug content. The product can be formulated with HFA 152a or HFO 1234ze in a coated canister.
Another well-known pharmaceutical company, Astrazeneca, holds different provisional patent rights on an pMDI formulation based on the HFO 1234ze propellant containing from one to four active ingredients selected from LAMA, LABA, short-acting beta-agonist (SABA), ICS and a non-corticosteroid anti-inflammatory agent. The API particles are suspended in the propellant together with phospholipidic microparticles [Citation58,Citation59]. The manufacturing of these Aerosphere particles follows the procedure disclosed in WO 2010/138862 [Citation60] starting from a perfluorooctyl bromide emulsion stabilized by 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC). It is demonstrated that the inclusion of Aerosphere particles led to a uniform aerodynamic distribution of GP, budesonide (BUD) and FF unlike the formulation without phospholipid particles. When the triple combination (160 + 7.2 + 5 µg ex valve of BUD-GP-FF) with DSPC particles in HFO 1234ze was actuated, the following respirable doses were released: 82.0, 3.8 and 2.6 µg for BUD, GP and FF, respectively, with MMAD for all the APIs around 3.7 µm. This performance was no different from that achieved by HFA 134a indicating a relatively easy transition of the platform from one propellant to the other. Conversely, if the three drugs as microcrystals were suspended in HFO 1234ze without DSPC, a respirable dose more than double for all three APIs compared to the previous case was reported, indicating low stability and rapid precipitation of the suspension.
6. The players involved in the transition and regulatory aspects
Being a combination product, it is necessary that in the transition the companies supplying the device components also proceed toward demonstrating the compatibility of valves, canisters and actuators with the new formulations. In this sense, many material suppliers for pMDIs have already presented data to support their commitment and many of these studies have already been conducted in collaboration with formulation developers.
Koura and Honeywell are currently the two suppliers that have the propellants available in pharmaceutical grade. However, the availability of these pharmaceutical grade propellants from alternative suppliers may grow in the future. Naturally, IP related to the manufacturing process and the availability of the toxicology package may hinder the number of new players in the field.
As regard the material suppliers, Honeywell performed a compatibility test joined with Aptar with the purpose of evaluating the behavior of Aptar metering valve when immersed in HFO 1234ze. Based on the test results generated to date, no notable differences have been observed between the mechanical properties of the materials (upper and lower stems, bodies, rings, springs and elastomers) immersed HFO 1234ze in comparison with the materials immersed in HFA 134a and HFA 227ea [Citation61]. At the same time, Aptar quantified the levels of leachables of their DF30 valve. Materials of construction included polyoxymethylene (POM) and polybutylene terephthalate (PBT) plastics as well as ethylene-propylene-diene monomer (EPDM) and elastomeric cyclo-olefin-copolymer (COCe) gaskets. The pMDIs were filled with one propellant (HFA 134a, HFA 152a or HFO 1234ze) without API. The comparison for volatile leachables (tetrahydrofuran (THF) and formaldehyde) was performed. The level of THF leachable was higher for HFA152a (50–60 µg/can) compared to that of HFA 134a and HFO 1234ze (20 µg/can) due to the slightly stronger solvent properties of HFA 152a compared the others. As regards the formaldehyde leachable in contact with HFA 152a, it was shown an increase between time zero and T3 months (to 50–100 µg/can), then the value tended to stabilize up to 24 months of storage. In summary, both the leachable levels were found to be within the acceptable toxicity limits as defined by the ‘Derived No Effect Level,’ i.e. the level of exposure to a substance above which humans should not be exposed [Citation62].
Overall, this period of change brings many important choices for supplier companies, each must decide if they want to be involved in the transition. This interest must be robust and strong to avoid the risk that pharmaceutical companies remain without the device components throughout the phases of product development.
Finally, there are very few suppliers of pharmaceutical grade new propellant, and they will be able to stipulate exclusivity contracts for their products included in specific formulations with pharmaceutical companies. This exclusivity mechanism represents a means of controlling the freedom for other companies to operate.
From a regulatory perspective, the different authorities need to develop guidance for the transition based on the experience gained from the CFC-HFA previous transition and generic pMDI introduction. In this respect, EMA has recently released a draft document for comments to address the introduction of new propellants (EMA/CHMP/83033/2023).
In particular, clinical data on local tolerance and clinical safety studies of the novel propellant are required. Regarding local tolerance, data on ciliary function on healthy volunteers and airway sensitivity reaction in asthmatic patients should be provided. The safety study duration should be at least three months and of a size of 300 subjects with the purpose to detect adverse events such as bronchoconstriction and others. The pMDI product under investigation should ideally be a vehicle version of the final formulation and be compared to the one on the market. Then, additional data to demonstrate clinical equivalence need to be provided (stepwise approach).
Other regulatory authorities, e.g. FDA, are in the process of defining their requests. Anyway, it is strongly suggested that any company that wants to develop new formulations approaches the relevant regulatory authority to agree on the requested data package.
Currently, checking ‘clinicaltrial.gov’ appears that Astrazeneca is performing a phase III study focused on the safety of BUD/GP/FF (320/14.4/9.6 μg) pMDI in HFO 1234ze twice daily in participants with moderate to very severe COPD. The length of the study is 12 weeks, with an extension to 52 weeks in a subset of participants. Chiesi is performing a phase I study in adult healthy volunteers with the primary objective of assessing the effect of multiple doses of the HFA 152a propellant compared to HFA 134a on mucociliary clearance. Moreover, Chiesi is also running a phase IIa, single dose study to evaluate the potential for bronchoconstriction of the new HFA 152a propellant versus the marketed HFA 134a in adult subjects with mild asthma.
Finally, GlaxoSmithKline is running a phase I study to compare the pharmacokinetic of salbutamol administered via pMDIs containing propellants HFA 152a compared to HFA 134a in 28 healthy participants.
The companies that decide to invest first into product development and clinical studies may pave the way for those who come later; i.e. those able to apply for a Q1 compared to what is newly authorized. However, those who transition later to alternative propellants may instead need to navigate a newly created patent and exclusivity landscape.
The CFC-HFC transition had not led to an increase in the prices of pharmaceutical products. Similarly, it is conceivable that the transition to new propellants won’t have influence on the price of new products and therefore any impact on the costs for the health systems.
7. Conclusions
In this review article, we have summarized recent literature on the knowledge gained to date about the impact of the physico-chemical characteristics of the new low GWP propellants on the properties of the produced aerosols. In fact, the different characteristics of formulation vapor pressure, surface tension, viscosity, density, nonvolatile additives and device components lead to different particle distributions, drug delivery efficiencies and plume geometries of the emitted aerosols.
The future looks both challenging and promising; a number of newly published PCTs and documented academic works demonstrate the concrete possibility of obtaining highly performing, cost effective, clinically effective respirable pMDI products utilizing the new propellants.
8. Expert opinion
It hasn’t been long since the first HFA 134a-based pMDI, Qvar, a 3M product containing BDP, reached the market in 2000. Indeed, many people who have been involved in the transition from CFC to HFA, with knowledge and experience, are still active in industry.
All the knowledge gained during the last transition can be invested to provide patients with low environmental impact solutions that are still suitable for their needs.
The characterization of the atomization process and the quality of the formulations developed to date demonstrate the transition is highly achievable.
The greatest limitation of the current pMDI is represented by the very rigid dose loading constraint; resulting from the characteristics of the current propellants and volume of the metered dose. This has pushed the formulation of some compounds with relatively high dosages (>300 µg) toward dry powder formulations. To date, one of the main advantages accompanying this transition is that the new propellants are very versatile and allow, thanks to their greater solvent capacity, higher drug loading of APIs and surfactants than previously achieved. Thus, low potency APIs do not necessarily need to resort to DPI. Furthermore, the addition of ethanol allows to further increase the solubility of lipophilic compounds.
It is concluded that the greatest challenge is not represented by a technological problem of re-formulation but by the uncertainty of the regulation for placing products on the market and therefore by the balance between risk and benefit that companies have to face.
A questionable aspect that we would like to bring to the attention of the scientific community is the approach that is currently adopted for the formulation of the new low GWP-pMDIs. The main purpose is to create an in vitro product copy of the current commercialized pMDIs, but wouldn’t it be better, while huge resources are invested, to take steps forward to make them even more performing? For example, one idea would be to use valves with the lowest possible volume (compatible with the feasibility to fall into the product specifications) to further limit the volumes of HFA used. Secondly, why not try to limit the number of shots for the intake of the entire dose by doubling the drug load per delivery? Finally, the use of open-chamber valves that do not require priming and re-priming would be of further help in minimizing dose wastage.
In parallel, acting on different strategies aimed at increasing patient adherence would help to limit the number of used inhalers and their waste. The inclusion of dose counters, and easier doctor-patient support via telemedicine would be beneficial.
In conclusion, the change of the propellant will certainly help to reduce the carbon footprint of an pMDI but it is also true that all the activities of the entire life cycle of a product from cradle to the grave contribute to this parameter. So, it will only be by adopting a global approach from raw material extraction, to manufacturing, distribution, use, and disposal (or recycling) that pMDIs can truly decrease greenhouse gas emissions and consequently their impact on climate change.
Article highlights
Indication of the timescales required by the regulations for the replacement of the propellants of current pMDIs with sustainable ones;
Impact of the chemical-physical properties of the new propellants on atomization (phase I of aerosol formation);
Description of the formulation approaches of pMDI products with HFA152a and HFO1234ze: effect on phase II of aerosol formation (droplet evaporation);
IP landscape of patent filed focused on HFA152a and HFO1234ze;
Overview of the regulatory aspects suggested to date and clinical trials currently underway (safety, local tolerability and PK).
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to thank Gaetano Brambilla for his kind availability in providing valuable suggestions and comments on the manuscript.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Stein SW, Thiel CG. The history of therapeutic aerosols: a chronological review. J Aerosol Med Pulm Drug Deliv. 2017;30(1):20–41. doi: 10.1089/jamp.2016.1297
- Myrdal PB, Sheth P, Stein SW. Advances in metered dose inhaler technology: formulation development. AAPS Pharm Sci Tech. 2014 Apr;15(2):434–455. doi: 10.1208/s12249-013-0063-x
- Cataldo D, Hanon S, Peché RV, et al. How to choose the right inhaler using a patient-centric approach? Adv Ther. 2022 Mar;39(3):1149–1163. doi: 10.1007/s12325-021-02034-9
- U. Environmental Protection Agency. Market characterization of the U.S. Metered dose inhaler industry. no. September. 2021. https://www.epa.gov/sites/default/files/2021-03/documents/epa-hq-oar-2021-0044-0002_attachment_1-mdis.pdf
- European Environment Agency, Fluorinated greenhouse gases 2020. no. 15. 2020. Available from: https://eea.europa.eu
- Pernigotti D, Stonham C, Panigone S, et al. Reducing carbon footprint of inhalers: analysis of climate and clinical implications of different scenarios in five European countries. BMJ Open Respir Res. 2021;8(1):1–11. doi: 10.1136/bmjresp-2021-001071
- NICE. Asthma inhalers and climate change: what is this decision aid about ? 2022. https://www.nice.org.uk/guidance/ng80/resources/inhalers-for-asthma-patient-decision-aid-pdf-6727144573
- Usmani OS, Scullion J, Keeley D. Our planet or our patients—is the sky the limit for inhaler choice? Lancet Respir Med. 2019;7(1):11–13. doi: 10.1016/S2213-2600(18)30497-1
- Price D, Roche N, Christian Virchow J, et al. Device type and real-world effectiveness of asthma combination therapy: an observational study. Respir med. 2011;105(10):1457–1466. doi: 10.1016/j.rmed.2011.04.010
- Woodcock A, Janson C, Rees J, et al. Effects of switching from a metered dose inhaler to a dry powder inhaler on climate emissions and asthma control: post-hoc analysis. Thorax. 2022;77(12):1187–1192. doi: 10.1136/thoraxjnl-2021-218088
- Pritchard JN. The climate is changing for metered-dose inhalers and action is needed. Drug Des Devel Ther. 2020;14:3043–3055.
- Gnopeck M, Gary J, Herena DL, et al. Medicament delivery Formulations, devices and methods WO/2006/101882. 2006
- Koura. Zephex ® 152a Overview. https://www.zephex.com/wp-content/uploads/2023/04/Zephex-152a-Overview-V3.pdf
- Commission CPS. Hazardous substances and articles: administration and enforcement regulations. Code Fed Regul. 2011;2:1500–1503.
- Close J HR, Makar M, Boldt E, et al. HFO-1234ze(E): flammability characterization for metered dose inhaler manufacturing. Respiratory Drug Delivery. 2023; 211–216.
- Murray J MA, Doidge W. Anti-microbial properties of low-GWP pMDI Propellant P-152a. Respiratory Drug Delivery. 2023; 239–242.
- Leach C L. The CFC to HFA transition and its impact on pulmonary drug development: discussion. Respir Care. 2005;50(9):1207–1208.
- Parliament THEE, Council THE, The OF, et al. Regulation (EU) no 517/2014 of the European parliament and of the council of 16 April 2014 on fluorinated greenhouse gases and repealing regulation (EC) no 842/2006. Off J Eur Union. 2014;2014(517):195–230. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2014.150.01.0195.01.ENG
- Amendment to the Montreal Protocol on Substances that Deplete the Ozone Layer. No. 26369. 2016. p. 1–5. https://treaties.un.org/Pages/ViewDetails.aspx?src=IND&mtdsg_no=XXVII-2-b&chapter=27&clang=_en
- European Chemical Agency. Annex XV restriction report-proposal for a restriction per- and polyfluoroalkyl substances (PFASs). ECHA. 2023. https://echa.europa.eu/documents/10162/f605d4b5-7c17-7414-8823-b49b9fd43aea
- Öko-Institut. Support contract for an evaluation and impact assessment for amending regulation (EU) no 517/2014 on fluorinated greenhouse gases. CLIMA.A2/ETU/2019/0016/Impact assessment final report - annexes. no. 517. 2022. https://climate.ec.europa.eu/system/files/2022-04/f-gas_evaluation_report_en.pdf
- Stein SW, Myatt BJ, Cocks P, et al. Experimental and theoretical investigations of pMDI Atomization and plume characteristics using alternative propellants. Respiratory Drug Delivery. 2021;1:15–26.
- Stein SW, Myrdal PB. The relative influence of atomization and evaporation on metered dose inhaler drug delivery efficiency. Aerosol Sci Technol. 2006;40(5):335–347. doi: 10.1080/02786820600612268
- Mason-Smith N, Duke DJ, Kastengren AL, et al. Revealing pMDI Spray initial conditions: flashing, atomisation and the effect of ethanol. Pharm Res. 2017;34(4):718–729. doi: 10.1007/s11095-017-2098-2
- Dunbar C. An experimental and theoretical investigation of the spray issued from a pressurised metered-dose inhaler [Ph.D. Thesis]. Manchester (UK): University of Manchester; 1996.
- Clark AR. Metered atomisation for respiratory drug delivery [Ph.D. Thesis]. Loughborough (UK): Loughborough University; 1991.
- Gavtash B, Cooper A, Dexter S, et al. Development of a theoretical model to predict pMDI spray force, using alternative propellant systems. Drug Delivery To The Lungs. 2018;29:76–79.
- Leach CL, Davidson PJ, Hasselquist BE, et al. Lung deposition of hydrofluoroalkane-134a beclomethasone is greater than that of chlorofluorocarbon fluticasone and chlorofluorocarbon beclomethasone: a cross-over study in healthy volunteers. Chest. 2002 Aug;122(2):510–516. doi: 10.1378/chest.122.2.510
- Stein SW, Myrdal PB. A theoretical and experimental analysis of formulation and device parameters affecting solution MDI size distributions. J Pharm Sci. 2004;93(8):2158–2175. doi: 10.1002/jps.20116
- Vutlapalli S,Rao, Lingzhe ,Myatt, Ben et al. Saturated vapor pressure of HFA152a-ethanol and HFO1234ze(E)-ethanol binary mixtures. Drug Delivery To The Lungs. 2022;33:171–174.
- Gavtash B, Myatt B, O’shea H, Mason F, Lewis D, et al. Saturated vapour pressure (SVP) measurement of ethanol/HFA binary mixtures. J Aerosol Med Pulm Drug Deliv. 2015;29(3):A12–A12. doi: 10.1089/jamp.2016.ab01.abstracts
- Gavtash B, Versteeg HK, Stein SW. A validated theoretical approach to predict the effect of ambient temperature and ethanol concentration on the event-averaged pMDI droplet size. Drug Delivery To The Lungs. 2019;30:68–71.
- Shur J, Rossi I, Ganley W, et al. The formulators guide to transitioning to low global warming potential pMdis. Respiratory Drug Delivery. 2022;1:65–74.
- Rao L,Kusangaya AJ, Marasini N, et al. In-vitro evaluation of pMDI Spray development of HFA134a, HFA152a and HFO1234ze(E). Drug Delivery To The Lungs. 2022;33:179–182.
- Duke D, Myatt B, Rao L, et al. Optimizing spray formation in solution and suspension low GWP metered dose inhalers. Respiratory Drug Delivery. 2023;1:81–92.
- Smyth HDC. The influence of formulation variables on the performance of alternative propellant-driven metered dose inhalers. Adv Drug Deliv Rev. 2003;55(7):807–828. doi: 10.1016/S0169-409X(03)00079-6
- Chierici V, Cavalieri L, Piraino A, et al. Consequences of not-shaking and shake-fire delays on the emitted dose of some commercial solution and suspension pressurized metered dose inhalers commercial solution and suspension pressurized metered dose inhalers. Expert Opin Drug Deliv. 2020;17(7):1025–1039. doi: 10.1080/17425247.2020.1767066
- D’Angelo D, Chierici V, Quarta E, et al. No-shaking and shake-fire delays affect respirable dose for suspension but not solution pMdis. Int J Pharm. 2022;631(December):122478. 2023. doi: 10.1016/j.ijpharm.2022.122478
- Corr S, Noakes T. Pressurised metered dose inhaler propellants: going forward. Respiratory Drug Delivery. 2017;2:255–258.
- Williams RO, Rogers TL, Liu J. Study of solubility of steroids in hydrofluoroalkane propellants. Drug Dev Ind Pharm. 1999;25(12):1227–1234. doi: 10.1081/DDC-100102292
- Buttini F, Miozzi M, Balducci AG, et al. Differences in physical chemistry and dissolution rate of solid particle aerosols from solution pressurised inhalers. Int J Pharm. 2014 Apr;465(1–2):42–51. doi: 10.1016/j.ijpharm.2014.01.033
- Marasini N, Koala V, Rao L, et al. Solution-based pressurized metered dose inhaler formulations using HFA134a, HFA152a and HFO1234ze(E) propellants: analysis of size, aerosolization performance and particle morphology. Drug Delivery To The Lungs. 2022;33:167–170.
- Lewis DA, Green JL, Turner R, et al. HFA152a MDI design: matching the in-vitro performance of HFA227ea and HFA134a MDIs. Respiratory Drug Delivery. 2023;1:295–298.
- Lewis DA, Young PM, Buttini F, et al. Towards the bioequivalence of pressurised metered dose inhalers 1: design and characterisation of aerodynamically equivalent beclomethasone dipropionate inhalers with and without glycerol as a non-volatile excipient. Eur J Pharm Biopharm. 2014 Jan;86(1):31–37. doi: 10.1016/j.ejpb.2013.02.014
- Brambilla G GR, Ganderton D, Lewis DA, et al. The modulation of clouds generated by pMdis. J Aerosol Med Pulm Drug Deliv. 1999;12:119.
- Lewis DA, Green JL, Turner R, et al. Towards pharmaceutical equivalence: a comparison of three MDIs: HFA152a HFA134a and HFA227ea. Respiratory Drug Delivery. 2023;1:299–304.
- Buttini F, Glieca S, Carretta G, et al. A roadmap for constructing a beclomethasone pMDI Solution using HFA152a. Respiratory Drug Delivery. 2023;1:421–426.
- Sule A, Sule S, Mullington T, et al. Comparison of in-vitro performance characteristics of salbutamol pMDI with low GWP propellant (HFA-152a) vs the Current propellant (HFA-134a). Drug Delivery To The Lungs. 2022;33:204–207.
- Kay R, Obirek A, Wegrzyn W, et al. Accelerated stability studies with pMDIs of Respitab HFA-152a propellant dispersible salbutamol tablets. Drug Delivery To The Lungs. 2022;33:212–215.
- Corr S, Noakes TJ. Pharmaceutical Compositions. WO 2012/156711 Al. 2012.
- Corr S, Noakes TJ. Pharmaceutical composition. WO 2018/051131. 2018
- CORR S, Noakes TJ. Pharmaceutical Composition.WO 2018/051128. 2018.
- Corr S, Noakes TJ. Pharmaceutical composition. WO 2018/051132. 2018
- Zambelli E, Bonelli S, Copelli D, et al. Stainless Steel Can For Pressurized Metered Dose Inhalers. WO 2021/110239A1. 2021.
- Zambelli E. Pressurized metered dose inhalers comprising a buffered pharmaceutical formulation. WO 2021/151857. 2021
- Zambelli E. Pressurized metered dose inhalers comprising a buffered pharmaceutical formulation. WO 2021/165348. 2021
- Zambelli E, Bonelli S, Copelli D, et al. A pharmaceutical formulation for pressurized metered dose inhaler. WO 2022/074183. 2022
- Joshi V,Archbell, James, Lachacz, Kellisa et al. Compositions, methods and systems for aerosol Drug Delivery. WO 2023/283438 A. 2023
- Joshi V,Archbell, James, Lachacz, Kellisa et al. Compositions, methods and systems for aerosol Drug Delivery. WO 2023/283441 A1. 2023
- Vehring R, Hartman S, Smith ME, et al. Compositions for Respiratory delivery of active agents and associated methods and systems. WO 2010/138862 A2. 2010
- Decaire B, Conviser S, Sarrailh S, et al. Materials compatibility testing of Honeywell’s new low global warming potential propellants. https://Sustainability.Honeywell.com/Content/Dam/s.
- Le Corre B, Sarrailh S, Ferrao J. Investigation of leachables from pMdis containing propellants HFA 134a, HFA 152a and HFO 1234ze. Respiratory Drug Delivery. 2022;1:209–212.