1. Introduction
1.1. The ABCB1 transporters system
Efflux proteins are a member of the ATP-binding cassette (ABC) transporter superfamily, which groups various importer and exporter membrane proteins [Citation1]. The main efflux protein is ABCB1 (alternative terms: P-glycoprotein or P-gp, multidrug resistance or MDR1, CD243). ABCB1 is a 170-kDa membrane glycoprotein. It has two nucleotide binding domains. They are responsible for ATP hydrolysis. In the CNS, ABCB1 appears in neurons, astrocytes, and microglia [Citation1]. ABCB1 was also found on the luminal side of brain capillary endothelial cells of the blood brain barrier (BBB) [Citation1]. ABCB1 plays an eminent role in brain detoxification, as it protects against endogenous and exogenous toxin transport into the brain parenchyma [Citation1]. Certain pharmacological agents inhibit ABCB1 function in the CNS [Citation2]. Upregulated ABCB1 expression is also known in certain disorders, such as epilepsy or HIV-associated dementia. It may result from the disease process itself or from its chronic drug treatment [Citation3]. Experimental findings suggest that ABCB1 modulation at the BBB level may improve symptomatic drug effects, but will they influence the etiology or progression of Parkinson’s disease (PD) [Citation2,Citation3] ?
1.2. Parkinson’s disease
This second most common chronic neurodegenerative disease is characterized by a smoldering disease process with an individually variable appearance of predominant motor, vegetative, and psychopathological symptoms. Evidence accumulates that certain genetic or environmental factors and exposure to exogenous and endogenous toxins predispose for PD onset [Citation4]. Essential features are nigral depigmentation, misfolded α-synuclein protein enrichment in Lewy Bodies, microglial activation in combination with the most compelling neurochemical symptom, which is the nigrostriatal dopamine deficit. To date, the exact pathological mechanism is still unknown. The underlying pathophysiology of PD probably results from multi factorial causes, which predispose for PD onset alone or in combination. Inflammation, such as microglial activation with elevation of pro-inflammatory cytokines, and free radical generation play an important role and involve neurons, microglia, astrocytes, perivascular macrophages, and endothelial cells. This event cascade induce a decrease of ABCB1 expression and -function [Citation2,Citation3,Citation5].
2. Body
Accumulation of xenobiotics and diminution of detoxification processes are linked to onset and progression of PD. As an example, rotenone as one of the strongest available insecticides depletes dopaminergic neurons by inhibition of mitochondrial complex I and causes Lewy body-like inclusions in rats. Pesticides are substrates of efflux pumps and reduce their function, i.e. in the case of ABCB1 transporters. BBB function was described as normal in early PD [Citation6], but ABCB1 function in advanced treated PD patients has not yet been explored in detail [Citation7].
2.1. May chronic application of PD drugs influence ABCB1 transporter function?
Experimental findings on dopamine agonists, i.e. pergolide, bromocriptine or pramipexole, or levodopa demonstrated that they are inhibitors or substrates of the ABCB1 transporters system [Citation2]. Thus they limit its capacity for brain BBB efflux of pesticides or other toxins. As a result, progression of chronic neurodegeneration may occur due to central accumulation of exogenous and endogenous toxins. It is far from clear, whether neuronal death features in PD, such as protein misfolding, alpha synuclein enrichment etc. result from this slowly emerging intoxication [Citation7,Citation8]. Hypothetically, an existing compensatory efflux capacity gets exhausted to chronic high dosing of dopamine receptor stimulating compounds in the long-term [Citation8]. In this regard, it is noteworthy, that the ABCB1 function weakening verapamil is well known as onset of idiopathic PD onset facilitating or PD syndrome inducing compound [Citation7]. There is no interaction between ABCB1 function and the MAO-B inhibiting compounds selegiline, rasagiline, and safinamide [Citation9,Citation10]. All of them reduce oxidative stress. Therefore MAO-B inhibition is looked upon as ‘neuroprotective’ in PD. In the past, a lot of effort has been undertaken to demonstrate a delay of progression of the neurodegenerative process in PD patients with these agents [Citation11,Citation12]. Interestingly, they beneficially influenced disease progression when given alone. This delaying or protective effect got lost sooner of later, once they were applied together with other PD drugs and thus hypothetical toxin accumulation due to a consecutive, chronic impairment of ABCB1 function. A free radical scavenging compound, such as tocopherol, provided no benefit, which scrutinizes the concept of oxidative stress reduction for neuroprotection [Citation11]. However, treatment with selegiline plus levodopa was superior to levodopa plus placebo in terms of levodopa sparing over 5 years [Citation13]. Hypothetically, less cerebral toxin accumulation due to lower levodopa dosing and accordingly better ABCB1 mediated detoxification may be one reason. In contrast, monotherapy with pramipexole showed no progression delaying effect [Citation14]. To date, numerous further neuroprotective compounds are known. All of them more or less failed once the according to experimental research outcomes promising agents were tested in clinical trials. They were tested in PD patients on a chronic regimen with levodopa or dopamine agonists.
3. Expert opinion
ABCB1 is an efflux pump linked to protection at the brain level. PD drugs and xenobiotics are ABCB1 substrates. A decrease of ABCB1 function or its expression reduces the brain detoxification capacity. The vulnerability to neurotoxins goes up. The most commonly applied PD drugs, levodopa, or dopamine agonists, are substrates of ABCB1 at the BBB. Therefore they limit the efflux capacity of ABCB1. They increase brain exposure to pesticides and other exogenous and endogenous toxins. This mechanism may accelerate chronic neurodegeneration [Citation4,Citation7]. To date ongoing research rarely focus on the BBB role and its ABCB1 transporters in context with chronic PD drug therapy [Citation7]. A genetic predisposition on adequate ABCB1 function is known and well documented in experimental PD models [Citation3]. However, its impact may be low in view of the heterogeneity of drug absorption,-response, and -combination in the daily maintenance of PD patients. Moreover changes of concomitant drugs and titration of PD drugs themselves are frequent in the daily clinical routine of management of PD patients. Therefore these therapeutic habits will further considerably blur the genetic influence on BBB transporters efflux [Citation7].
3.1. Hypothesis: function of ABCB1 transporters in PD is a double edged sword
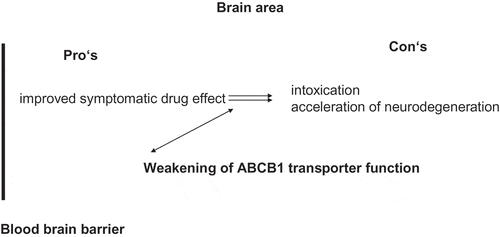
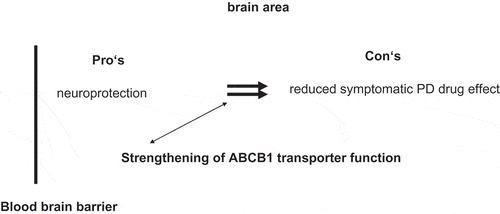
On the one hand, most dopamine substituting drugs directly weaken ABCB1 function. Thus chronic drug treatment may result in acceleration of toxin mediated neurodegenerative processes with an increased vulnerability for dementia, psychosis, or drug induced delirium. These phenomena often appear with high dosing of PD drugs in advanced PD stages. A certain threshold for this exhausted compensation capacity of ABCB1 function is likely. Easy to apply biological markers are not known for the determination of the barrier between toxin enrichment as a result of drug treatment induced ABCB1 exhaustion and still adequate ABCB1 mediated detoxification (). On the other hand, a drug induced ABCB1 impairment will deteriorate the efflux of dopamine substituting compounds, elevate the drug disposition in the brain and thus will provide a longer symptomatic benefit on impaired motor behavior in PD. This approach sounds alluring in terms of decreasing the amount of dopamine substituting drugs necessary for adequate control of disturbed motor behavior in PD patients ().
3.2. Future therapeutic approaches
It is necessary, that function of ABCB1 transporters is generally more considered in the future. As an example, the available MAO-B inhibitors do not impair this efflux transporters system. Therefore these compounds should be applied initially and then together with dopamine agonists or levodopa, if they are tolerated. In addition to their symptomatic effect on motor symptoms, their use should nearly be mandatory, because they help to spare dopamine substitution with levodopa or dopamine agonists. As inductor of ABCB1 transporters function looks promising the agent St John’s wort. As herbal medicine, it is well accepted by patients and it also acts as a MAO inhibitor [Citation15]. Another complementing approach would be to reduce the intake of pesticides or related toxic compounds. Here, a new therapeutic concept, i.e. change to diet with low pesticide content or specific modifications of the intestinal microbiome for reduction of pesticide absorption, might be successful in the long-term. Moreover clinicians should be more aware of compounds with a negative impact on ABCB1 function, when they treat additional disorders in PD patients. Agents, such as atorvastatin, carvedilol, erythromycin, or verapamil, are known as inhibitors. Drug alternatives exist. They have no impact or even work as inducers, such as rifampin or cotrimazole [Citation15]. To date drugs are often used in combination with levodopa or dopamine agonist without considerations on the putative exhaustion of the compensation potential of the ABCB1 transporters system in the clinic.
3.3. Conclusion
There is an important role of ABCB1 in the drug treatment of PD, but not in terms of inhibition of this efflux transporters system for elevation of PD drug disposition and their symptomatic efficacy. This could result in an accelerated progression of the chronic neurodegenerative process due to an impaired toxin efflux over the BBB. Future research should focus on induction of the ABCB1 efflux system for detoxification, as most dopamine substituting PD drugs are at least AB1CB1 substrates. One has to accept that these inducers will reduce symptomatic drug efficacy to a certain extent, but the still hypothetical benefit would be a slowing of PD progression in the long term.
Highlights
ABCB1 is an efflux pump linked to protection at the brain level.
Dopamine agonists and levodopa impair ABCB1 transporter function.
Drug induced ABCB1 impairment will deteriorate the efflux dopamine substituting compounds.
No markers are known for determination of the shift from ABCB1 mediated detoxification to toxin enrichment due ABCB1 exhaustion.
Exhausted ABCB1 efflux capacity increases brain exposure to pesticides and other toxins, all of which accelerate chronic neurodegeneration.
This box summarizes key points contained in the article.
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Vautier S, Milane A, Fernandez C, et al. Interactions between antiparkinsonian drugs and ABCB1/P-glycoprotein at the blood-brain barrier in a rat brain endothelial cell model. Neurosci Lett. 2008;442:19–23.
- Dutheil F, Jacob A, Dauchy S, et al. ABC transporters and cytochromes P450 in the human central nervous system: influence on brain pharmacokinetics and contribution to neurodegenerative disorders. Expert Opin Drug Metab Toxicol. 2010;6:1161–1174.
- Vautier S, Fernandez C. ABCB1: the role in Parkinson’s disease and pharmacokinetics of antiparkinsonian drugs. Expert Opin Drug Metab Toxicol. 2009;5:1349–1358.
- Tanner CM, Ross GW, Jewell SA, et al. Occupation and risk of Parkinsonism: a multicenter case-control study. Arch Neurol. 2009;66:1106–1113.
- Blum-Degen D, Müller T, Kuhn W, et al. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett. 1995;202:17–20.
- Häussermann P, Kuhn W, Przuntek H, et al. Integrity of the blood-cerebrospinal fluid barrier in early Parkinson’s disease. Neurosci Lett. 2001;300:182–184.
- Bartels AL, Willemsen AT, Kortekaas R, et al. Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson’s disease, PSP and MSA. J Neural Transm (Vienna). 2008;115:1001–1009.
- Müller T. Detoxification and antioxidative therapy for levodopa-induced neurodegeneration in Parkinson’s disease. Expert Rev Neurother. 2013;13:707–718.
- Müller T. Pharmacokinetic drug evaluation of safinamide mesylate for the treatment of mid-to-late stage Parkinson’s disease. Expert Opin Drug Metab Toxicol. 2017;13:693–699.
- Pingili R, Vemulapalli S, Mullapudi SS, et al. Pharmacokinetic interaction study between flavanones (hesperetin, naringenin) and rasagiline mesylate in wistar rats. Drug Dev Ind Pharm. 2016;42:1110–1117.
- Parkinson Study Group. Impact of deprenyl and tocopherol treatment on Parkinson’s disease in DATATOP patients requiring levodopa. Ann Neurol. 1996;39:37–45.
- Olanow CW, Rascol O, Hauser R, et al. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med. 2009;361:1268–1278.
- Przuntek H, Conrad B, Dichgans J, et al. SELEDO: a 5-year long-term trial on the effect of selegiline in early Parkinsonian patients treated with levodopa. Eur J Neurol. 1999;6:141–150.
- Schapira AH, McDermott MP, Barone P, et al. Pramipexole in patients with early Parkinson’s disease (PROUD): a randomised delayed-start trial. Lancet Neurol. 2013;12:747–755.
- Kim RB. Drugs as P-glycoprotein substrates, inhibitors, and inducers. Drug Metabolism Reviews. 2002;34:47–54.