ABSTRACT
Introduction
: Emerging studies suggest that antibiotic pharmacokinetics (PK) are difficult to predict in critically ill patients. The high intra- and inter-patient PK variability makes it challenging to accurately predict the appropriate dosage required for a given patient. Identifying patients at risk could help clinicians to consider more individualized dosing regimens and perform therapeutic drug monitoring. We provide an overview of relevant predictors associated with target (non-)attainment of β-lactam antibiotics in critically ill patients.
Areas covered
: This narrative review summarizes patient and clinical characteristics that can help to predict the attainment of target serum concentrations and to provide guidance on antimicrobial dose optimization. Literature was searched using Embase and Medline database, focusing on β-lactam antibiotics in critically ill patients.
Expert opinion
: Adequate concentration attainment can be anticipated in critically ill patients prior to initiating empiric β-lactam antibiotic therapy based on readily available demographic and clinical factors. Male gender, younger age, and augmented renal clearance were the most significant predictors for target non-attainment and should be considered in further investigations to develop dosing algorithms for optimal β-lactam therapy.
1. Introduction
Severe bacterial infections are a major challenge in the intensive care unit (ICU) because of their high prevalence and mortality. Early adequate antimicrobial therapy improves the likelihood of clinical cure and survival rates [Citation1–3]. However, dosage guidelines for most antibiotics are derived from pharmacokinetic (PK) studies in healthy volunteers, and do not consider the significant changes in PK and pathogen susceptibility that are common to the critically ill patient. For example, changes in drug clearance and/or volume of distribution can lead to significant changes in the plasma drug concentration [Citation4,Citation5], resulting in predetermined pharmacokinetic/pharmacodynamic (PK/PD) targets not being achieved and thus a higher treatment failure rate [Citation6]. Furthermore, critically ill patients can undergo rapid physiological changes, such as altered fluid status, changes in serum albumin concentrations, end-organ dysfunction, systemic inflammatory response syndrome (SIRS), and microvascular failure [Citation4,Citation7]. These factors imply that antibiotic dosing in critically ill patients demands a thorough assessment and the need for an individualization from initiation of the therapy and during the course of treatment [Citation8,Citation9].
β-lactam antibiotics (penicillins, cephalosporins, monobactams, and carbapenems) are amongst the most commonly used antibiotics to treat severe infections in the ICU because of their broad spectrum, low likelihood of drug-drug interactions, and wide therapeutic range. These antibiotics display a time-dependent activity. The pharmacodynamic index associated best with a high probability of successful outcome is the percentage of time (T) of the dosing interval in which the unbound (free, ƒ) serum antibiotic concentration remains above the minimum inhibitory concentration (% ƒT > MIC). For β-lactams, the ƒT > MIC value needed for bactericidal activity is between 40% and 70% in in vitro infection models [Citation10,Citation11], this has been confirmed in patients with nosocomial pneumonia for both ceftazidime and ceftobiprole [Citation12,Citation13]. However, clinical data suggest optimal efficacy is achieved at 100% ƒT > MIC in critically ill patients [Citation14–17].
Achieving the high ICU targets is not easy, particularly when fixed conventional β-lactam dosing regimens are used. Although β-lactam antibiotics have a relatively wide therapeutic window, simply increasing the standard dosing for this group of antibiotics in all critically ill patients is not an optimal strategy, since high dosing regimens might result in trough levels associated with overexposure and toxicity [Citation18]. Looking at the current standard approach, dose adjustment and optimization is made only based on indication and adjusted for renal function. Moreover, appropriate antimicrobial therapy refers not only to a suitable drug choice in terms of spectrum of activity, but also to an adequate dosing regimen. Thus, it appears necessary to individualize β-lactam dosing regimens in critically ill patients. Accordingly, identifying patients at risk could guide clinicians to consider more individualized dosing regimens and incorporate therapeutic drug monitoring (TDM) when needed.
The purpose of this review is to examine recent evidence on relevant demographic and clinical characteristics predicting β-lactam exposure in critically ill patients and to provide dosing recommendations.
2. Methodology
A literature search was conducted in August 2020 without a restriction of the publication date. Two databases (Medline All Ovid and Embase) were searched to assess literature on risk factors to predict target concentration prior to initiating empiric β-lactam therapy in critically ill patients. The search was additionally limited to English-language articles. Detailed research terms can be found in supplementary Table S1.
2.1. Eligibility criteria and study selection
Studies reporting the relationship between patient or clinical variables and target (non-)attainment at the time of β-lactam antibiotics treatment initiation in critically ill patients were eligible for inclusion. Titles and abstracts were screened to identify relevant publications. Articles were excluded if they assessed pediatric patients, or were clinical cases, reviews, letters or editorials. Reference lists of eligible studies were searched for additional studies. The references from the database were imported into a reference manager (Endnote X9®).
2.2. Data extraction
We extracted the following data from each included study: author, year of publication, study antibiotics, number of participants, and the effect of the predictor on β-lactam target attainment. The estimates for the multivariate regressions examining the association of target attainment with predictor variables were extracted. For the effect size we have reported the odds ratios (ORs) and the 95% confidence intervals (95% CI). In studies in which the relationship with target non-attainment was investigated, the OR was converted to the inversed OR (1/OR).
3. Predictors for β-lactam exposure
3.1. Study selection
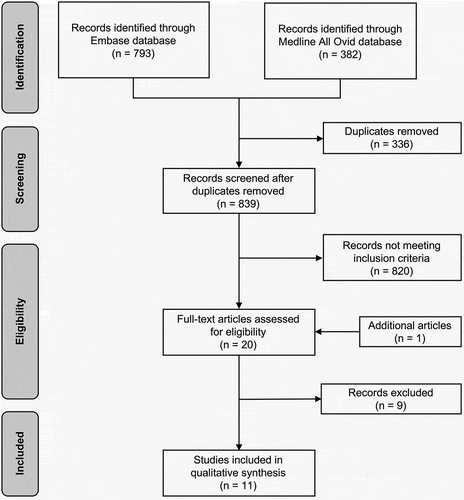
shows a flowchart of the selection process by which articles were identified. Using the search process described above, 839 studies remained once duplicates were removed. A total of 20 studies were included for the full-text assessment. Of these, 11 studies were found that met the inclusion criteria, representing 11 different β-lactam antibiotics (3 penicillins, 6 cephalosporins, and 3 carbapems) [Citation16,Citation19–28]. Only four studies were primarily designed to assess the relationship between drug concentrations or target attainment and risk factors [Citation19,Citation21,Citation24,Citation27]. Almost all studies were partly or fully performed in European hospitals (n = 10, 91%). Taken together, results suggest that β-lactam exposure is associated with a wide range of demographic and clinical characteristics (). Details on the factors that predicted the achievement of the PK/PD targets (Cmin> MIC, 50% (ƒ)T > MIC, 100% (ƒ)T > MIC, and 100% (ƒ)T > 4× MIC are shown .
Figure 2. Demographic and clinical factors associated with β-lactam target attainment. The thickness of the arrow is a representation of the associated evidence. The up arrows indicate that the probability of target attainment increases, the down arrows indicate that the probability of target attainment decreases
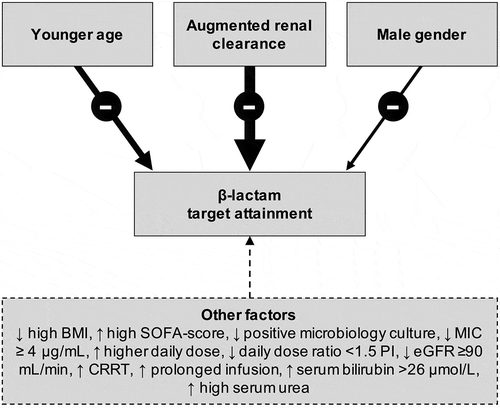
Table 1. Predictors for beta-lactam target non-attainment extracted from literature
3.2. Demographic predictors
We found two demographic characteristics that can be used at the start of empirical antibiotic therapy to potentially increase the chance of target attainment. Firstly, male gender is significantly associated with target non-attainment [Citation19,Citation20,Citation27]. On average, men have a larger volume of distribution (plasma volume and intra-/extracellular water) and a higher drug clearance, possibly explaining the observed effect of gender on drug exposure [Citation29]. Furthermore, male gender is thought to offer an underlying physiological reserve to critically ill patients and contribute to target non-attainment by facilitating augmented renal clearance (ARC) [Citation30]. Although gender is easy to implement in predictor models for target attainment, future studies should be designed with a primary focus on this topic to better understand the basic mechanisms of gender differences and the implications for clinical management.
Age is the second demographic predictor that was found to be significantly correlated with target attainment [Citation20,Citation27]. This association is related to the presence of reduced renal function, which is common in older patients.
3.3. Clinical predictors
We found various clinical characteristics that can be used at the start and during empirical antibiotic therapy to optimize target attainment. Evidence suggests that renal function is among the most important clinical factor to contribute to target non-attainment at the time of antibiotic initiation [Citation16,Citation19–25,Citation27,Citation28].
Traditionally, renal function in critically ill patients has been routinely assessed with the objective of detecting renal impairment and adjusting drug doses. Nevertheless, ARC has also been identified in ICU patients [Citation30,Citation31]. As a result, patients with presumed ‘normal’ or increased renal function are at risk of target non-attainment [Citation32]. The PK of critically ill patients can be significantly altered due to an increased cardiac output with resultant of increased renal blood flow and this may lead to ARC of solutes and drugs [Citation33,Citation34]. Furthermore, as β-lactam antibiotics are hydrophilic compounds and are predominantly cleared by the kidney, high renal function, as observed in ARC, contributes significantly to suboptimal target attainment [Citation16]. Although there is no final consensus as to what defines ARC of drugs, a recent definition suggests ARC when the creatinine clearance (CLCr) exceeds 130 mL/min per 1.73 m2 [Citation35]. Udy et al. examined the CLCr and β-lactam trough concentrations of 58 intensive care unit (ICU) patients, CLCr values ≥130 mL/min/1.73 m2 were associated with trough concentrations less than the MIC of the antibiotic needed to inhibit the targeted micro-organism in 82% of patients [Citation32]. Imani et al. assessed the performance of eGFR as an independent predictor for target non-attainment using a ROC curve and found an eGFR threshold value of ≥71.5 mL/min/1.73 m2 had a sensitivity and specificity of 77% and 65%, respectively [Citation27]. Furthermore, Carrié et al. reported that eGFR ≥170 mL/min were significantly associated with ƒT < 4× MIC [Citation23]. The ability to rapidly predict the risk of target non-attainment in patients with ARC using available eGFR has considerable clinical value. Moreover, the emergence of ARC itself has been associated with a wide range of factors. One that has most consistently been linked to a high risk of ARC is younger age [Citation30,Citation36].
The use of renal replacement therapy (RRT) during β-lactam therapy also shows a strong and significant association with target attainment [Citation19]. Not surprisingly, considering that β-lactam antibiotics are predominantly cleared via renal elimination. At the same time, these patients may be at risk for overexposure and toxicity due to the reduced elimination. Furthermore, in an obese patient cohort, RRT was identified as an independent risk factor for overdose and a protective factor for target attainment [Citation26]. Predicting β-lactam concentrations during treatment with RRT (intermittent, prolonged or continuous) seem to be challenging, as both volume of distribution and total drug clearance are affected, and both parameters may be significantly disturbed during critical illness. In addition, it is important to realize that the effect of RRT on target attainment may be unpredictably affected by for example the type of membrane, device settings, and intensity.
Higher doses and prolonged infusions are also clear predictors for target attainment. Imani et al. found that prescribed daily antibiotic dose ≥ 1.5 times the product information (PI) recommendations were associated with better target attainment [Citation27]. Higher total daily dose is associated with the achievement of 100% ƒT >4× MIC for piperacillin [Citation20]. Moreover, Carrie et al. showed that in critically ill patients with ARC, higher than licensed dosing regimens of β-lactam antibiotics may be safe and effective in reducing the rate of therapeutic failure [Citation37]. The total daily dose is not associated with achievement of 100% ƒT >MIC, because there was not a wide range of doses used in the studies, or alternatively, because the dose adjustments that were made for different levels of renal function prevented this being significant. However, in ARC patients, higher dosing than the licensed dosing regimens of β-lactam antibiotics may be safe and effective in reducing the rate of therapeutic failure [Citation37]. Furthermore, the use of prolonged (extended or continuous) infusion is significantly associated with the achievement target attainment [Citation20,Citation24]. In dosing simulations studies, extended or continuous infusion has also been demonstrated to increase the changes of target attainment [Citation38–41].
Obesity has previously been proposed to be a risk factor for altered β-lactam concentrations in both non-critically ill and critically ill patients [Citation42–45]. High body mass index (BMI) was a significant risk factor for target non-attainment [Citation19]. With the increased prevalence of obesity in Western societies and no dosing guidelines available for critically ill obese patients, ensuring adequate β-lactam therapeutic concentrations is considered to be a serious challenge for clinicians.
Finally, there are several other significant but less prominent clinical predictors for target attainment. Target non-attainment is more frequently observed in patients with lower sequential organ failure assessment (SOFA) scores [Citation27]. However, the presence of severe illness and especially the SIRS response, has also been shown to impact the volume of distribution for some antibiotics [Citation46,Citation47], making increased dosing and close monitoring necessary. Furthermore, positive microbiology cultures seem to be associated with target attainment [Citation27]. Lastly, positive correlation was found between target attainment and high serum concentrations of bilirubin and urea [Citation19,Citation28].
3.4. Antimicrobial dosing strategies
For β-lactam antibiotics, an increase in %ƒT > MIC can be achieved particularly by increasing the number of daily doses or by providing extended or continuous infusion. Furthermore, dosing individualization based on population PK models and patient factors known to influence antimicrobial PK increases the probability of achieving therapeutic drug exposure while at the same time avoiding toxic concentrations. However, optimizing antimicrobial therapy still represents a complex challenge given the wide and unpredictable variability of antibiotic concentrations in critically ill patients. Indeed, the complexity and dynamic nature of critically ill patients make associations of clinical variables and the considered risk of target non-attainment difficult to apply without supporting tools. To enable the practical application of significant relationships between risk factors and β-lactam exposure, and consequently target attainment, risk assessment tools could provide guidance. Ehmann et al. developed an easy-to-use tool, MeroRisk Calculator, for the risk assessment of target non-attainment based on the renal function [Citation48].
3.4.1. Workflow for dose individualization
Refined dosing strategies for antimicrobials are necessary to enhance the probability of achieving drug concentrations that increase the likelihood of clinical success in critically ill patients [Citation49]. A workflow involving several steps is proposed to achieve optimal dosing in these patients (). Firstly, antibiotic selection must be based on both relevant patient and pathogen factors. Subsequently, the selection of the correct dosing regimen takes place using tools such as guideline and dosing nomograms. In addition, dose individualization in critically ill patients based dose simulations and patient factors increases the probability of achieving therapeutic drug exposures, while at the same time avoiding toxic concentrations. Pending the result TDM, a higher daily dose of β-lactam antibiotics at the onset of treatment should be considered, especially in the most critically ill patients and in those with preserved renal function. Particularly in the case of patients with ARC, evidence is building up regarding the clinical impact of ARC and the potential need for increased doses in critically ill patients [Citation50–52]. Finally, appropriate PK models coupled to PD targets can be used to improve dosing regimens based on adaptive feedback through TDM.
AUC/MIC: the ratio of the area under the concentration–time curve to MIC; Cmax/MIC: the ratio of maximum drug concentration to MIC; MIC: minimum inhibitory concentration; PK/PD: pharmacokinetic/pharmacodynamic; T> MIC: the duration of time that the drug concentration remains above the MIC during a dosing interval; TDM: Therapeutic drug monitoring.
3.4.2. Pharmacodynamic targets and outcome
The optimal pharmacodynamic target (PDT) is still not clearly defined for β-lactam antibiotics. The used PDTs in the included studies vary between 50% and 100% ƒT >MIC and 50–100% ƒT >4xMIC. However, 100% ƒT > MIC target attainment is reported in only 40% to 60% of critically ill patients treated with β-lactam antibiotics [Citation5,Citation19,Citation53].
To maximize the probability of clinical efficacy in critically ill patients, unbound plasma concentration from one up to four times the MIC for 100% of the dosing interval (100% ƒT > 1–4× MIC) is recommended [Citation54–59], although the correlation with improved clinical outcomes is not well established. Further increasing the exposure does not appear to increase the rate and extent of bacterial killing or positive clinical outcome [Citation25,Citation60].
For continuous infusions, a random concentration of at least 4xMIC is suggested. Toxicity of β-lactam antibiotics is rare, but can be serious. Neurotoxicity, especially convulsions, hallucinations, myoclonus and confusion, is described due to high concentrations of β-lactam antibiotics [Citation61–65]. To avoid potentially toxic effects, dose reduction is arbitrarily recommended when the unbound trough levels exceeds 8–10xMIC [Citation9,Citation66,Citation67].
There are two types of interventions commonly used to optimize beta-lactam exposure, which are modifying beta-lactam administration by increasing the duration of the infusion and/or TDM and adjusting the dose based on serum levels. However, the clinical impact on patient’s prognosis using this strategy in critically ill patients is not yet fully demonstrated. The results from a multicenter randomized controlled trial investigating the effect of TDM of beta-lactams on clinical outcomes in critically ill patients are expected [Citation68].
3.4.3. Prolonged infusion of β-lactams
Extended and continuous infusion of β-lactams is associated with better target attainment and cure rates in critically ill patients [Citation69]. Recent meta-analyses have shown an association between extended infusion of β-lactams and lower mortality rates in critically ill patients with severe sepsis [Citation70,Citation71]. Especially for piperacillin-tazobactam and meropenem, extended or continuous infusion is strongly recommended based on high-quality evidence [Citation71]. Prolonged infusion of β-lactams can facilitate in dose optimization in the critically ill patient are at risk for target non-attainment.
4. Conclusion
We provide an overview of evidence on factors associated β-lactam exposure in critically ill patients. Early identification of patients at risk when initiating empirical antibiotic therapy based on demographic and clinical risk factors has considerable clinical value. Based on the findings of this study, male gender, younger age, and augmented renal clearance are the most significant predictors for target non-attainment of β-lactam antibiotics. Furthermore, these factors could be considered when developing algorithms to help optimize antibiotics therapy.
5. Expert opinion
Patients admitted to the ICU represent a highly heterogeneous population ranging from young trauma patients to postsurgical patients and elderly medical patients. This heterogeneity is well known to result in high variability in PK parameters. Β-lactam are hydrophilic antimicrobials, which experience increased volume of distribution, generally require a loading dose in patients with sepsis regardless of renal function. One should remind that patient’s clinical condition may change rapidly during the ICU stay, toward either improvement or degradation, which may subsequently lead to altered PK parameters.
Identifying at-risk patients when initiating therapy is a first step in dose optimization. However, since PK parameters vary considerably in ICU patients during therapy, exposure should be monitored in this population. TDM combined with population PK models and dosing simulation can be used to interpret the complex and changing PK parameters in critically ill patients to improve target attainment. Yet, TDM of β-lactam antibiotics is not structurally performed due to the wide therapeutic window of these agents and the lack of concrete dose recommendations in relation to the measured drug levels. However, in recent years, the increasing resistance to β-lactam antibiotics and the association with low levels has increased the relevance of TDM with β-lactam antibiotics. β-lactam TDM is recommended after the onset of treatment, after any change in dosage, and in the event of a significant change in the patient’s clinical condition [Citation9,Citation72].
In the present review some suggestions and solutions are offered based on the current knowledge. For the strong predictors regarding target attainment, we advise their integration into practice. Currently, the expansion of screening software provides an important tool to assist clinicians in the detection and management of under or overdosing. Yet, for demographic and clinical factors, and even for strongly substantiated associations, translation into clinical recommendations is still lacking in clinical practice. Our findings imply the need for dosing intensification in patients identified to be at risk of target non-attainment. Understanding which factors are responsible for the variability of β-lactam exposure would help predict and adjust the dosing strategy in each patient during the ICU stay and therefore optimize antimicrobial effectiveness. Thus, it appears possible to adjust the β-lactam dosage by taking into account demographic and clinical factors, until the plasma concentration data are available.
Article highlights
This review provides an overview of important predictors for β-lactam target (non)-attainment in critically ill patients.
Adequate target attainment can be anticipated in critically ill patients prior to initiating empiric β-lactam antibiotic therapy based on readily available demographic and clinical factors.
Male gender, younger age, and augmented renal clearance are the most significant predictors for β-lactam target non-attainment.
A higher daily dose of β-lactam antibiotics at the onset of treatment should be considered in the most critically ill patients and in those with preserved renal function or augmented renal clearance.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Conception and design: AA, TE, RE, and BK. Analysis and interpretation of the data: AA. AA wrote the first draft of the manuscript. All authors contributed to subsequent drafts and gave final approval of the version to be published.
ExpOpin_DM_T_Suppl._Material__Methods_R1.docx
Download ()Acknowledgments
The authors wish to thank dr. Wichor M. Bramer from the Erasmus MC Medical Library for help in developing the search strategies.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637..
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596..
- Leone M, Bourgoin A, Cambon S, et al. Empirical antimicrobial therapy of septic shock patients: adequacy and impact on the outcome. Crit Care Med. 2003;31(2):462–467.
- Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient–concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev. 2014;77:3–11.
- Taccone FS, Laterre PF, Dugernier T, et al. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care. 2010;14(4):R126..
- Fujii M, Karumai T, Yamamoto R, et al. Pharmacokinetic and pharmacodynamic considerations in antimicrobial therapy for sepsis. Expert Opin Drug Metab Toxicol. 2020;16(5):415–430..
- Roberts JA, Abdul-Aziz MH, Lipman J, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14(6):498–509..
- Goncalves-Pereira J, Paiva JA. Dose modulation: a new concept of antibiotic therapy in the critically ill patient? Journal of Critical Care . 2013;28(4):341–346.
- Guilhaumou R, Benaboud S, Bennis Y, et al., Optimization of the treatment with beta-lactam antibiotics in critically ill patients—guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique—SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation—SFAR). Crit Care. 23(1):104. 2019. .
- Craig WA. Pharmacokinetic/Pharmacodynamic Parameters: rationale for Antibacterial Dosing of Mice and Men. Clinl Infect Dis. 1998;26(1):1–12.
- Craig WA. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis. 1995;22(1–2):89–96.
- Muller AE, Punt N, Mouton JW. Optimal exposures of ceftazidime predict the probability of microbiological and clinical outcome in the treatment of nosocomial pneumonia. J Antimicrob Chemother. 2013;68(4):900–906.
- Muller AE, Punt N, Mouton JW. Exposure to ceftobiprole is associated with microbiological eradication and clinical cure in patients with nosocomial pneumonia. Antimicrob Agents Chemother. 2014;58(5):2512–2519.
- McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents. 2008;31(4):345–351.
- Abdul-Aziz MH, Lipman J, Mouton JW, et al. Applying pharmacokinetic/pharmacodynamic principles in critically ill patients: optimizing efficacy and reducing resistance development. Semin Respir Crit Care Med. 2015;36(1):136–153.
- Huttner A, Von Dach E, Renzoni A, et al. Augmented renal clearance, low β-lactam concentrations and clinical outcomes in the critically ill: an observational prospective cohort study. Int J Antimicrob Agents. 2015;45(4):385–392.
- Li C, Du X, Kuti JL, et al. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother. 2007;51(5):1725–1730.
- Imani S, Buscher H, Marriott D, et al. Too much of a good thing: a retrospective study of β-lactam concentration-toxicity relationships. J Antimicrob Chemother. 2017;72(10):2891–2897.
- Abdulla A, Dijkstra A, Hunfeld NGM, et al., Failure of target attainment of beta-lactam antibiotics in critically ill patients and associated risk factors: a two-center prospective study (EXPAT). Crit Care. 24(1):558. 2020. .
- Alobaid AS, Brinkmann A, Frey OR, et al., What is the effect of obesity on piperacillin and meropenem trough concentrations in critically ill patients? J Antimicrob Chemother. 71(3):696–702. 2016. .
- Carlier M, Carrette S, Roberts JA, et al., Meropenem and piperacillin/tazobactam prescribing in critically ill patients: does augmented renal clearance affect pharmacokinetic/pharmacodynamic target attainment when extended infusions are used? Crit Care. 17(3):3. 2013. .
- Carrié C, Legeron R, Petit L, et al. Higher than standard dosing regimen are needed to achieve optimal antibiotic exposure in critically ill patients with augmented renal clearance receiving piperacillin-tazobactam administered by continuous infusion. Journal of Critical Care. 2018;48:66–71. .
- Carrié C, Petit L,D, Houdain N, et al., Association between augmented renal clearance, antibiotic exposure and clinical outcome in critically ill septic patients receiving high doses of β-lactams administered by continuous infusion: a prospective observational study. Int J Antimicrob Agents. 51(3):443–449. 2018. .
- De Waele JJ, Lipman J, Akova M, et al., Risk factors for target non-attainment during empirical treatment with β-lactam antibiotics in critically ill patients. Intensive Care Med. 40(9):1340–1351. 2014. .
- Dhaese SAM, Thooft ADJ, Farkas A, et al. Early target attainment of continuous infusion piperacillin/tazobactam and meropenem in critically ill patients: a prospective observational study. Journal of Critical Care. 2019;52:75–79.
- Hites M, Taccone FS, Wolff F, et al., Case-control study of drug monitoring of β-lactams in obese critically ILL patients. Antimicrob Agents Chemother. 57(2):708–715. 2013. .
- Imani S, Buscher H, Day R, et al., An evaluation of risk factors to predict target concentration non-attainment in critically ill patients prior to empiric β-lactam therapy. Eur J Clin Microbiol Infect Dis. 37(11):2171–2175. 2018. .
- Woksepp H, Hällgren A, Borgström S, et al., High target attainment for β-lactam antibiotics in intensive care unit patients when actual minimum inhibitory concentrations are applied. Eur J Clin Microbiol Infect Dis. 36(3):553–563. 2017. .
- Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48(3):143–157.
- Bilbao-Meseguer I, Rodríguez-Gascón A, Barrasa H, et al. Augmented Renal Clearance in Critically Ill Patients: a Systematic Review. Clin Pharmacokinet. 2018;57(9):1107–1121.
- Baptista JP, Martins PJ, Marques M, et al. Prevalence and Risk Factors for Augmented Renal Clearance in a Population of Critically Ill Patients. J Intensive Care Med. 2018;35(10):1044–1052.
- Udy AA, Varghese JM, Altukroni M, et al. Subtherapeutic initial β-lactam concentrations in select critically Ill patients: association between augmented renal clearance and low trough drug concentrations. Chest. 2012;142(1):30–39..
- Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37(3):840–851.
- Udy AA, Roberts JA, Boots RJ, et al. Augmented Renal Clearance. Clin Pharmacokinet. 2010;49(1):1–16.
- Baptista JP, Udy AA, Sousa E, et al. A comparison of estimates of glomerular filtration in critically ill patients with augmented renal clearance. Crit Care. 2011;15(3):R139..
- Akers KS, Niece KL, Chung KK, et al. Modified Augmented Renal Clearance score predicts rapid piperacillin and tazobactam clearance in critically ill surgery and trauma patients. J Trauma Acute Care Surg. 2014;77(3 Suppl 2):S163–70.
- Carrié C, Chadefaux G, Sauvage N, et al. Increased β-Lactams dosing regimens improve clinical outcome in critically ill patients with augmented renal clearance treated for a first episode of hospital or ventilator-acquired pneumonia: a before and after study. Crit Care. 2019;23(1):379..
- Lodise TP Jr., Lomaestro B, Drusano GL. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin Infect Dis. 2007;44(3):357–363.
- Shea KM, Cheatham SC, Wack MF, et al. Steady-state pharmacokinetics and pharmacodynamics of piperacillin/tazobactam administered by prolonged infusion in hospitalised patients. Int J Antimicrob Agents. 2009;34(5):429–433.
- Crandon JL, Ariano RE, Zelenitsky SA, et al. Optimization of meropenem dosage in the critically ill population based on renal function. Intensive Care Med. 2011;37(4):632–638.
- Dulhunty JM, Roberts JA, Davis JS, et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: a multicenter double-blind, randomized controlled trial. Clin Infect Dis. 2013;56(2):236–244..
- Hites M, Taccone FS, Wolff F, et al. Broad-spectrum β-lactams in obese non-critically ill patients. Nutr Diabetes. 2014;4(6):e119–e..
- Kays MB, Fleming MR, Cheatham SC, et al. Comparative pharmacokinetics and pharmacodynamics of doripenem and meropenem in obese patients. Ann Pharmacother. 2014;48(2):178–186.
- Payne KD, Hall RG 2nd. Dosing of antibacterial agents in obese adults: does one size fit all? Expert Rev Anti Infect Ther. 2014;12(7):829–854.
- Alobaid AS, Hites M, Lipman J, et al. Effect of obesity on the pharmacokinetics of antimicrobials in critically ill patients: a structured review. Int J Antimicrob Agents. 2016;47(4):259–268.
- Bone RC, Fisher CJ Jr., Clemmer TP, et al. Sepsis syndrome: a valid clinical entity. Methylprednisolone Severe Sepsis Study Group. Crit Care Med. 1989;17(5):389–393.
- MacGregor RR, Gibson GA, Bland JA. Imipenem pharmacokinetics and body fluid concentrations in patients receiving high-dose treatment for serious infections. Antimicrob Agents Chemother. 1986;29(2):188–192.
- Ehmann L, Zoller M, Minichmayr IK, et al., Role of renal function in risk assessment of target non-attainment after standard dosing of meropenem in critically ill patients: a prospective observational study. Crit Care. 21(1):1. 2017. .
- Tängdén T, Ramos Martín V, Felton TW, et al. The role of infection models and PK/PD modelling for optimising care of critically ill patients with severe infections. Intensive Care Med. 2017;43(7):1021–1032..
- Udy AA, Putt MT, Shanmugathasan S, et al. Augmented renal clearance in the Intensive Care Unit: an illustrative case series. Int J Antimicrob Agents. 2010;35(6):606–608.
- Tröger U, Drust A, Martens-Lobenhoffer J, et al. Decreased meropenem levels in Intensive Care Unit patients with augmented renal clearance: benefit of therapeutic drug monitoring. Int J Antimicrob Agents. 2012;40(4):370–372.
- Baptista JP, Sousa E, Martins PJ, et al. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int J Antimicrob Agents. 2012;39(5):420–423.
- Roberts JA, Paul SK, Akova M, et al. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58(8):1072–1083..
- Wong G, Brinkman A, Benefield RJ, et al. An international, multicentre survey of β-lactam antibiotic therapeutic drug monitoring practice in intensive care units. J Antimicrob Chemother. 2014;69(5):1416–1423..
- Tam VH, McKinnon PS, Akins RL, et al. Pharmacodynamics of cefepime in patients with Gram-negative infections. J Antimicrob Chemother. 2002;50(3):425–428.
- Aitken SL, Altshuler J, Guervil DJ, et al. Cefepime free minimum concentration to minimum inhibitory concentration (fCmin/MIC) ratio predicts clinical failure in patients with Gram-negative bacterial pneumonia. Int J Antimicrob Agents. 2015;45(5):541–544..
- De Waele JJ, Carrette S, Carlier M, et al. Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: a randomised controlled trial. Intensive Care Med. 2014;40(3):380–387..
- Delattre IK, Taccone FS, Jacobs F, et al. Optimizing β-lactams treatment in critically-ill patients using pharmacokinetics/pharmacodynamics targets: are first conventional doses effective? Expert Rev Anti Infect Ther. 2017;15(7):677–688..
- Scharf C, Liebchen U, Paal M, et al. The higher the better? Defining the optimal beta-lactam target for critically ill patients to reach infection resolution and improve outcome. J Intensive Care. 2020;8(1):86..
- Richter DC, Frey O, Röhr A, et al. Therapeutic drug monitoring-guided continuous infusion of piperacillin/tazobactam significantly improves pharmacokinetic target attainment in critically ill patients: a retrospective analysis of four years of clinical experience. Infection. 2019;47(6):1001–1011..
- Smith NL, Freebairn RC, Park MA, et al. Therapeutic drug monitoring when using cefepime in continuous renal replacement therapy: seizures associated with cefepime. Crit Care Resusc. 2012;14(4):312–315.
- Gangireddy VG, Mitchell LC, Coleman T. Cefepime neurotoxicity despite renal adjusted dosing. Scand J Infect Dis. 2011;43(10):827–829.
- Lam S, Gomolin IH. Cefepime neurotoxicity: case report, pharmacokinetic considerations, and literature review. Pharmacotherapy. 2006;26(8):1169–1174.
- Chapuis TM, Giannoni E, Majcherczyk PA, et al. Prospective monitoring of cefepime in intensive care unit adult patients. Crit Care. 2010;14(2):R51..
- Beumier M, Casu GS, Hites M, et al. Elevated β-lactam concentrations associated with neurological deterioration in ICU septic patients. Minerva Anestesiol. 2015;81(5):497–506.
- Wong G, Briscoe S, McWhinney B, et al. Therapeutic drug monitoring of β-lactam antibiotics in the critically ill: direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J Antimicrob Chemother. 2018;73(11):3087–3094..
- Roberts JA, Ulldemolins M, Roberts MS, et al. Therapeutic drug monitoring of β-lactams in critically ill patients: proof of concept. Int J Antimicrob Agents. 2010;36(4):332–339..
- Abdulla A, Ewoldt TMJ, Hunfeld NGM, et al. The effect of therapeutic drug monitoring of beta-lactam and fluoroquinolones on clinical outcome in critically ill patients: the DOLPHIN trial protocol of a multi-centre randomised controlled trial. BMC Infect Dis. 2020;20(1):57..
- Lee YR, Miller PD, Alzghari SK, et al. Continuous Infusion Versus Intermittent Bolus of Beta-Lactams in Critically Ill Patients with Respiratory Infections: a Systematic Review and Meta-analysis. Eur J Drug Metab Pharmacokinet. 2018;43(2):155–170..
- Roberts JA, Abdul-Aziz MH, Davis JS, et al. Continuous versus Intermittent β-Lactam Infusion in Severe Sepsis A Meta-analysis of Individual Patient Data from Randomized Trials. Am J Respir Crit Care Med. 2016;194(6):681–691.
- Vardakas KZ, Voulgaris GL, Maliaros A, et al. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: a systematic review and meta-analysis of randomised trials. Lancet Infect Dis. 2018;18(1):108–120.
- Abdul-Aziz MH, Alffenaar J-WC, Bassetti M, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a Position Paper#. Intensive Care Med. 2020;46(6):1127–1153.

![Figure 3. General steps to obtain antibiotic dose optimization (Modified form [54])](/cms/asset/b10a3d31-314b-465a-bbf6-bf58b01a5ef8/iemt_a_1879049_f0003_b.gif)