ABSTRACT
Background
Oral semaglutide comprises the glucagon-like peptide-1 analog, semaglutide, and sodium N-(8-[2-hydroxybenzoyl] amino) caprylate (SNAC). Levothyroxine has similar dosing conditions to oral semaglutide. This trial investigated if oral semaglutide co-administered with levothyroxine affects thyroxine (T4) exposure and if multiple placebo tablets co-administered with oral semaglutide affect semaglutide exposure.
Research design and methods
In this one-sequence crossover trial, 45 healthy subjects received levothyroxine (600 μg single-dose) alone, or with concomitant SNAC 300 mg or concomitant oral semaglutide 14 mg at steady-state. Subjects also received oral semaglutide 14 mg at steady-state alone or with five placebo tablets once-daily for 5 weeks.
Results
A 33% increase in total T4 exposure was observed with levothyroxine/oral semaglutide vs levothyroxine alone, but baseline-corrected maximum concentration (Cmax) was unaffected. SNAC alone did not affect total T4 exposure, whereas Cmax was slightly decreased. A 34% decrease in semaglutide exposure was observed when oral semaglutide was co-administered with placebo tablets, and Cmax also decreased.
Conclusions
Levothyroxine pharmacokinetics were influenced by co-administration with oral semaglutide. Monitoring of thyroid parameters should be considered when treating patients with both oral semaglutide and levothyroxine. Oral semaglutide exposure was influenced by co-administration with multiple tablets, which is addressed in the dosing guidance.
1. Introduction
Oral semaglutide, a glucagon-like peptide-1 receptor agonist (GLP-1RA), has been shown to improve glycemic control and reduce body weight in patients with type 2 diabetes (T2D) [Citation1–7]. Oral semaglutide is a novel co-formulation of semaglutide with the absorption enhancer sodium N-(8-[2-hydroxybenzoyl] amino) caprylate (SNAC), which facilitates the absorption of semaglutide across the gastric mucosa in a transcellular manner [Citation8]. Administration of oral semaglutide should be in the fasting state [Citation9] and with at least a 30-min delay before eating, drinking, or consuming other oral medications [Citation9,Citation10], as data suggest that its absorption in the stomach is affected by the presence of food and water [Citation11].
Many patients with T2D take several different oral medications for comorbidities such as hypertension, hyperlipidemia, and cardiovascular disorders [Citation11]. Hypothyroidism is another frequent chronic condition, which has a prevalence ranging from 7% to 37% in patients with T2D [Citation12–16]. Levothyroxine, a synthetic form of the thyroid hormone thyroxine (T4), is used as a thyroid hormone replacement therapy and is one of the most commonly prescribed oral drugs [Citation17]. Levothyroxine is indistinguishable from the endogenous T4 hormone. T4 is metabolized through deiodination to the biologically more active triiodothyronine (T3) in the liver and other peripheral tissues [Citation18]. Endogenous T4 and T3 are nearly entirely bound to plasma proteins, primarily thyroid-binding globulin (TBG) [Citation18]. Levothyroxine has a narrow therapeutic index [Citation19]. It is absorbed in the small intestine and its bioavailability is reduced by food; hence, it is also recommended that levothyroxine be administered in the fasting state [Citation20–22].
Due to similar dosing conditions for levothyroxine and oral semaglutide, part A of this trial investigated whether the pharmacokinetics (PK) of T4 were affected when levothyroxine was co-administered with oral semaglutide compared to administration alone. Part B of this trial investigated if co-administration of multiple other tablets affects the exposure of semaglutide, using five placebo tablets as a model, as this is considered to be relevant, based on the number of tablets typically taken by a patient with T2D.
2. Patients and methods
This was an open-label, one-sequence, crossover, single-center, multiple-dose, two-part trial for which all subjects provided written informed consent before participating in any trial-related activities. The trial was conducted in accordance with the Declaration of Helsinki [Citation23], the International Conference on Harmonisation Good Clinical Practice [Citation24], and the Food and Drug Administration (FDA) 21 Code of Federal Regulations 312.120 [Citation25]. An independent ethics committee (Landesamt für Gesundheit und Soziales Ethik-Kommission des landes Berlin, Berlin, Germany) reviewed and approved the protocol, consent form, and subject information sheets, according to local regulations by appropriate health authorities.
2.1. Trial population
Subjects were male or female aged 18–50 years with a body mass index of 20.0–29.9 kg/m2 and considered to be generally healthy based on medical history, physical examination, electrocardiogram, clinical laboratory parameters, and vital signs. Key exclusion criteria included hypersensitivity to trial products or related products, pregnancy, history or presence of thyroid or cardiovascular disease, and history of previous major surgery involving the stomach (see Table S1).
2.2. Trial design
According to FDA and European Medicines Agency guidance for drug–drug interaction studies, the systemic exposure of the perpetrator drug (oral semaglutide) should generally be the exposure obtained with the highest recommended dose under therapeutic (steady-state) conditions [Citation26,Citation27]. Consequently, the current study used the highest therapeutic dose of oral semaglutide (14 mg) studied in the phase III trials [Citation1–7,Citation28]. As oral semaglutide tablets contain 300 mg of the absorption enhancer, SNAC, this was the dose used when SNAC alone was co-administered with levothyroxine.
Dosing of oral semaglutide, levothyroxine, and placebo tablets was done in the morning in the fasting state, at least 30 min before the first meal of the day, with up to half a glass of water (maximum 120 mL/4 fluid oz). The trial products were taken simultaneously or immediately after each other (within 2 min). Safety and tolerability, including any treatment-emergent adverse events and hypoglycemia, were assessed.
To minimize the length of the trial, part A and B overlapped, which is reflected in .
Figure 1. Trial design
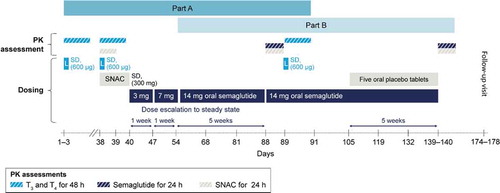
2.2.1. Part A
Single supratherapeutic doses of levothyroxine 600 µg were administered in accordance with FDA guidelines for bioequivalence studies, in three treatment periods: (1) alone, (2) co-administered with tablets containing SNAC alone 300 mg, and (3) co-administered with oral semaglutide 14 mg at steady-state (). To limit gastrointestinal (GI) adverse events (AEs), oral semaglutide was dose-escalated as follows: 3 mg for 1 week, then 7 mg for 1 week, followed by 14 mg for 5 weeks to reach steady-state (7 weeks total) (). To minimize carry-over, washout periods of at least 35 days (based on the approximate 6–7 day half-life of levothyroxine [Citation18]) were included between each levothyroxine dose [Citation25,Citation29].
PK sampling of thyroid hormones was performed for 48 h after levothyroxine dosing: every 30 min until 3 h post-dose, at 4 h post-dose, every 2 h until 12 h post-dose, every 6 h until 36 h post-dose, and at 48 h post-dose. PK sampling of SNAC was performed for 24 h after co-administration of levothyroxine with SNAC alone 300 mg: every 10 min until 1 h post-dose, every 30 min until 3 h post-dose, at 4 h, 6 h, 12 h, and 24 h post-dose.
2.2.2. Part B
Oral semaglutide 14 mg was administered to steady-state in two treatment periods: (1) alone, and (2) co-administered with five placebo tablets (). To minimize levothyroxine carry-over from part A, and to achieve a steady-state before the first semaglutide and SNAC PK assessment, oral semaglutide was dosed for 7 weeks after the second levothyroxine dose, as described in Section 2.2.1. To ensure a similar washout period before the next semaglutide and SNAC PK assessment (i.e. the assessment with co-administration with five placebo tablets), and to achieve a new steady-state, oral semaglutide was dosed for 7 weeks after the final levothyroxine dose: 14 mg alone (for 2 weeks) and co-administered with placebo tablets (for 5 weeks).
PK sampling of semaglutide was performed for 24 h: every 30 min until 1 h post-dose, every 1 h until 3 h post-dose, then at 6 h, 12 h, and 24 h post-dose. PK sampling of SNAC was performed for 24 h at the time points described in Section 2.2.1.
2.3. Trial endpoints
2.3.1. Part A
The primary endpoint was the baseline-corrected area under the serum concentration–time curve (bcAUC) for total T4 from 0 to 48 h after a single dose of levothyroxine (bcAUC0–48h,T4,SD). Supportive secondary endpoints included: bcAUCs for total T3 (bcAUC0–48h,T3,SD), free T4 (bcAUC0–48h,FT4,SD), and free T3 (bcAUC0–48h,FT3,SD); baseline-corrected maximum serum concentration (bcCmax) of total T4 (bcCmax,T4,SD), total T3 (bcCmax,T3,SD), FT4 (bcCmax,FT4,SD), and FT3 (bcCmax,FT3,SD); and time to maximum serum concentration (tmax) for total T4, total T3, FT4, and FT3, each from 0 to 48 h after a single dose of levothyroxine.
Following levothyroxine dosing, T4 and T3 PK were assessed by comparing baseline-corrected total T4 and total T3 in the different treatment periods. Baseline correction was obtained from the average of three total T4 or total T3 pre-dose measurements [Citation30], and was done because levothyroxine is indistinguishable from endogenous T4 in vivo and in bioanalytical assays. Circulating FT4 and FT3 were also measured, as this is the active fraction of the hormones.
TBG levels were analyzed prior to levothyroxine dosing on days 1, 38, and 89. Thyroid-stimulating hormone levels and body weight were also measured at regular intervals throughout the trial.
2.3.2. Part B
The primary endpoint was the area under the plasma concentration–time curve (AUC) for semaglutide during a dosing interval (0–24 h) at steady-state (AUC0–24h,sema,SS). Supportive secondary endpoints included SNAC AUC, and semaglutide and SNAC maximum serum concentration (Cmax,sema,SS) and tmax during a dosing interval (0–24 h) at steady-state.
Supportive secondary safety endpoints (applicable to part A and part B of the trial) included the number of treatment-emergent adverse events, treatment-emergent hypoglycemic episodes, changes in physical examination, vital signs, electrocardiogram, and laboratory safety parameters.
2.4. Biochemical analysis
2.4.1. Part A
Validated liquid chromatography-mass spectrometry (LC-MS/MS) assays were used to investigate total serum concentrations of T4 and T3. The assays used automated solid-phase extraction, isocratic LC conditions, and Sciex API 5,000 triple quadropole mass spectrometers. The assay ranges were 3.21–1,287.22 nmol/L (2.5–1,000 ng/mL) and 0.38–153.61 nmol/L (0.25–100 ng/mL) for T4 and T3, respectively. Serum concentrations of FT4 and FT3 were measured using validated Chemiluminescent Microparticle Immunoassays (Abbott GmbH Diagnostika, Wiesbaden, Germany) on the architect i2000SR automated analyzer from Abbott. The assay ranges were 8.04–66.75 pmol/L and 2.37–28.72 pmol/L for FT4 and FT3, respectively.
2.4.2. Part B
Validated LC-MS/MS assays for semaglutide and SNAC bioanalysis have previously been described [Citation31,Citation32].
2.5. Statistical analysis
Because within-subject standard deviations are much larger for the semaglutide primary endpoints (part B) than for the T4 primary endpoints (part A), the T4 endpoints had little effect on the sample size calculations – these were driven by the semaglutide endpoints (part B). The sample size was chosen to achieve a combined power of at least 80% to show ‘no-effect’ of co-administration with placebo tablets on both the primary endpoints AUC0–24h,sema,SS and Cmax,sema,SS.
With 36 completing subjects with evaluable AUC0–24h,sema,SS and Cmax,sema,SS, the combined power of concluding ‘no-effect’ of co-administration with placebo tablets was 80%, assuming a 0.37 within-subject standard deviation and that the true ratio in AUC and Cmax was 91–110%.
Based on the above calculations, 36 subjects with evaluable AUC0–24h,sema,SS and Cmax,sema,SS were deemed sufficient for the trial. As the trial had a long duration and the withdrawn subjects were not to be replaced, 45 subjects were planned to be enrolled in the trial.
Two analysis sets were defined in accordance with ICH-E9 guidance [Citation33]. Both the full analysis set and the safety analysis set comprised all subjects who were exposed to at least one dose of a trial product and subjects contributed to the evaluation ‘as treated’; however, in exceptional cases, subjects could be eliminated from the full analysis set.
2.5.1. Part A
The primary endpoint was log-transformed and analyzed using an analysis of variance model. Fixed effects were subject and treatment (levothyroxine). Differences in log-transformed values were back-transformed to original scale and presented as ratios together with the corresponding two-sided 90% confidence intervals (CIs). For the endpoint bcAUC0–48h,T4,SD, no effect was concluded if the CIs fell entirely within the pre-specified ‘no effect’ interval of 0.80–1.25 (with/without oral semaglutide or with/without SNAC alone).
When performing the baseline correction of the total and free T4 and T3 profiles, some of the values became negative. Since it is not possible to calculate the geometric mean of negative values, the baseline-corrected profiles were calculated using the arithmetic mean. The endpoints bcAUC0–48h,T3,SD, bcAUC0–48h,FT4,SD, bcAUC0–48h,FT3,SD, bcCmax,T4,SD, bcCmax,T3,SD, bcCmax,FT4,SD, and bcCmax,FT3,SD were planned to be analyzed and presented similarly to the analysis of the primary endpoint bcAUC0–48h,T4,SD. However, no prespecified ‘no effect’ limits were used for these endpoints.
2.5.2. Part B
The primary endpoint was log-transformed and analyzed using an analysis of variance model. Fixed effects were subject and treatment (oral semaglutide). Differences in log-transformed values were back-transformed to original scale and presented as ratios together with the corresponding two-sided 90% CIs. For the endpoint AUC0–24h,sema,SS, the ‘no effect’ limits were widened to 0.7000–1.4286 (with/without five placebo tablets) due to the broad therapeutic window and the high variability in exposure of oral semaglutide [Citation34,Citation35].
3. Results
3.1. Demographics
Healthy male and female subjects (N = 45) were exposed to trial products and 43 subjects completed the trial. One subject withdrew consent due to personal reasons and one subject withdrew due to a non-serious AE of nausea, which occurred during the oral semaglutide dose-escalation period and was moderate in severity. All 45 subjects were included in the full analysis set and the safety analysis set. Baseline characteristics are presented in .
Table 1. Baseline characteristics
3.2. Pharmacokinetics
3.2.1. Part A
3.2.1.1. Effect of oral semaglutide on thyroxine
Geometric mean and baseline-corrected arithmetic mean concentration−time profiles of total T4 and FT4 when levothyroxine was administered alone or co-administered with oral semaglutide 14 mg are presented in , respectively. The estimated ratio of bcAUC0–48h for total T4 when levothyroxine was administered with/without oral semaglutide was 1.33 [1.25; 1.42]90% CI, which was not within the pre-specified ‘no effect’ interval of 0.80–1.25. The estimated ratio of bcAUC0–48h for FT4 when levothyroxine was administered with/without oral semaglutide was 1.31 [1.23; 1.39]90% CI ().
Figure 2. Total T4 profiles after a single dose of levothyroxine 600 mg administered alone or co-administered with oral semaglutide 14 mg at steady-state
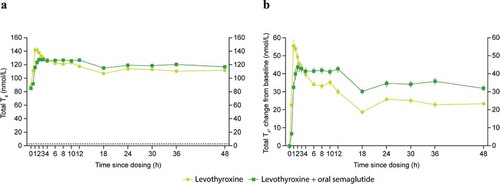
Figure 3. FT4 profiles after a single dose of levothyroxine 600 mg administered alone or co-administered with oral semaglutide 14 mg at steady-state
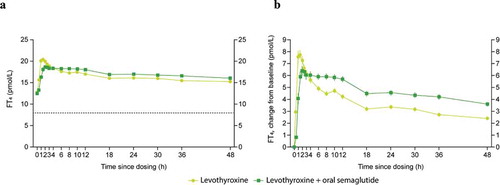
Table 2. PK baseline-corrected endpoints of levothyroxine after a single dose either administered alone or co-administered with steady-state oral semaglutide 14 mg or SNAC. (a) Total T4; (b) Free T4; (c) Total T3; (d) Free T3
Cmax of total T4 and FT4 were not affected by co-administration with oral semaglutide. The estimated ratio of bcCmax of total T4 when levothyroxine was administered with/without oral semaglutide was 0.88 [0.81; 0.94]90% CI, which was within the pre-specified ‘no effect’ interval. The estimated ratio of bcCmax of FT4 when levothyroxine was administered with/without oral semaglutide was 0.91 [0.84; 0.98]90% CI ().
The tmax for total T4 and FT4 appeared to occur slightly later when levothyroxine was co-administered with oral semaglutide compared to when administered alone: 3.0 h vs 1.0 h and 2.5 h vs 1.5 h, for total T4 and FT4, respectively ().
Statistical analyses for total and free T3 were performed by estimating the treatment difference (levothyroxine co-administered with oral semaglutide vs levothyroxine alone) rather than the treatment ratio due to some endpoints becoming negative for some subjects after baseline correction. Baseline total T3 and FT3 concentrations were higher when levothyroxine was administered alone compared to when co-administered with oral semaglutide. There was a significant increase in bcAUC0–48h and bcCmax for total T3; the estimated treatment differences (ETDs) were 6.20 nmol∙h/L [4.40; 7.99]95% CI and 0.05 nmol/L [0.01; 0.10]95% CI, respectively. There was also a significant increase in bcAUC0–48h and bcCmax for FT3; the ETDs were 15.92 pmol∙h/L [11.73; 20.11]95% CI and 0.20 pmol/L [0.11; 0.28]95% CI, respectively ().
3.2.1.2. Effect of SNAC alone on thyroxine
There was no effect on bcAUC0–48h for total T4 when single-dose levothyroxine was administered with/without SNAC alone; the estimated ratio was 0.97 [0.90; 1.04]90% CI (). The bcCmax of total T4 was slightly decreased (estimated ratio 0.85 [0.79; 0.92]90% CI) when levothyroxine was co-administered with SNAC alone compared with administration of levothyroxine (). Other PK endpoints, including bcAUC0–48h,FT4,SD, bcAUC0–48h,T3,SD, bcCmax,T3,SD, bcAUC0–48h,FT3,SD, and bcCmax,FT3,SD, were not affected when levothyroxine was co-administered with SNAC alone (). The tmax for total T4 appeared to occur at a similar time (median 1.5 h vs 1.0 h when levothyroxine was administered with/without SNAC alone, respectively.
3.2.2. Part B
3.2.2.1. Effect of five oral placebo tablets on semaglutide
The geometric mean profile for oral semaglutide co-administered with/without five oral placebo tablets is shown in . The estimated ratio of semaglutide AUC0–24h at steady-state (with/without placebo tablets) was 0.66 [0.56; 0.78]90% CI, which was not within the pre-specified ‘no effect’ interval of 0.7000–1.4286. The estimated ratio of semaglutide Cmax (with/without placebo tablets) was 0.68 [0.57; 0.80]90% CI, which was not within the pre-defined ‘no effect’ interval of 0.7000–1.4286. The tmax for semaglutide appeared to occur at a similar time (1 h) for oral semaglutide administered with/without placebo tablets ().
Figure 4. Semaglutide 14 mg profiles at steady-state alone or co-administered with five oral placebo tablets
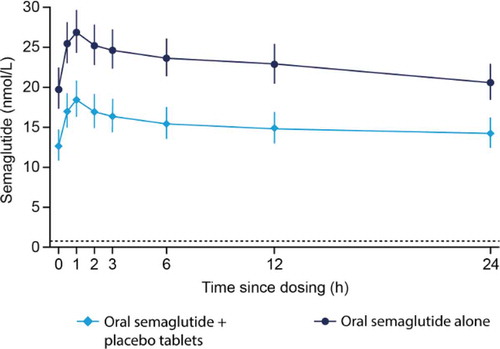
Table 3. PK endpoints of steady-state oral semaglutide (a) and SNAC (b) either administered alone or after co-administration with five oral placebo tablets
3.2.2.2. Effect of five oral placebo tablets on SNAC
There was a significant decrease in SNAC exposure when oral semaglutide was co-administered with placebo tablets compared to when oral semaglutide was administered alone. The estimated ratio of SNAC AUC0–24h at steady-state was 0.92 [0.86; 0.98]95% CI. In contrast, there was a statistically significant increase in the SNAC Cmax when oral semaglutide was co-administered with placebo tablets compared to when oral semaglutide was administered alone; the estimated ratio was 1.29 [1.04; 1.59]95% CI. The tmax for SNAC appeared to occur slightly earlier when oral semaglutide was co-administered with placebo tablets (0.35 h) compared to when oral semaglutide was administered alone (0.67 h) ().
3.3. Other measurements
As expected, thyroid-stimulating hormone levels were suppressed following administration of single-dose levothyroxine, but had reverted to baseline levels by the following assessment (Figure S1). TBG appeared to be similar across all three treatment periods in part A (data not shown). The mean change in body weight was −4.8 kg from the first day to the last day of oral semaglutide administration (Figure S2).
3.4. Safety and tolerability
A total of 226 AEs were reported in 35 (77.8%) subjects during the entire trial (parts A and B). The majority of AEs were mild to moderate in severity. There was one serious AE of severe hypoglycemia during the dose escalation of oral semaglutide, considered by the investigator as possibly related to oral semaglutide. There were two AEs requiring additional data collection: non-serious increases in blood creatinine phosphokinase >10 × upper limit of normal (one during the oral semaglutide alone period, and one during the levothyroxine period). Two subjects withdrew from the trial; one due to moderate nausea during the dose escalation of oral semaglutide and one due to personal reasons. There were no deaths reported in this trial.
The most frequently reported AEs were GI disorders (122 events in 23 [51.1%] subjects), including nausea (39 events in 19 [42.2%] subjects), eructation (13 events in 9 [20.0%] subjects), and vomiting (20 events in 8 [17.8%] subjects). The GI disorders were more common during the dose escalation of oral semaglutide. The second most frequently reported AEs were nervous system disorders (34 events in 20 [44.4%] subjects), including headache (28 events in 17 [37.8%] subjects) and dizziness (3 events in 3 [6.7%] subjects). The nervous system disorders were more common during the oral semaglutide and placebo period, and the levothyroxine period. The majority of headaches (the most common nervous system disorder AE) were considered by the investigator to be possibly or probably related to levothyroxine. An overview of AEs is provided in Table S2.
4. Discussion
4.1. Part A
GLP-1RAs are effective therapies for the treatment of T2D. Oral semaglutide, a novel co-formulation of semaglutide with the absorption enhancer SNAC, has shown significant improvements in glycemic control and body weight compared with placebo [Citation1–3,Citation5,Citation29]. Absorption of oral semaglutide takes place in the stomach and is affected by the presence of food and water; therefore, administration should be in the fasting state [Citation8]. Levothyroxine is a frequently used oral thyroid hormone replacement therapy for patients with hypothyroidism [Citation17], a common condition in patients with T2D [Citation12–16]. As levothyroxine and oral semaglutide share similar dosing conditions and thus, are likely to be co-administered, the effect of co-administration on T4 exposure was investigated. The trial results showed that overall exposure of baseline-corrected total T4 was increased by co-administration with oral semaglutide, with an average increase in bcAUC0–48h,T4,SD of 33%. This was accompanied by a later tmax when co-administered with oral semaglutide compared to levothyroxine alone. When levothyroxine was co-administered with the absorption enhancer SNAC, there was no obvious effect on T4 PK endpoints, except for a slight reduction in bcCmax of total T4. Given the lack of effect of SNAC alone, the increase in overall exposure of bctotal T4 with oral semaglutide co-administration could be attributed to the GLP-1 component of oral semaglutide. This is in line with other GLP-1RAs increasing exposure of co-administered oral drugs [Citation36]. One hypothesis for this is that a delay in gastric emptying caused by GLP-1RAs results in some co-administered oral drugs having a longer transit time in the GI tract, and consequently a longer time-window for absorption. This can also lead to a later tmax, which was indeed observed when levothyroxine was co-administered with oral semaglutide, but not with SNAC alone. A delay in gastric emptying has been shown with both subcutaneous and oral semaglutide [Citation37–39].
The effect of co-administration of levothyroxine with oral semaglutide on total T3 and FT3 was analyzed, and bcAUC was found to be increased with co-administration (which is in line with the T4 results). There was no obvious effect on T3 PK endpoints for levothyroxine co-administered with SNAC alone. Interpretation of these results is difficult, as the baseline concentrations of total T3 and FT3 were higher in the levothyroxine alone treatment period than after co-administration with SNAC alone or oral semaglutide. The results should therefore be interpreted with caution, as the differences from baseline are small and there is a relatively high variance in these measurements.
Previous trials have shown that oral semaglutide does not have any clinically relevant effect on the PK of other commonly administered drugs [Citation32,Citation40,Citation41]. Differences were observed in exposure of certain drugs when co-administered with oral semaglutide compared to administration alone, e.g. AUC of metformin increased by 32% [Citation40], AUC of furosemide increased by 28%, and Cmax decreased by 34% [Citation41]. These alterations were deemed clinically insignificant due to the wide therapeutic index of metformin and furosemide. Furthermore, no changes were seen with drugs with a narrow therapeutic index, such as warfarin and digoxin. The findings in the present trial are in line with these results, of no or only minor increases in exposure of co-administered drugs, and further support the hypothesis that the cause of the increase is due to the delayed gastric emptying effects of semaglutide.
The trial was designed according to FDA and European Medicines Agency guidelines for drug–drug interaction studies and for levothyroxine bioequivalence studies, where use of healthy subjects and a single supratherapeutic dose is suggested. These factors should be considered when interpreting these results in a clinical context, as patients with thyroid dysfunction are likely to receive a lower daily dose of levothyroxine than that given in the present trial. This dose was selected to ensure measurable concentration difference, while considering endogenous thyroid levels. The use of a single dose of levothyroxine also means that the thyroid axis did not reach steady-state, which should be considered when interpreting the results clinically. Finally, the body weights in the three levothyroxine dosing periods were slightly different (mean body weights were 77.8 kg, 79.2 kg, and 75.5 kg prior to the levothyroxine, levothyroxine + SNAC, and levothyroxine + oral semaglutide periods, respectively), which may have impacted the levothyroxine results, as body weight can affect the regulation of the thyroid axis [Citation42,Citation43]. Further trials in patients with thyroid dysfunction would be necessary to understand the clinical implications.
4.2. Part B
Many patients with T2D take several different medications for comorbidities, therefore, at certain times, multiple tablets may be present in the stomach. Oral semaglutide is absorbed in the stomach and its absorption is known to be negatively affected by the presence of food and water; therefore, the consequences of other substances in the stomach should be considered in order to guide the patients optimally. As such, the current trial investigated if the co-administration of oral semaglutide with multiple tablets affected the exposure of semaglutide, using five placebo tablets as a model. The total exposure of semaglutide was decreased by 34% and Cmax was decreased by 32% when oral semaglutide was dosed with five oral placebo tablets compared to administration of oral semaglutide alone. This indicates that co-administration of oral semaglutide with multiple oral medications may reduce semaglutide absorption. The effect on SNAC exposure was less pronounced. The observed effects on the semaglutide PK could be due to the presence of the placebo tablets in the stomach causing physical displacement of the oral semaglutide tablet from the stomach surface or a change in the gastric motility as a result of the slightly larger stomach content. Alternatively, the additional tablets may disrupt the close spatial proximity of semaglutide and SNAC at the site of absorption, which is crucial for the absorption enhancement to take place [Citation8]. The dosing instructions for oral semaglutide include a recommendation to avoid food, drink, and other oral medications for at least 30 min after oral semaglutide intake [Citation9,Citation10], and these data highlight the importance of following these instructions closely. The safety and tolerability profile of oral semaglutide was consistent with previous trials [Citation1–7,Citation31,Citation35,Citation40]. A higher proportion of subjects experienced GI AEs in the current trial than in the those reported by Bækdal et al. [Citation31] and Granhall et al. [Citation35], but in the Bækdal 2019 trial (which had a similar oral semaglutide dose-escalation regime to the present study), 63.5% of the subjects experienced GI AEs [Citation40] compared with the 51.5% reported in the present trial. This could be due to the higher doses of oral semaglutide (5, 10, and 20 mg) in the Bækdal 2019 trial.
5. Conclusions
To conclude, overall exposure of baseline-corrected total T4 was increased when levothyroxine was co-administered with oral semaglutide at steady-state. Monitoring of thyroid parameters should be considered when treating patients with oral semaglutide and levothyroxine concomitantly. Absorption of oral semaglutide is reduced by co-administration with multiple tablets, which is addressed in the dosing guidance recommendation that other oral medication should be dosed at least 30 min after oral semaglutide administration. Safety and tolerability were found to be consistent with previous clinical trials with oral semaglutide and no unexpected safety findings were observed.
Declaration of interest
Camilla Hauge, Simon Jensen, and Tine A. Bækdal are employees and shareholders in Novo Nordisk A/S. Marie-Louise Hartoft-Nielsen was an employee of Novo Nordisk A/S at the time of the conduct of the trial and manuscript preparation, and is a shareholder of Novo Nordisk A/S. Astrid Breitschaft did not receive direct payments or support for participating in the manuscript but is an employee of PAREXEL International GmbH, which received funding from Novo Nordisk A/S to conduct the trial. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Ethical approval
An independent ethics committee reviewed and approved the protocol, consent form, and subject information sheets, according to local regulations by appropriate health authorities. The trial was conducted in accordance with the Declaration of Helsinki.
Author contributions
Study conception: Camilla Hauge, Tine A. Bækdal and Marie-Louise Hartoft-Nielsen; Study design: all authors; Data analysis: Simon Jensen; Interpretation of data: all authors. All authors gave the final approval of the version to be published and agreed to be accountable for all aspects of the work.
Previous data presentation
Data in this manuscript were previously presented at the Endocrine Society annual meeting (ENDO), March 23–26, 2019, New Orleans, LA, USA. The abstract is published as: Hauge C et al. J Endocrine Soc 2019;3(Suppl.):SAT-140.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Supplemental Material
Download MS Word (178 KB)Acknowledgments
This trial was sponsored by Novo Nordisk A/S (trial registration: NCT02920385). Writing support was provided by Lindsay Uglow of Axis, a division of Spirit Medical Communications Group Ltd, and was supported by Novo Nordisk A/S. The authors would like to thank all the participants, investigators, and trial-site staff who were involved in the conduct of the trials, Karen Boje Pedersen (Novo Nordisk A/S) for reviewing the manuscript, and Michael Pilgaard Andersen (Novo Nordisk A/S) for support with the bioanalyses.
Supplementary materials
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724–1732.
- Mosenzon O, Blicher TM, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):515–527.
- Rodbard HW, Rosenstock J, Canani LH, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42(12):2272–2281.
- Zinman B, Aroda VR, Buse JB, et al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care. 2019;42(12):2262–2271.
- Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394(10192):39–50.
- Pieber TR, Bode B, Mertens A, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):528–539.
- Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321(15):1466–1480.
- Buckley ST, Bækdal TA, Vegge A, et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med. 2018;10(467):eaar7047.
- European Medicines Agency. Rybelsus summary of product characteristics. May 2020 . [cited 2020 Dec 8]. Available from: https://www.ema.europa.eu/en/documents/product-information/rybelsus-epar-product-information_en.pdf.
- Food and Drug Administration. Rybelsus prescribing information. September 2019. [cited 2020 Dec 8]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/213051s000lbl.pdf.
- Indu R, Adhikari A, Maisnam I, et al. Polypharmacy and comorbidity status in the treatment of type 2 diabetic patients attending a tertiary care hospital: an observational and questionnaire-based study. Perspect Clin Res. 2018;9(3):139–144.
- Jali MV, Kambar S, Jali SM, et al. Prevalence of thyroid dysfunction among type 2 diabetes mellitus patients. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2017;11 Suppl 1: S105–S108.
- Barmpari ME, Kokkorou M, Micheli A, et al. Thyroid dysfunction among Greek patients with type 1 and type 2 diabetes mellitus as a disregarded comorbidity. J Diabetes Res. 2017;2017:6505814.
- Zhu Y, Xu F, Shen J, et al. Prevalence of thyroid dysfunction in older Chinese patients with type 2 diabetes – a multicenter cross-sectional observational study across China. PLoS One. 2019;14(5):e0216151.
- Song F, Bao C, Deng M, et al. The prevalence and determinants of hypothyroidism in hospitalized patients with type 2 diabetes mellitus. Endocrine. 2017;55(1):179–185.
- Talwalkar P, Deshmukh V, Bhole M. Prevalence of hypothyroidism in patients with type 2 diabetes mellitus and hypertension in India: a cross-sectional observational study. Diabetes Metab Syndr Obes. 2019;12:369–376.
- IMS Institute for Healthcare Informatics. Medicines use and spending in the U.S. A review of 2015 and outlook to 2020. 2016. [cited 2020 Nov 10]. Available from: https://morningconsult.com/wp-content/uploads/2016/04/IMS-Institute-US-Drug-Spending-2015.pdf.
- Colucci P, Yue CS, Ducharme M, et al. A review of the pharmacokinetics of levothyroxine for the treatment of hypothyroidism. Eur Endocrinol. 2013;9(1):40–47.
- Wartofsky L. Levothyroxine: therapeutic use and regulatory issues related to bioequivalence. Expert Opin Pharmacother. 2002;3(6):727–732.
- Virili C, Antonelli A, Santaguida MG, et al. Gastrointestinal malabsorption of thyroxine. Endocr Rev. 2019;40(1):118–136.
- Virili C, Brusca N, Capriello S, et al. Levothyroxine therapy in gastric malabsorptive disorders. Front Endocrinol (Lausanne). 2021;11:621616.
- Medicines and Healthcare Products Regulatory Agency. Levothyroxine summary of product characteristics (Mercury Pharma). February 2016. [cited 2020 Nov 10]. Available from: http://www.mhra.gov.uk/home/groups/par/documents/websiteresources/con676411.pdf.
- World Medical Association. Declaration of Helsinki. Recommendations guiding medical doctors in biomedical research involving human subjects. 48th WMA General Assembly; 1996 October 22–26; Somerset West, Western Cape Province, South Africa.
- International Conference on Harmonisation. ICH harmonised tripartite guideline: guideline for good clinical practice E6 (R1). ICH. June 10, 1996.
- Food and Drug Administration. FDA Code of Federal Regulations, 21 Code of Federal Regulations 312.120. Foreign clinical studies not conducted under an IND. FDA; 2014.
- Food and Drug Administration. Guidance for industry - drug interaction studies - study design, data analysis, implications for dosing and labeling recommendations. Draft guidance. FDA; February 2012.
- European Medicines Agency. Guideline on the investigation of drug interactions. EMA; 2012.
- Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–851.
- Food and Drug Administration. Guidance for industry - levothyroxine sodium tablets - in vivo pharmacokinetic and bioavailability studies and in vitro dissolution testing. FDA; 2000.
- Food and Drug Administration. Draft guidance on levothyroxine sodium. FDA; 2014.
- Bækdal TA, Thomsen M, Kupcova V, et al. Pharmacokinetics, safety, and tolerability of oral semaglutide in subjects with hepatic impairment. J Clin Pharmacol. 2018;58(10):1314–1323.
- Baekdal TA, Breitschaft A, Navarria A, et al. A randomized study investigating the effect of omeprazole on the pharmacokinetics of oral semaglutide. Expert Opin Drug Metab Toxicol. 2018;14(8):869–877.
- International Conference on Harmonisation. ICH Harmonised Tripartite Guideline E9. statistical principles for clinical trials, step 4 version. ICH; February 5, 1998.
- Davies M, Pieber TR, Hartoft-Nielsen ML, et al. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA. 2017;318(15):1460–1470.
- Granhall C, Sondergaard FL, Thomsen M, et al. Pharmacokinetics, safety and tolerability of oral semaglutide in subjects with renal impairment. Clin Pharmacokinet. 2018;57(12):1571–1580.
- Hurren KM, Pinelli NR. Drug-drug interactions with glucagon-like peptide-1 receptor agonists. Ann Pharmacother. 2012;46(5):710–717.
- Meier JJ, Gallwitz B, Salmen S, et al. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab. 2003;88(6):2719–2725.
- Hjerpsted JB, Flint A, Brooks A, et al. Semaglutide improves postprandial glucose and lipid metabolism, and delays first-hour gastric emptying in subjects with obesity. Diabetes Obes Metab. 2018;20(3):610–619.
- Dahl K, Brooks A, Almazedi F, et al. Oral semaglutide improves postprandial glucose and lipid metabolism, and delays gastric emptying, in subjects with type 2 diabetes. Diabetes Obes Metab. 2021;23(7):1594–1603.
- Baekdal TA, Borregaard J, Hansen CW, et al. Effect of oral semaglutide on the pharmacokinetics of lisinopril, warfarin, digoxin, and metformin in healthy subjects. Clin Pharmacokinet. 2019;58(9):1193–1203.
- Jordy AB, Breitschaft A, Christiansen E, et al. Oral semaglutide does not affect the bioavailability of the combined oral contraceptive ethinylestradiol/levonorgestrel. Diabetes. 2018;67(Supplement 1):1135-P.
- Agnihothri RV, Courville AB, Linderman JD, et al. Moderate weight loss is sufficient to affect thyroid hormone homeostasis and inhibit its peripheral conversion. Thyroid. 2014;24(1):19–26.
- Roef G, Lapauw B, Goemaere S, et al. Body composition and metabolic parameters are associated with variation in thyroid hormone levels among euthyroid young men. Eur J Endocrinol. 2012;167(5):719–726.
