ABSTRACT
Introduction
Idiosyncratic drug-induced liver injury (iDILI) is a challenging and unpredictable multifactorial condition. At present, validated preclinical models for the prediction of the hepatotoxic potential of a given drug are scarce.
Areas covered
This review intends to sum up the current knowledge about in vitro (including hepatocyte 2D cultures, cocultures with non-parenchymal cells, 3D configurations and non-typical closer to reality in vitro models), in vivo (covering models for immunological and oxidative stress features, humanized mouse-based and non-rodent models) and in silico approaches for iDILI modeling, highlighting the recent advances in each topic.
Expert opinion
The future strategy for iDILI modeling should be patient-centered. Future animal and cell-based models, with more predictive value, will be easier to design by using a more translational approach based on mechanisms demonstrated in humans. Genetic and epigenetic information gathered from iDILI patients, together with data from in vitro and in vivo studies, could be used to develop sophisticated predictive in silico models to find compounds with iDILI potential. Collecting genetic, metabolic, and biomarker data from patient cohorts might be another option to create a ‘fingerprint’ characteristic of people at risk, allowing for the development of new, mechanistic strategies to enhance iDILI in vitro evaluation.
1. Introduction
Drug-induced liver injury (DILI) consists of adverse reactions to drugs and herbal and dietary supplements (HDS), with important industry and clinical implications, as it has been responsible for a considerable fraction of drug withdrawals from the market and during drug development [Citation1].
DILI has been traditionally classified into two different entities: intrinsic and idiosyncratic DILI (hereinafter, iDILI). The first one refers to the predictable, dose-dependent toxicity and it occurs shortly after the patient exceeds a threshold defined for some drugs, such as acetaminophen, which accounts for ~ 50% of acute liver failure (ALF) in Europe and ~ 40% in the USA [Citation2,Citation3].
On the other hand, iDILI is unpredictable, has a longer latency period, and, although a threshold dose is necessary to induce the cascade of events leading to liver damage, it is considered to be non-dose dependent [Citation4]. Despite having a low incidence (14–19 cases per 100,000 inhabitants) [Citation5], iDILI is responsible for 11% of ALF cases in the USA [Citation6].
Recently, a different type of DILI denominated ‘indirect DILI’ has been proposed. This entity is believed to be caused by the drugs mechanism of action (e.g. activation of a harmful immune response by immunomodulatory agents) rather than by an unpredictable/idiosyncratic combination of conditions or the inherent toxic nature of the agent [Citation7].
The differences between different types of liver injury are vague, and the majority of drug-induced liver responses are regarded as idiosyncratic. Research over the past few years has shown that there are host susceptibility factors that influence the risk of liver damage and, at the same time, for drugs that are thought to cause idiosyncratic liver damage, there may be a dose threshold.
IDILI presents significant clinical and diagnostic challenges. Due to the absence of specific biomarkers, the Roussel Uclaf Causality Assessment Method (RUCAM) scale is still the most used methodology for the assessment of iDILI, although it has some limitations, such as doubtful reproducibility [Citation8,Citation9]. A new electronic version (revised electronic causality assessment method, [RECAM]) has recently been proposed to improve iDILI diagnosis through an increase of objectivity and clarity [Citation10].
Since the development of iDILI depends on a combination of host and environmental factors and drug properties, it is considered a multifactorial condition [Citation11]. Indeed, the ‘multiple determinant hypothesis’ proposes that the more risk factors are present simultaneously, the greater the likelihood that iDILI develops [Citation12]. Although genetic predisposing factors have been described, only a very few people that carry them develop this disease, which reveals that other factors influence the occurrence of iDILI [Citation13]. Underlying mechanisms of iDILI may involve the formation of chemically reactive metabolites (CRM) [Citation14] which can produce direct damage on the cell, interfere with bile acids (BAs) transport, or result in oxidative stress, which leads to harmful responses that may disrupt the hepatocyte homeostasis [Citation15]. Drugs can also bind covalently to proteins and form immunogenic adducts that act as neo-antigens capable of triggering an adaptive immune response [Citation16]. This reaction is amplified due to the release of damage-associated molecular patterns (DAMPs) that interact with antigen-presenting cells activating the innate immune response to trigger an inflammatory process, cell death pathways and, ultimately, liver injury [Citation17,Citation18]. In addition, increased intestinal permeability may lead to the leak of pathogen-associated molecular patterns (PAMPs) that are known to stimulate the immune system activation and foster a chronic immune process in the liver [Citation19].
The uncertainty in the diagnosis of iDILI and the lack of complete knowledge about its pathophysiology highlight the need for the establishment and characterization of appropriate iDILI models. Moreover, experimental approaches could assist the detection of biomarkers for the early diagnosis of iDILI at the initial stages of pre-clinical trials, as well as in pharmacovigilance. Most studies investigating serum DILI biomarkers have been performed in intrinsic acetaminophen (APAP)-induced liver injury animal models, but there is still a lack of useful preclinical tools for the prediction and diagnosis of iDILI [Citation20].
Therefore, it is important to develop reliable in vitro, in vivo and in silico iDILI models, to advance in the understanding of gaps for diagnosis, prediction, and cellular/molecular mechanisms that are still to be solved [Citation21]. Additionally, current models need refinement to be approved by regulatory agencies and the industry. Here, we review the in silico, in vitro and in vivo models with a focus on iDILI, discussing the advantages and drawbacks of each approach, and mentioning predictive information that could be extrapolated for use in current iDILI issues in the clinical scenario.
2. An optimal strategy for approaching iDILI modeling
The broad spectrum of complex factors attributed to iDILI onset makes it virtually impossible to predict the disease using a single model. Although the best predictive values are achieved when different configurations of in vitro or in vivo models are combined, currently available modeling strategies still lack high predictive capacity for many hepatotoxic drugs [Citation22]. However, whether the goal is to predict the disease or to model its mechanisms, some specific features of the disease are susceptible to be studied following the proper modeling methodology.
Reproducing iDILI features requires the establishment of a carefully designed strategy (), on which the first step should be to delimit the purpose of the research. For example, the choice of the appropriate model will vary depending on whether we want to predict the disease, assess comorbidities, identify genetic susceptibility factors, evaluate the effect of physicochemical properties of drugs, investigate specific molecular mechanisms, etc. It is essential to know which endpoint of the disease and drug we are interested in analyzing in the models. For instance, the study of immune system activation usually requires configurations that include at least two cell types, and desirably, more complex systems that encompass the role of the peripheral adaptive immune system.
Figure 1. Optimal workflow for designing an iDILI modeling strategy. The proposed workflow for iDILI modeling is a flexible circuit that attempts to consider different aspects of experimental design, from the objectives to the choice of the most appropriate setup. Early decisions in the preclinical approach to iDILI include establishing the purpose of the study and the choice of drugs to be used to replicate the liver damage in models. More specific biological aspects such as the processes involved in phenotypic and functional characterization are then considered. The models are categorized into in silico, in vitro and in vivo. The latter two are ordered according to their complexity. Estimation of complex physiological parameters may require the use of in vivo models, while in vitro configurations will be more appropriate for chemical and cellular processes. Combined testing, data integration and correlation with human studies will be desirable to improve the quality of the models, especially when hepatotoxic risk prediction is the main objective.
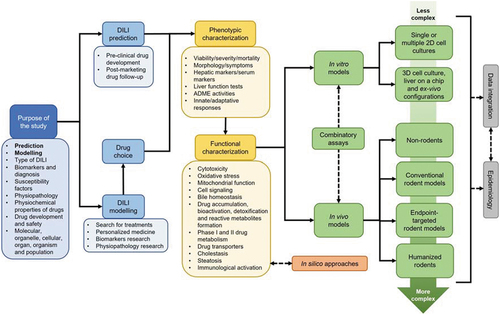
If the purpose of the study is not limited to the prediction of iDILI during drug development, the choice of drug is of crucial importance. According to the Food and Drug Administration (FDA), iDILI can potentially be produced by approximately 750 documented drugs [Citation23], belonging to very different classes and mechanisms of action. Ideally, new predictive models of iDILI should be tested on a broad battery of drugs, including drugs with high hepatotoxic risk and drugs with low hepatotoxic potential, to assess their predictive capacity. It is the subject of much scientific effort to establish consensus drugs for iDILI modeling, although this remains a partially unanswered question. The Prospective European Drug-induced Liver Injury Network (ProEuro DILI Network), which arises from a European Cooperation in Science and Technology (COST) action, launched in 2018 (https://proeurodilinet.eu), has among its goals to establish a list of appropriate control compounds for testing new in vitro models. Probably the most common drug in DILI modeling studies is still APAP, which has been defined as an archetypal drug model for intrinsic DILI [Citation24]. However, although APAP can also produce iDILI, the cases of hepatotoxicity detected are mostly intrinsic through mitochondrial dysfunction, and therefore, its modeling may not fit the study of other mechanisms of drugs also associated with idiosyncratic reactions [Citation25].
Hepatotoxicity screening is not a novel endeavor during drug development. Pharmaceutical companies have been using various strategies to detect this phenomenon for decades. Regardless of the cell or animal model chosen, the main endpoint pursued by approaches has traditionally been the detection of cytotoxicity following drug exposure. In recent years, an increasing number of companies are adding endpoints such as generation of reactive metabolites, BAs transporters inhibition and mitochondrial toxicity to the screening assays, achieving improved sensitivity and specificity [Citation26].
To develop consensus-based predictive models of iDILI, Weaver and collaborators recently established a fairly detailed 3-tier roadmap, on which current available models are organized by complexity [Citation22]. Two-dimensional (2D) cultures and computational tools were included in tier 1, three-dimensional (3D) configurations in tier 2, and more complex systems, such as customized animal models, in tier 3. The authors considered that tier 1 models are good enough for addressing immediate chemical and biological effects of drugs, whereas experiments requiring longer drug exposures and more physiological conditions should be addressed by models in tier 2. Tier 3 satisfies studies that require the inclusion of specific biological variables and patient-derived factors. Therefore, the usefulness, and therefore the adequacy, of each model will depend directly on the endpoint or function to be studied. The primary goal of this system is to achieve an improved early drug safety evaluation, but their approach may be of great use to any investigators who want to design an iDILI modeling experiment.
3. In vitro models
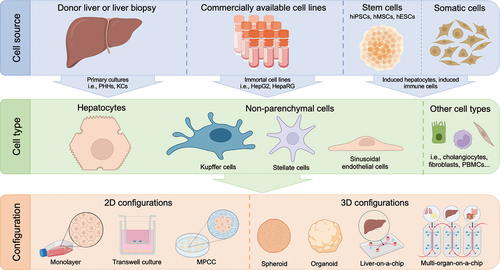
In vitro iDILI modeling refers to the establishment of a cell-based system mimicking as accurately as possible the conditions of iDILI patients. There is a wide variety of in vitro models, differing in complexity (). The simpler models allow studying specific endpoints while the more complex models reproduce the disease in a closer fashion to the in vivo situation. The simplest in vitro models to study iDILI consist of 2D hepatocyte cultures, in which the direct effect of the drug on these cells can be studied. Advancing in complexity, 2D cocultures of hepatocytes together with other cell types (i.e. endothelial cells, immune cells, or fibroblasts) can be established. Although 3D models improve liver functions and drug-metabolizing capacity over 2D cultures, their complexity and difficult reproducibility prevent their incorporation into pharma industries protocols.
Figure 2. Cell sources, types and culture configurations involved in iDILI modeling in vitro. Cell cultures used to study iDILI can be obtained from a variety of sources, such as liver biopsies, immortalized hepatoma-derived lines or stem cells. The type of cells used depends on the study to be performed, being hepatocytes the most usual, either in monoculture or cultured together with other non-parenchymal cell types. However, there are cultures of other cell types that are being used to study this disease, such as fibroblasts or PBMCs. Finally, the cell culture configuration determines the studies that can be performed and the level of complexity of the assay. The simplest are two-dimensional monolayer cultures and, from there, various levels of complexity can be reached, such as the microfluidic models that simulate the relationship between various organs, the multi-organ-on-a-chip.
3.1. Ease-to-use 2D hepatic cell monocultures
Hepatocytes are the parenchymal cells of the liver, so they will ultimately be the cells affected when liver damage occurs. Therefore, the simplest way to approximate the damage due to iDILI is using a 2D hepatic culture.
The hepatocytes used to establish these cultures can be derived from animals or humans. Although human cell cultures are the most commonly used, animal cells have the great advantage that the process of obtaining primary hepatic cells is much simpler than in humans. However, hepatic animal cells have significant drawbacks in simulating human liver tissue, as these cells do not express the mature phenotype of hepatocytes and this affects principally drug metabolism and transport [Citation27,Citation28]. Hence, we will mainly discuss in vitro cultures of human cells.
3.1.1. Primary human hepatocytes (PHHs): chasing the golden standard
Primary Human Hepatocytes (PHHs) are cells directly obtained from human livers, either from resections, tissue unsuitable for transplantation or biopsies. Cell culture of PHHs is considered the gold standard in liver modeling, as these cells have the great advantage of maintaining liver-specific functions, metabolic activity and morphology at levels similar to the human organ in vivo. On the other hand, there are several downsides to the use of PHHs. First, obtaining these cells is a complicated process, and variation in isolation procedures may cause differences in the experiment outcomes, making the standardization of the established models difficult. In addition, PHHs are the cells that best retain liver function but only for short periods of time when cultured in suspension, as they rapidly lose their functionality and morphology. There are several strategies to slightly slow down this process. For example, PHHs can be cultured in collagen-coated plastic dishes, extending the useful life of these cells from hours to some days [Citation29].
PHHs cultured in 2D have been widely used as iDILI models for toxicity testing. Nevertheless, the specificity and sensitivity of these cultures for predicting iDILI are not excessively high, as they range between 50 and 70% and between 70 and 85% respectively. This is due to several reasons, including a shortage of donors to account for the variability that occurs between patients or the choice of different concentrations of the hepatotoxic drug [Citation30]. Some examples of drugs classified as of major concern for iDILI used to model the disease by using PHHs cultured in 2D are amiodarone, flutamide and ketoconazole [Citation31].
Different endpoints can be studied in PHHs cultures to approximate the cell damage, including cytotoxicity (typically calculated by the amount of ATP in cell lysates), urea production, albumin secretion, the study of the activity of genes involved in drug metabolism, or organelle involvement (such as reactive oxygen species [ROS] increase or mitochondrial damage [Citation32,Citation33]).
Despite PHHs being the gold standard due to their similarities to the functioning liver, we believe that more work needs to be done to achieve more practically useful 2D monoculture models. One option could be to immortalize these cells so that they can be maintained in long-term cultures, as already done by Su et al. [Citation34]. This type of culture with PHHs has already been used to study various diseases, such as liver tumors and inflammatory diseases [Citation35]. However, to our knowledge, it has not yet been used to model hepatotoxicity.
3.1.2. Hepatoma-derived cell lines: tackling some issues
Another approach to the study of iDILI involves the use of liver tumor-derived cells. These cells benefit from the advantage that they have been extensively studied and are therefore very well characterized. In addition, they preserve their liver morphology and are easy to maintain in culture, which makes it easier to carry out long-term studies [Citation29]. However, it is important to bear in mind that immortalized cell lines dedifferentiate in culture, which normally implies a loss of specific functions. Moreover, immortalized lines are derived from a single donor, thus the inter-donor variations that occur in iDILI cannot be studied.
Although there are several immortalized cell lines used in hepatotoxicity assays, the most used are HepaRG and HepG2. In a 2017 study by Xiugong Gao & Yitong Liu [Citation36], the differences at the transcriptomic level of several of these cell lines were compared and the authors concluded that the HepaRG line showed the highest similarity to PHHs. Moreover, they maintain many of the organotypic functions, including the expression of some drug-metabolizing enzymes and transporters (DMETs) (i.e. CYP450) at levels close to those of PHHs [Citation37]. This cell line has the characteristic that it can yield two cell types, mature hepatocyte-like cells (HLCs), and biliary epithelial cells (BEPs). It should be noted that although HepaRG cell cultures are the most similar model to PHHs in terms of metabolic studies, they seem to move away from them when studying the activation of apoptosis or necrosis, having reduced sensitivity in the detection of hepatotoxic drugs [Citation29].
On the other hand, HepG2 cell line has a low expression level of some DMETs, which is its major disadvantage compared to HepaRG cell line. However, this problem can be partially overcome by transforming HepG2 cells with adenoviruses containing some of the genes encoding for the proteins that are poorly expressed [Citation38–40]. HepG2 cells have been widely used to study various endpoints related to hepatotoxicity, such as cytotoxicity, mitochondrial damage or the bile salt export pump (BSEP), inhibition, as reviewed by Walker et al. [Citation26]. It is noteworthy that in some of the models shown, the specificity reaches values close to 100%. A large number of hepatotoxic drugs (e.g. inducers of reactive metabolite formation) have been tested in HepG2 and HepaRG cells, such as amodiaquine, clozapine, diclofenac and isoniazid [Citation41].
The use of these cell cultures is very common in hepatotoxicity assays attempting to assess iDILI risk, as they are widely available. We believe that, although their characteristics do not exactly mimic the in vivo liver ones, they constitute a good model to study this disease.
3.1.3. Induced hepatocytes: targeting interdonor variability
As mentioned before, one of the major drawbacks of immortalized cell lines is that all cells come from the same donor, thus the factors that are dependent on the individual are not taken into account. To overcome this problem, one of the existing possibilities is to use cells derived from different donors and, through different techniques, transform them into induced hepatocytes. These techniques can be categorized into two groups, human-induced stem cell-derived hepatocyte-like cells (hiHLCs) and transdifferentiation-induced hepatocytes (hiHeps). The characteristics exhibited by these cells have recently been reviewed by Jin et al. [Citation42].
The hiHLCs group includes hepatocyte-like cells derived from three types of stem cells, human embryonic stem cells (hESCs), human induced pluripotent stem cells (hiPSCs) and human mesenchymal stem cells (hMSCs). In Jin et al.’s review, it is discussed that the current methods to obtain hiHLCs only manage to obtain cells that show a phenotype far remote from PHHs at several levels, such as drug metabolism or gene expression. Although scientists are striving to improve the characteristics of these cells, it is necessary to establish a consensus on the differentiation protocols to obtain cells useful for modeling hepatic toxicity.
On the other hand, first transdifferentiation-induced hepatocytes were obtained through direct reprogramming of fibroblasts (as established by Huang et al. in 2014 [Citation43]). Although hiHeps become more mature than those obtained by stem cell differentiation, they are still functionally distant from PHHs, but some strategies are being developed to make up for this shortfall. Fibroblasts can be differentiated into liver progenitor cells with unlimited propagation capacity which later differentiate into hiHeps, acquiring characteristics much more similar to those of PHHs, and being able to identify the hepatotoxic potential of some drugs [Citation44]. As an alternative, Fu et al. [Citation45] established a protocol to obtain progenitor cells from PHHs (which could then differentiate back into hepatocytes), but could not maintain them in culture indefinitely, since they suffered abnormalities at high passages.
Thanks to this strategy, we believe that this model will constitute a paradigm shift in iDILI research. When these cell cultures are perfected, it will be feasible to obtain liver models from patients directly, without the need to perform a liver biopsy. In the preclinical field, these cultures might become useful predictive models for hepatotoxicity. Furthermore, we believe the use of these cells will make it possible to move towards personalized medicine, being a useful tool that can be used in the clinic as a prognostic method.
3.2. Moving forward in complexity: hepatocytes-non parenchymal cells cocultures in 2D
Although most liver cells are hepatocytes, non-parenchymal cells (NPCs) account for 20% of the liver mass. These include Kupffer cells (KCs), hepatic stellate cells (HSCs), and liver endothelial cells (LSECs), among others. Therefore, when it is intended to use in vitro models that more closely resemble the situation in a real liver, one of the options available is to coculture some of these cell types together with hepatocytes.
Recently, Tasnim et al. [Citation46] reviewed the most commonly used systems for liver cells coculture. This article highlights that, in addition to the cell type used, the disposition of the different cell types also affects the performance of the in vitro model. Among the different possibilities, the authors define three types: 1) mixed cultures on a single surface area; 2) transwell cultures, where the different cell types are separated by a membrane that allows the exchange of soluble substances; 3) and micropatterned cocultures (MPCCs), where hepatocytes grow over an extracellular matrix and the rest of the cell types grow surrounding them.
The review also discusses that cocultures of HSCs with KCs and PHHs have the advantage of improving sensitivity in hepatotoxicity testing compared to that of the PHHs monoculture, when treating both of them with drugs such as amiodarone, bosentan, fialuridine and tolcapone. The authors finally describe a new model under development that looks very promising: coculture of hepatocytes and immune cells derived from iPSCs.
Although the characteristics of the hepatocytes are improved with respect to the monoculture, the hepatic cell types used are the ones explained in the previous section, so the disadvantages remain the same (scarcity of PHHs donors, no inter-patient variability in hepatoma-derived cell lines, etc.). We would like to highlight a very interesting article carried out by Ware et al. in 2015, in which the authors engineered a MPCC using HLCs from multiple donors and murine fibroblasts, and performed hepatotoxicity assays achieving sensitivity values (65%) very similar to those obtained with PHHs MPCCs (70%). This gives us a hint as to what might be the way forward in this area, as many of the problems associated with using PHHs could be avoided [Citation47].
3.3. Improving liver functions: 3D cell configurations in iDILI modeling
Spatial configuration is essential for hepatocytes to execute their physiological functions, including those related to drug metabolism. 2D cultures fail to provide this setup, which translates into a less mature phenotype, poorer metabolizing capacity, reduced longevity, and ultimately, limited ability to model iDILI accurately. Regardless of the source of the hepatocytes (PHHs, hepatoma-derived or HLCs), 3D in vitro cultures have been shown to improve liver functions and drug-metabolizing capacity over 2D. These structures may include other NPCs in addition to hepatocytes, better recapitulating the native physiology of the liver. In the case of PHHs, 3D configuration produces an extension cell lifespan, which is traditionally a major limitation in the use of these cells for modeling iDILI [Citation48,Citation49].
The 3D structures most successfully used for toxicity testing are the ones either formed by typical organ development and chemical processes (organoids) or by aggregation of cells (spheroids) [Citation50]. For example, PHHs spheroids showed improved sensitivity and specificity (69 and 100 %, respectively) in a hepatotoxicity screening assay of 123 drugs [Citation51]. More recently, Shinozawa et al. [Citation52] developed a sophisticated methodology, by which high predictive values were achieved by measuring viability, cholestatic and mitochondrial toxicity on pluripotent stem cells-derived human liver organoids (HLO) treated with 238 different drugs. Following a similar approach, a recent work applied omics and morphology techniques for iDILI risk prediction using HLOs [Citation53].
Establishing proper scaffolds for the 3D configuration is important to design a good model, from the formation of an extracellular matrix to sandwich configurations. For example, a carefully designed collagen-based 3D PHHs model, consisting of three different layers, demonstrated superior predictive sensitivity for 122 drugs compared to all previously reported in vitro models [Citation54]. Recently, Liu et al. [Citation55] designed a model system providing biomatrix scaffolds to liver spheroids showing remarkable results in toxicity screening assays of iDILI drugs. To achieve better in vitro architectures, bioprinting technologies also seem to be a powerful tool. Bouwmeester et al. [Citation56] combined human liver-derived epithelial organoids with a biofabrication approach, developing liver bioprinted constructs very promising for toxicity testing.
IDILI 3D cultures have enabled better integration of complex optical technologies into in vitro disease modeling. Cell imaging has been used to evaluate hepatotoxic responses and to monitor the cell responses during drug exposure [Citation57,Citation58]. In addition, 3D cultures have been pioneers for testing drug-induced liver fibrosis by using compounds such as methotrexate [Citation59]. We believe that 3D configurations are going to be the most powerful tools for in vitro modeling. However, despite all the promising potential of these in vitro tools commented above, most of them have not been incorporated into industry protocols yet, and there is a need for more comparative and validation analyses with better integration of NPCs and immune cells.
3.4. Non-typical in vitro models: closer to liver physiology
In order to gain a better understanding of the physiology and pathophysiology of the liver, in vitro models have to recreate the hepatic microenvironment.
In the last few years, efforts have been made to emulate the human liver with the organ-on-a-chip technology. Microfluidic devices called liver-on-a-chip systems are used to culture diverse hepatic cells in micrometric compartments with constant fluid flow to mimic the physiological processes of the liver. These systems allow an in vivo-like dynamic flow, the manipulation of spatial and temporal parameters and mimic liver zonation. However, manufacturing these systems is complex and expensive, since many different parameters such as oxygen and chemical gradients, medium flow, mass transfer of nutrients, mechanical forces, cell-cell interactions, etc. should be taken into consideration [Citation60]. Several groups have created different livers-on-a-chip to detect adverse reactions to drugs and to study drug metabolism. Chao Ma et al. [Citation61] described a 3D liver lobule-like microtissue that better reproduced the metabolism of three iDILI drugs (APAP, isoniazid, and rifampicin) than other conventional in vitro models, measured by comparing the activity of CYP1A2 and uridine 5’-diphospho-glucuronosyltransferase (UGT) enzymes. Moreover, this liver-on-a-chip could detect different adverse reactions of drug-drug interactions previously reported. More recently, Rubiano et al. [Citation62] showed that the liver-on-a-chip configuration with primary hepatocytes and different batches of KCs could reproduce the hepatotoxic effects of trovafloxacin.
Individual organs-on-a-chip can be joined together and form multi-organ systems to account for multi-organ impacts on a compounds toxicity. These systems can model important processes including drug absorption and metabolism, as well as organ-organ interactions. For example, Bricks et al. [Citation63] developed a microfluidic biochip integrating different compartments, simulating the intestinal unit (Caco-2 TC7 cells) and the hepatic unit (HepG2 C3A cells). This multi-organ-on-a-chip was able to model the transport of the drug phenacetin through the intestine unit and its biotransformation into paracetamol in the hepatic unit.
However, although the use of organs-on-a-chip is very promising, precision-cut liver slices (PCLS) are currently thought to be the most physiologically accurate in vitro model for liver research [Citation64]. PCLS preserve tissue architecture and includes all types of liver cells, maintaining cell-cell interactions. However, in static culture systems, PCLS show a rapid loss in hepatic functioning. Currently, the functional lifetime of PCLS can be extended using microfluidic devices [Citation65]. Diseases’ pathophysiology can be studied in slices from healthy PCLS exposed to toxic stimuli such as drugs, herbs, or reactive metabolites, in the case of DILI. In this sense, Hadi et al. [Citation66] developed an iDILI model using human PCLS co-incubated for 24 h with iDILI-related drugs (ketoconazole, clozapine) and lipopolysaccharide (LPS). The combination of both drugs with LPS reduced glutathione (GSH) levels in PCLS while increasing LPS-induced tumor necrosis factor (TNF) release, suggesting that PCLS could help determine the contribution of inflammation in iDILI development.
3.5. The role of the immune system in iDILI. Can it be addressed in vitro?
The multifactorial development of iDILI makes its modeling using cell cultures challenging. However, the immune system is thought to play a key role in the development of the disease, so a great effort is being made to integrate the communication between the liver cells, that will secrete DAMPs when exposed to hepatotoxicants, and the immune cells, that will sense these molecules and elicit a response.
In this regard, several interesting studies have been conducted, such as the one by Oda et al. in 2016 [Citation67]. HepG2 and HepaRG cells were treated in monoculture with a large number of iDILI-causing drugs (such as diclofenac or isoniazid). The conditioned medium (after drug exposure) was put in contact with HL-60 immune cells, and the response of these cells was measured at a transcriptional level, quantifying the expression of genes associated to immune activation. The assay obtained very high sensitivity values, between 80 and 96%, while specificity was lower, between 51 and 63%. We believe that this is a very interesting model, which has served as a reference for the development of new studies using other more physiologically relevant cell types and cultures.
In 2017, Kato & Uetrecht [Citation68] investigated the effect of amodiaquine and nevirapine on the DAMPs production capacity of hepatic spheroids derived from the FLC-4 line (hepatocellular carcinoma), as well as the ability of these DAMPs to activate the inflammasome in THP-1 macrophages. They found out that, at least for some iDILI-causing drugs, there is a direct connection between the release of DAMPs, the inflammasome activation and the subsequent immune response. Also in 2017, Ogese et al. [Citation69] used a similar approach, exposing monocultures of PHHs to amoxicillin, flucloxacillin or isoniazid and using the supernatant to investigate their effect on a co-culture of dendritic cells and naive T cells.
A well-known approach to apply in vitro modeling of iDILI into the clinics is the use of patient‐derived cells, such as PBMCs. In this sense, the lymphocyte transformation test (LTT) has historically proven helpful to diagnosing drug hypersensitivity in vitro [Citation70]. Recently, a modified LTT measuring granzyme B and different cytokines was proposed to diagnose iDILI associated with an adaptive immune response and identify the responsible drug. However, except for one patient who was treated with isoniazid, this strategy was unable to reliably demonstrate causality [Citation71].
In conclusion, there are already in vitro models that serve to study the relationship between the immune system and liver cells, although there is still room for improvement. In fact, we believe that one of the most interesting approaches would be the use of cells derived from patients who have had an episode of iDILI, both HLCs (or even PHHs if a liver biopsy is performed), NPCs, and immune cells, since they can constitute models that reproduce many of the factors that cause iDILI. In addition, we believe that direct diagnostic applications for the clinic can be derived from these approaches. An example of this would be the establishment of a system in which a hepatic culture is exposed to the drug suspected of having produced iDILI and the conditioned supernatant is exposed to immune cells from the patient. Analyzing the response could potentially lead to the identification of the causative agent of the episode and the achievement of a certain diagnosis of iDILI in those cases where other concomitant medications represent a challenge.
4. In vivo models of iDILI
One of the main challenges for modeling iDILI is the limited knowledge of the mechanisms behind the disease. For progressing in the understanding of the physiopathology and being able to reproduce and predict iDILIs multifactorial nature, various efforts have been made to develop relevant animal DILI models. In theory, in vivo models can reflect in a more holistic way the different variables involved in the onset of iDILI clinical manifestations, such as the relationship between different organs, or the role of the immune system.
Leaving aside mechanistic studies based on the administration of high hepatotoxic doses of drugs to rodents, such as the APAP or carbon tetrachloride (CCl4) intrinsic DILI models [Citation72,Citation73], probably the most straightforward in vivo strategy to model iDILI would be to use a large panel of mice that includes sufficient genetic variability to ensure the presence of disease susceptibility factors, mimicking the heterogenicity found in the human population. This model might seem like a good strategy to approach the interpersonal variability, low incidences, and unknown mechanisms. However, it is highly probable that if this approach were applied to current preclinical drug research protocols, it would not be robust enough to predict adverse effects in humans due to species differences and lack of reproducibility. Indeed, only partial success was obtained when this approach was used to try to model APAP or isoniazid-iDILI [Citation74,Citation75]. In addition, this model seems unpractical for the number of animals needed, low fidelity and lack of validation on iDILI drugs.
Therefore, more refined in vivo modeling iDILI strategies have been developed. Some approaches are aimed to study specific endpoints of the disease, such as the immune system or oxidative stress, through genetic or pharmacological manipulation of the animals. Other more complex strategies contemplate the humanization of rodents to include variables specific to human iDILI ().
Table 1. Summary of main advantages and disadvantages of in vivo iDILI models.
4.1. Models of immunological features
Increasing evidence indicates that a pro-inflammatory situation is conducive to the onset and progression of iDILI [Citation76]. Following this hypothesis, the inflammagen murine model has been able to reproduce some degree of liver injury using non-toxic iDILI drug doses, for example, amiodarone or diclofenac. The main principle of the model consists in the application of the drug in combination with bacterial LPS, which promotes an inflammatory reaction by binding to Toll-like receptors (TLR), resulting in an increased susceptibility to develop iDILI [Citation77,Citation78]. The major limitation of this model is that the immune response observed is mainly mediated by neutrophils, excluding other typical iDILI immune features, such as the role of monocytes or the adaptive immune system. In addition, although the results of these studies are impressive and may have opened the door to formulate new theories about the inflammatory origin of iDILI, the endotoxemia hypothesis has not been confirmed for most drugs causing iDILI in humans.
In this sense, a model that may allow a better understanding of immune reactions of human iDILI is the immune tolerance suppressed model. The model is based on the disruption of the livers inherent high immunological tolerance [Citation79]. To achieve this goal, many authors specifically target two immune checkpoints, PD-1 and CTLA-4, which are key factors in the maintenance of immune tolerance. The technique consists of using PD-1 knockout mice, which are treated with anti-CTLA-4 antibodies in combination with the drug under study. Although with restrained severity, this model has been able to simulate the liver damage produced by drugs such as amodiaquine, isoniazid, nevirapine or green tea extracts [Citation80,Citation81]. Additionally, it has been able to discern iDILI drugs from non-iDILI drugs [Citation82]. In all cases, mononuclear cell infiltration of the liver was observed, and CD8 T cells played a key role in the injury. Although there is no clear evidence that iDILI in humans involves a breakdown of liver immune tolerance for most drugs, there is a growing concern for immuno-checkpoint inhibitor antitumor drugs. These immunotherapies are showing great success in cancer treatment, but at the cost of an increased risk of iDILI [Citation83]. This model may be a promising tool for the study of safer immunotherapies against cancer.
Non-altered mice have also been used to study the mechanisms of drug-induced hepatotoxicity in a dose-dependent manner. In this way, the role of some immune factors in liver damage induced by diclofenac or carbamazepine has been further clarified [Citation84,Citation85]. In addition, there is currently no approved pharmacological treatment for iDILI, and the best option is still discontinuation of the culprit drug. In the case of intrinsic injury caused by APAP, animal models are greatly contributing to the search for therapeutic compounds, such as methane, ceria nanozymes or Poria Cocos polysaccharides, which have recently shown hepatoprotective effects in APAP-DILI mice models [Citation86–88].
4.2. Models of oxidative stress features
The plethora of oxidative stress-related processes manifested in iDILI can range from the uncontrolled accumulation of ROS, mitochondrial dysfunction, or endoplasmic reticulum (ER) stress of hepatocytes [Citation25]. A strategy to model iDILI by attending to the in vivo role of oxidative stress is to directly affect the generation of ROS or the antioxidant pathways of the hepatocytes. For example, genetically altered mice with a heterozygous deficiency in mitochondrial superoxide dismutase (Sod2) have been used to achieve increased sensitivity to liver injury by trovafloxacin or troglitazone [Citation89,Citation90]. In both cases, the mitochondrial aconitase activity of the mice was decreased, affecting mitochondrial function. In these mice, an increase in mitochondrial GSH levels was detected, as an attempt to compensate for the oxidative damage generated. Precisely, another strategy to model iDILI in vivo consists of targeting GSH levels, reducing the antioxidant potential of hepatocytes under stress. The most prominent strategies to reduce GSH in mice are based on knocking out the enzyme g-glutamylcysteine synthetase (g-GCS), responsible for the de novo synthesis of GSH, or its pharmacological inhibition in healthy rodents [Citation91]. GSH depletion has been used as a strategy to successfully model flutamide-induced hepatotoxicity in rats [Citation92]. Regarding ER, alterations in the ER stress signaling pathway in mice have been useful in determining the involvement of this phenomenon in iDILI. For example, knock-out mice for CHOP (a pro-apoptotic gene regulated by ER stress) were used to demonstrate the role of ER stress in APAP-DILI [Citation93].
Clearly, however, the independent approach to the role of oxidative stress is not sufficient to fully model iDILI. A recent study succeeded in establishing a mouse model of azathioprine-induced liver injury, demonstrating that, in addition to oxidative reactions, inflammatory processes contributed to the later stages of iDILI by this drug [Citation94]. More comprehensive approaches allow a better understanding of the disease and direct research towards the development of possible therapies. One way to achieve a higher degree of complexity in iDILI modeling is to reduce interspecies differences in murine models through humanization.
4.3. Humanized models
Humanization is probably the most forward-looking strategy to address interspecies issues while considering more accurately host-dependent factors in iDILI using in vivo models. The generation of human-like liver functionality in rodents is typically achieved by the engraftment of human cells into an immunodeficient host or by generating transgenic mice carrying human drug metabolism genes or susceptibility factors.
The opportunity to use PHHs or HLCs from selected donors as a source of hepatocytes to humanize mice enables the inclusion of specific human factors of interest in murine models in a very personalized approach. For example, reconstitution of murine livers using cells from donors with different CYP450 genotypes produced metabolites expected for each genotype studied after diclofenac treatment [Citation95]. These models appear to have great potential for drug development, which became evident in the case of fialuridine. TK-NOG chimeric mice with humanized livers were able to detect hepatotoxicity by this drug, which caused high toxicity in humans during clinical trials, but normal animal models failed to detect in preclinical studies [Citation96]. Although it is still an approach that is not widely implemented, chimeric mice are increasingly being used in pharmacological studies [Citation97]. Chimeric mice have been particularly valuable at predicting cholestatic injuries and might be option when considering modeling this type of injury. For example, TK-NOG mice with humanized liver predicted bosentan-induced cholestatic liver toxicity, a phenomenon that could not be predicted by other animal models [Citation98]. PXB-mice®, a commercially available mice strain with humanized liver, recently served to detect compounds with cholestatic iDILI potential among 45 different drugs with high sensitivity and specificity [Citation99].
The previously cited models miss many of the immuno-mediated reactions consequence of using immunodeficient mice. Therefore, a step forward in the refinement of these models in the context of iDILI is the double humanization, that is, obtaining mice with both human-like liver and immune system. One of the pioneers’ models based on the simultaneous introduction of human hepatocytes and human CD34+ hematopoietic precursors was achieved by Washburn et al. [Citation100], and since then, different mice strains and strategies for cell engraftment have been developed. The primary utility of these models was intended for studies of viral infections, such as hepatitis C virus (HCV) or human immunodeficiency virus (HIV), since the lack of thymus humanization and relatively low rates of liver repopulation make them less optimal for iDILI studies. To achieve the human functionality of T cells, the humanized bone marrow-liver-thymus (BLT) mouse was developed. In addition to liver cells and hematopoietic precursors, these mice are also transplanted with human thymus stem cells. This model demonstrated the production of human chemokines in hepatocytes and maintained co-localization of human immune cells in the mouse liver, such as macrophages, dendritic cells, NK cells and other T cells [Citation101]. BLT mice have been used to study adverse effects produced by the drug nivolumab, an immune-checkpoint inhibitor known to trigger hepatitis [Citation102], or muromonab, known to cause cytokine release syndrome, an event which usually is not detected in preclinical in vitro and in vivo procedures [Citation103].
Besides human cells engraftment, genetic humanization of rodents through the introduction of single or multiple human leucocyte antigens (HLAs) alleles, or genes involved in human DMETs pathways has been useful for toxicological studies and has provided insight into the mechanisms underlying iDILI for various drugs [Citation104,Citation105]. However, it should be noted that in some cases these are not sufficient to replicate the disease, and the entire genetic background of the mouse is still very influential [Citation106].
4.4. Non-rodent models
Although rodents are by far the most widespread animal models in iDILI research, other animal-based models such as zebrafish, sheep, pigs, dogs, and monkeys have also provided useful insights into the disease. Interestingly, in some cases, dogs have shown to be a better predictor of human iDILI than rodents [Citation21]. However, the use of higher vertebrates carries a greater economic cost and ethical burden.
One of the simplest in vivo models described for approaching iDILI is the zebrafish (Danio rerio). The advantages of using these organisms to model iDILI over rodents were already discussed by Vliegenthart et al. [Citation107]. Briefly, although the zebrafish liver is physiologically different from that of humans, its similar cellular and subcellular processes allow the study of iDILI from a metabolic, histological and biomarker point of view, at a much lower cost and simplified manipulation. Zebrafish have been shown to possess human-like metabolic reactions for several drugs, such as the formation of the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) after treatment with APAP [Citation108]. To test whether zebrafish could be a good early-stage screening tool, studies exposing the larvae or adult zebrafish to multiple hepatotoxic and non-hepatotoxic drugs, have demonstrated high specificity and predictivity [Citation109,Citation110]. Using imaging and fluorescence techniques, transgenic zebrafish models have also been used to investigate the mechanisms of iDILI by drugs such as isoniazid and tetracycline and explore new hepatoprotective compounds [Citation111,Citation112].
Despite the practical convenience in using zebrafish as an iDILI model in vivo, the inter-species differences will be greater than using other mammals closer to humans. In addition, exposure to drugs by dissolving them in water may be inaccurate and some genes related to drug metabolism in zebrafish are regulated by the circadian cycle, which may affect the results [Citation107]. In addition, the involvement of the zebrafish immune system in iDILI is still unclear. Therefore, the usefulness of this model may rely on its ability to complement other in vivo or in vitro studies.
In conclusion, there are many examples where animal models are indispensable for the detection of adverse drug reactions and pharmacokinetic studies in the preclinical field. In the case of iDILI, the usefulness of these models has been mainly focused on intrinsic reactions. Indeed, although numerous models have been capable of modeling certain aspects of iDILI physiopathology, they fail to predict disease or correlate results with human studies. The reason for this poor predictive potential could be due to inter-species differences, but also because experimental designs still generate conditions far removed from the complexity involved in the occurrence of idiosyncratic injury in humans. A clear example is the case of troglitazone, where rodent studies did not show sufficient evidence of danger signals for iDILI risk in humans, while in vitro studies were able to echo this hazard risk [Citation113]. This demonstrates that, despite the efforts made in recent years, there is still a long way to go to refine and standardize animal models before they can be routinely implemented for iDILI detection in preclinical guidelines.
5. In silico models to predict iDILI
The term ‘in silico’ related to modeling refers to computer-based approaches to perform experimentation through the creation of computational models that could be used to make predictions, suggest hypotheses or become the input for further analysis that could complement in vivo and in vitro research [Citation114]. Specifically, in silico models in iDILI are used to predict the potential for a single or several drugs to produce hepatotoxicity.
In silico models are generally more controllable and less expensive than in vivo and in vitro models, and can be used to screen a large number of drugs within a short time even before they are isolated or synthesized [Citation115]. Despite that, iDILI-specific in silico models are currently scarce – as most of the models have been developed for intrinsic properties of the molecule and not for complex endpoints due to the lack of understanding of mechanisms of idiosyncratic hepatotoxicity – and the validity of the majority has not yet been demonstrated [Citation116].
The strategies for in silico hepatotoxicity prediction have been mainly based on the chemical structure of the drug, and can be divided into statistical and expert system approaches. In the first case, models are built from a training dataset of structures and their associated toxicity data, using an automated algorithm to obtain quantitative models for hepatotoxicity prediction. In the second one, the use of the understanding of the experts about toxicological mechanisms is employed to outline the relationship between biological activity and chemical structure, and it results in the development of not statistically-derived models such as structural alerts and pharmacophores [Citation117–119].
Statistical models are usually called quantitative structure-activity relationships (QSAR). Their development is faster than that of an expert system – since it requires the integration and study of literature sources so they are evidence-based [Citation119] – and they allow the study of a huge amount of drugs and drug-like compounds simultaneously. Over the years, these systems have been improved through the training with several toxicity data (e.g. from the US FDA Human Liver Adverse Effects Database [HLAED] [Citation120]). In actual fact, QSAR-based models can perform sensitivities from 39 to 89 % [Citation121], specificities from 65 [Citation122] to 94 % [Citation120][] and accuracies from 59 [Citation122] to 84 % [Citation120] for predicting iDILI. However, QSAR models may be sometimes incapable of capturing the complex mechanisms of iDILI, as they are built based only on the physicochemical properties of the drug without taking other factors into account [Citation123]. The most frequent statistic algorithms in these models are discriminant analysis, Artificial Neutral Networks [Citation124], Bayesian models [Citation114], k-Nearest Neighbor, Random Forest [Citation125] and specialist QSAR software [Citation121][]. Indeed, some of them are based on new artificial intelligence approaches to perform more accurate predictions on DILI [Citation126].
An example of an expert-based approach is ‘evaluation of drug-induced serious hepatotoxicity’ (eDISH), released in 2004 by the FDA which uses biochemical liver values from patients that have taken a given drug for assessing the dangerousness of it and, lastly, for preventing the cancellation of the trials of a safe drug [Citation117]. Also, there exist some hybrid models that combine statistical approaches with clinical data and features of expert models, such as DILIsym® software. It is built based on literature and/or experimental analyzed data and simulates possible mechanistic interactions and phenomena between a drug and the liver, such as inhibition of BAs efflux or oxidative stress. This software has provided important progress for in silico models to predict iDILI [Citation106].
Although the chemical structure of the drug is the information more commonly used for generating in silico iDILI models, genomic and transcriptomic profiles of iDILI patients, as well as cell and tissue images (i.e. presence of specific features like mitochondrial damage and oxidative stress in PHHs cultures) can be useful to complement and improve these predictive models [Citation96,Citation126]. In connection with this idea, Koido et al. [Citation127] developed a ‘polygenicity-in-a-dish’ approach to evaluate iDILI risk. The authors established a polygenic risk score (PRS) for iDILI by combining the effects of multiple previously reported genome-wide loci linked with iDILI risk and cellular hepatotoxicity activation mechanisms, such as oxidative stress.
In silico models have a higher false-positive rate rather than a false negative rate to prevent the release of dangerous drugs into the market. However, this strategy could be detrimental for the pharmaceutical industry, since it can stop the development of a harmless drug [Citation124]. Therefore, more balanced in silico models are crucial to obtain an accurate prediction of iDILI potential for a given drug. Due to the multifactorial nature of iDILI, more clinical data from well-phenotyped patients are essential to designing better models. In this sense, databases collecting suspected cases of iDILI are critical. Current iDILI Registries and Networks are summarized in .
Table 2. Principal studies, databases and registries of iDILI cases.
6. Omics technologies in iDILI modeling
Since there is not a single mechanism of action of a certain drug to induce iDILI, -omics techniques that consider one aspect of the cellular biology globally are needed to better understand the complex pathophysiology of this entity.
The study of the influence of genotypes in iDILI episodes, mainly by genome-wide association studies (GWAS), has identified multiple HLA haplotypes associated with increased risk of iDILI development. These associations are generally drug-specific. Currently, HLA alleles with strong iDILI associations are HLA-B*57:01 for flucloxacillin-iDILI [Citation138] and abacavir-iDILI [Citation139] and HLA DRB1*15:01-DQB1*06:02 for amoxicillin-clavulanic-associated hepatotoxicity [Citation140–142]. Interestingly, a recent GWAS study identified a novel association between HLA-A*33:01 allele and iDILI risk due to different drugs such as terbinafine, fenofibrate, and ticlopidine [Citation143]. Some of the identified HLA risk alleles for iDILI have a negative predictive value (NPV) > 95%, which enables the use of HLA genotyping to rule out iDILI diagnosis. However, usually HLA risk alleles have a very low positive predictive value (PPV), which restricts the use of genetic testing in clinical practice.
Moreover, the single nucleotide polymorphism (SNP) rs2476601 of the protein tyrosine phosphatase non-receptor type 22 (PTPN22) gene has been recently associated with iDILI due to different drugs [Citation144].
However, transcriptomics and/or metabolomics are the most used strategies to develop a more accurate predictive or prognostic model of iDILI, as these techniques are able to account global cellular changes elicited by specific drugs.
To understand the pathogenesis of iDILI, it is of vital importance to identify what changes in gene expression potential hepatotoxic drugs induce. However, up to now only a few in vitro models have been established for predicting human iDILI based on transcriptomics data [Citation145,Citation146]. Although these studies were able to distinguish hepatotoxic drugs from non-liver-toxic compounds based on transcriptome expression analysis of hepatoma cells or hepatocytes, it is important to take into account that under physiological conditions, liver NPCs can also suffer drug toxicity and secrete DAMPs that could affect transcriptome of hepatocytes. Therefore, cocultures of liver NPC-PHHs would be potentially more useful for toxigenomics analysis in iDILI.
In the context of liver damage, transcriptomics of extracellular vesicles (EVs) is gaining great importance, since miRNAs are the molecules associated with EVs that are showing the most promise as biomarkers for liver disorders [Citation147]. A recent study describing miRNA changes in sera of subjects with iDILI showed that miR-122 was the most significantly elevated in iDILI subjects compared to controls [Citation148]. However, although miR-122 is high liver-specific, its levels can be associated to liver injuries with different aetiologies. Moreover, there is a lack of preclinical studies to understand the function of EVs and their content in iDILI.
On the other hand, metabolomics is an emerging technology that holds promise to initiate precision medicine in iDILI, enhancing also the detection of novel biomarkers [Citation149]. The first group to ever apply metabolomics to in vitro modeling of a human cell line were Ruiz-Aracama et al. [Citation150]. Since then, metabolite profiles of human hepatic cells treated with potential hepatotoxic drugs have revealed patterns of metabolome changes that are consistent for different liver toxicity mechanisms [Citation151,Citation152]. Very recently, Quintás G. et al. [Citation153] performed a metabolomic analysis of serum samples from cholestatic, hepatocellular, and mixed iDILI patients, in order to find potential biomarkers to detect and distinguish the different iDILI clinical phenotypes. The authors observed that BAs and glycerophospholipids were the most relevant metabolites to discriminate among the cholestatic and hepatocellular iDILI phenotypes. Moreover, metabolomic data together with information about severity and progression of the iDILI episode was used to build a bioinformatics model for a precise monitoring of an iDILI case.
Another important area of research in iDILI is the role of human intestinal microbiota. Drugs can alter intestinal microbiota and increase intestinal permeability, releasing bacterial products into the blood stream, which act as costimulatory signals for the innate immune system activation [Citation154]. Microbiota diversity has been suggested as a potential iDILI risk factor that could influence susceptibility and outcome. In fact, it has been hypothesized that dysbiosis could be a reason for the high number of detected iDILI cases induced by antibiotics, which are known to cause temporal alterations in the microbiota [Citation13]. Analyzing microbiota variability in iDILI through next generation sequencing would provide new mechanistic hypotheses, facilitate iDILI diagnosis and establish profiles of individual hepatotoxicity risk. It could also provide information on new potential targets for personalized iDILI treatments, an area that has had limited therapeutic advances in recent years.
Although the characterization of the diversity and richness of bacterial communities in iDILI patients are of great importance, these results may provide limited information on the phenotypic consequences of variations in the intestinal microbiota. This is why new technologies, such as stool metabolomics, are being applied to expand the study of the microbiota and focus not only on composition but also on microbial function.
7. Conclusions and future directions
In this review, we intended to summarize the current preclinical approaches in iDILI modeling and the future directions in the field. The different experimental designs stablished so far to model intrinsic DILI have been increasing in complexity in order to be useful to study iDILI. However, given the large number of drugs causing iDILI that evade detection in current preclinical testing, new models for predicting iDILI and determining the molecular pathways that cause the disease are necessary.
In silico approaches to predict hepatotoxicity can be used before preclinical studies, reducing the cost of iDILI risk assessment and allowing the analysis of multiple chemical compounds at once. However, since both the features of the drugs and the specific aspects of the patients must be taken into consideration, there is still a shortage of well-validated computational models for iDILI prediction. Developing predictive models using the most advanced approaches introduces additional challenges in terms of collecting more datasets to test the models, hence a coordinated effort is being undertaken to improve iDILI registries.
Because of iDILIs complexity, future directions in the field lead to a mix of in silico drug behavior modeling and in vitro drug testing on human cell lines. However, even the most sophisticated in vitro systems lack several useful characteristics that are required to accurately predict iDILI.
For this reason, in vivo models are a crucial component of the iDILI preclinical research. There is currently no commonly accepted animal model to predict iDILI, given the difficulties in establishing a link between animal indicators of hepatotoxicity and human iDILI [Citation155], as well as the great variability in drug absorption, distribution, metabolism and excretion between species. Humanized mice models are considered to be the most promising for investigating iDILI since they can detect human-specific liver injury while also producing human-specific metabolites in vivo [Citation156]. Specifically, mixed humanized mouse models, which combine both humanized liver and immune system are emerging to detect human-specific iDILI and could be a successful strategy in the future.
8. Expert opinion
Current preclinical models do not fully replicate the complexity of human iDILI. Although animal models could provide insight into the pathophysiology and biomarkers of iDILI, establishing animal models to generate predictive screening assays is really challenging. From our point of view, a preferable strategy would be to develop more complex and physiological in vitro models. Nevertheless, the growing number of advanced hepatic in vitro models demands harmonization of the in vitro approaches used to identify iDILI risk. The ultimate goal in iDILI modeling field would be establishing reproducible protocols and decision points to elaborate regulatory guides.
The main critical task which is missing would be to correctly classify the reference drugs used in iDILI preclinical assays. Moreover, achieving a unified criteria about the range of concentrations to use and consensus guidelines to interpret the results of preclinical studies has to be a priority for translational researchers [Citation157]. Establishing sets of reference drugs categorized by their iDILI concern, on the other hand, is one of the key challenges in the field due to the limited number of afflicted individuals and the variability of phenotypic presentation.
The drug concentrations and time of treatment are necessary to be standardized too. Short-term incubation systems are commonly employed in prospective compound testing, although longer-term incubation strategies may better reflect the nature of iDILI. However, there is still no agreement on how to choose the concentrations to test and the assessment time points. Since most hepatotoxic drugs exhibit considerable cytotoxicity within the 100-fold maximum plasma concentration (Cmax) range, using multiples of the Cmax of the studied drugs is currently a practical approach [Citation32]. This strategy, on the other hand, presupposes that the ratio of test drugs in blood or plasma in vivo is the same in cell culture medium in vitro [Citation158]. Furthermore, such a wide dose range within the same drug may reflect various toxicity pathways [Citation30].
On the other hand, it is critical to define what mechanistic endpoints relevant to human iDILI are best represented in each in vitro system [Citation159]. It is also necessary to select reference drugs related to the specific mechanisms, if known, by which they produce iDILI (e.g. valproic acid produces mitochondrial damage). A change of paradigm is crucial, since cytotoxicity should not be the principal endpoint to detect iDILI; the release of DAMPs that eventually activates immune system response requires liver cell stress activation, but not always liver cell death. For this reason, other advanced endpoints such as formation of CRM, alterations of BAs homeostasis, mitochondrial damage, ER stress, oxidative stress, immune system activation or different -omics approaches (e.g. RNA-seq, epigenetics, and proteomics) could further support the better prediction of hepatotoxicity. Therefore, a consensus guideline of a series of in vitro tests and endpoints elaborated by teams of international experts is crucial for the future of iDILI research.
Regarding this approach, in a recent study, Rusyn, I. et al. [Citation160] developed a consensus expert opinion on the Key Characteristics (KC) of known hepatotoxicants, intending to define a finite set of properties of agents known to affect the liver. The authors proposed that identifying KC of hepatotoxicants could help to better design cellular and molecular assays to model and predict iDILI. In this study, 12 KC were identified. Some examples of these consensus KC are a) it is reactive and/or is metabolized; b) causes the death of liver cells or c) triggers immune-mediated responses in the liver, between others.
This idea aligns with the general thought that a multiparametric-multi-model approach determining different endpoints and combining multiple in vitro assays (according to those KC involved in human iDILI) with an in silico modeling of drug behavior and clinical information from patients is a promising tool in iDILI research in the coming years. In a recent study performed by Sankalp et al. [Citation161], in vivo data in combination with in silico predictions models, based on in vitro experiments, were used for modeling hepatic steatosis.
The combination of the data obtained from preclinical assays with -omics information obtained from iDILI patients is going to be critical, since translating the knowledge gained from patients to cell-based systems could also provide clues about iDILI mechanisms. In line with this idea, new in vitro approaches must consider host-specific factors. The use of patient-derived cells in iDILI modeling would allow researchers to investigate the impact of host factors on disease progression.
The utilization and characterization of patient-derived HLCs, either by differentiation of hiPSCs [Citation162,Citation163] or by direct reprogramming of somatic cells [Citation43,Citation164,Citation165] are two promising approaches in iDILI research and translation of knowledge to clinical practice, which is the ultimate goal in the field. The use of these cells in combination with other cell types derived from the same patient may constitute a paradigm shift in personalized medicine applied to iDILI. Since the genetic background of these cells is identical to that of donor cells, panels of iDILI HLCs with particular genetic polymorphisms might be used to assess patient-specific liver adaptability after cellular stress, taking into account that iDILI in humans is greatly influenced by individual genetic traits. Moreover, patient-derived HLCs can be used to screen patient-tailored drugs. However, the high variability in DMETs activities in patient-derived cells hinders obtaining stable functional cells for their use in hepatotoxicity research.
Patient-derived cells as an in vitro model could also be critical in studying the role of the immune system in iDILI. Despite the general agreement on the immune systems crucial involvement in iDILI pathophysiology [Citation16,Citation68,Citation166], a huge limitation that prevent research in iDILI modeling is that there is no standardized screening technique for detecting drugs that could induce immune-mediated iDILI. Cell culture approaches targeted at analyzing primary T-cell responses to drugs have been established in recent attempts to examine the function of the immune system in iDILI in vitro. Usui et al. [Citation167] exposed a panel of 14 HLA-typed human donors to diverse drugs, showing that T-cell stimulation with some of these drugs was biased toward donors expressing specific HLA alleles.
Therefore, from our point of view, the future strategy for iDILI modeling should be patient-centered. Collecting genetic, metabolic, and biomarker data from large patient cohorts might be another option to create a ‘fingerprint’ characteristic of people at risk, allowing for improved patient stratification and/or the development of new mechanistic strategies to enhance iDILI in vitro evaluation. A recent study by Dirven et al. [Citation113] reinforces this idea. In such study, the authors investigated the role of preclinical studies in predicting human iDILI by applying three approaches: i) systematic literature review of in vivo studies on rosiglitazone or troglitazone (two anti-diabetic drugs with very different toxicological profiles); ii) in vitro data on troglitazone and rosiglitazone retrieved from the US EPA ToxCast database and iii) troglitazone‐ and rosiglitazone‐related iDILI cases retrieved from the World Health Organization (WHO) Vigibase. The authors found that neither published animal nor human research could accurately anticipate the potential of troglitazone to constitute an iDILI risk in people, whereas in vitro results could detect the risk. Their analysis shows that mechanistic approaches have a lot of promise to complement the insufficiently conclusive animal safety data. Moreover, from our point of view, new and refined preclinical in vitro models could be also determinant for the discover of specific biomarkers for iDILI and the achievement of a definite iDILI diagnosis in the clinics, which facilitates the choice of appropriate therapies for patients.
The use of patients’ data obtained by standard multi-omics approaches is helping to improve the design, predictivity, reliability, and reproducibility of in vivo, in vitro and in silico models for iDILI. However, from our point of view, iDILI modeling will evolve in the future thanks to emerging strategies allowing a more complete analysis of diseases’ pathophysiology, such as 1) high content imaging, which is performed to study multiple cell parameters (mitochondrial damage, oxidative stress, etc.) at the same time, using fluorescent probes; 2) single-cells technologies (i.e. transcriptomics of individual cells) which can be very useful when there is heterogeneity between cells, which is characteristic of hepatocytes and 3) coculturing different cell types to study the interaction between different hepatic cells and their specific function.
Article highlights
Idiosyncratic drug-induced liver injury (iDILI) is an unpredictable multifactorial condition whose underlying mechanisms are not well-known. The development and validation of iDILI models for prior-to-marketing drug hepatotoxicity testing is a growing necessity.
Preclinical detection of iDILI is a complex and poorly standardized process. Designing an appropriate model requires a solid understanding of the purpose of the study and the functional characterization to be determined.
A wide range of in vitro models is currently available for modeling iDILI. When choosing which model to use, the source of the cells, the cell types that will be involved in our culture and the model configuration have to be taken into account.
Conventional in silico iDILI models have been used to screen a large number of drugs within a short time, since they have been mainly based on the chemical structure of the drug. However, more sophisticated in silico models should include multi-omics data from iDILI patients and information obtained from in vitro experiments to improve their predictive potential.
Conventional in vivo models have provided important pathophysiological information about the disease, especially regarding immunological and oxidative stress features. Models of increasing complexity, such as humanized rodent models, are being able to better account for the multifactorial nature of iDILI, although their validation and utility on a large scale are still unclear.
In the near future, the development of preclinical iDILI models will involve a change of paradigm: knowledge of iDILI mechanisms obtained from patients’ data will help to improve the design, predictivity, reliability, and reproducibility of in vivo and in vitro models for iDILI.
This box summarizes key points contained in the article.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Conceptualization: RJ Andrade and M Villanueva Paz; writing—original draft preparation: M Villanueva Paz, A Segovia-Zafra, DE Di Zeo-Sanchez, and G Matilla-Cabello; writing—review and editing: M Villanueva Paz, A Segovia-Zafra, DE Di Zeo-Sanchez, G Matilla-Cabello, JM Pinazo-Bandera, RJ Andrade and MI Lucena; supervision: M Villanueva Paz and RJ Andrade.
Additional information
Funding
References
- Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006 Feb 16; 354(7):731–739.
- Andrade RJ, Chalasani N, Bjornsson ES, et al. Drug-induced liver injury. Nat Rev Dis Primers. 2019 Aug 22;5(1):58.
- Bunchorntavakul C, Reddy KR. Acetaminophen-related hepatotoxicity. Clin Liver Dis. 2013 Nov;17(4):587–607.
- Lewis JH. The art and science of diagnosing and managing drug-induced liver injury in 2015 and beyond. Clin Gastroenterol Hepatol. 2015 Nov;13(12):2173–89.
- Stephens C, Robles-Diaz M, Medina-Caliz I, et al. Comprehensive analysis and insights gained from long-term experience of the Spanish DILIRegistry. J Hepatol. 2021 Jul;75(1):86–97.
- Reuben A, Tillman H, Fontana RJ, et al. Outcomes in adults with acute liver failure between 1998 and 2013: an observational cohort study. Ann Intern Med. 2016 Jun 7; 164(11):724–732.
- Hoofnagle JH, Bjornsson ES, Longo DL. Drug-induced liver injury - types and phenotypes. N Engl J Med. 2019 Jul 18; 381(3):264–273.
- Aithal GP, Watkins PB, Andrade RJ, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011 Jun;89(6):806–815.
- Danan G, Benichou C. Causality assessment of adverse reactions to drugs–I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993 Nov;46(11):1323–1330.
- Hayashi PH, Lucena MI, Fontana RJ, et al. A revised electronic version of RUCAM for the diagnosis of DILI. Hepatology. 2022 Jan 11;76(1):18–31.
- Chen M, Suzuki A, Borlak J, et al. Drug-induced liver injury: interactions between drug properties and host factors. J Hepatol. 2015 Aug;63(2):503–514.
- Li AP. A review of the common properties of drugs with idiosyncratic hepatotoxicity and the “multiple determinant hypothesis” for the manifestation of idiosyncratic drug toxicity. Chem Biol Interact. 2002 Nov 10; 142(1–2):7–23.
- Dara L, Liu ZX, Kaplowitz N. Mechanisms of adaptation and progression in idiosyncratic drug induced liver injury, clinical implications. Liver Int. 2016 Feb;36(2):158–165.
- Park BK, Boobis A, Clarke S, et al. Managing the challenge of chemically reactive metabolites in drug development. Nat Rev Drug Discov. 2011 Apr;10(4):292–306.
- Noureddin N, Kaplowitz N. Overview of mechanisms of drug-induced liver injury (dili) and key challenges in DILI research. In: Chen M, Will Y, editors. Drug-Induced Liver Toxicity. New York: Springer New York; 2018. p. 3–18.
- Jee A, Sernoskie SC, Uetrecht J. Idiosyncratic drug-induced liver injury: mechanistic and clinical challenges. Int J Mol Sci. 2021 Mar 14; 22(6):2954.
- Iorga A, Dara L, Kaplowitz N. Drug-induced liver injury: cascade of events leading to cell death, apoptosis or necrosis. Int J Mol Sci. 2017 May 9; 18(5):1018.
- Zindel J, Kubes P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu Rev Pathol. 2020 Jan 24; 15(1):493–518.
- Lucena MI, Sanabria J, Garcia-Cortes M, et al. Drug-induced liver injury in older people. Lancet Gastroenterol Hepatol. 2020 Sep;5(9):862–874.
- McGill MR, Jaeschke H. Biomarkers of drug-induced liver injury: progress and utility in research, medicine, and regulation. Expert Rev Mol Diagn. 2018 Sep;18(9):797–807.
- Kuna L, Bozic I, Kizivat T, et al. Models of drug induced liver injury (DILI) - current issues and future perspectives. Curr Drug Metab. 2018;19(10):830–838.
- Weaver RJ, Blomme EA, Chadwick AE, et al. Managing the challenge of drug-induced liver injury: a roadmap for the development and deployment of preclinical predictive models. Nat Rev Drug Discov. 2020 Feb;19(2):131–148.
- Thakkar S, Chen M, Fang H, et al. The liver toxicity knowledge base (LKTB) and drug-induced liver injury (DILI) classification for assessment of human liver injury. Expert Rev Gastroenterol Hepatol. 2018 Jan;12(1):31–38.
- Fernandez-Checa JC, Bagnaninchi P, Ye H, et al. Advanced preclinical models for evaluation of drug-induced liver injury - consensus statement by the European Drug-Induced Liver Injury Network [PRO-EURO-DILI-NET]. J Hepatol. 2021 Oct;75(4):935–959.
- Villanueva-Paz M, Moran L, Lopez-Alcantara N, et al. Oxidative stress in drug-induced liver injury (DILI): from mechanisms to biomarkers for use in clinical practice. Antioxidants (Basel). 2021 Mar 5;10(3).
- Walker PA, Ryder S, Lavado A, et al. The evolution of strategies to minimise the risk of human drug-induced liver injury (DILI) in drug discovery and development. Arch Toxicol. 2020 Aug;94(8):2559–2585.
- Nautiyal M, Qasem RJ, Fallon JK, et al. Characterization of primary mouse hepatocyte spheroids as a model system to support investigations of drug-induced liver injury. Toxicol In Vitro. 2021 Feb;70:105010.
- Ullah I, Kim Y, Lim M, et al. In vitro 3-D culture demonstrates incompetence in improving maintenance ability of primary hepatocytes. Anim Cells Syst. 2017 September 03;21(5):332–340.
- Zeilinger K, Freyer N, Damm G, et al. Cell sources for in vitro human liver cell culture models. Exp Biol Med. 2016;241(15):1684–1698.
- Albrecht W, Kappenberg F, Brecklinghaus T, et al. Prediction of human drug-induced liver injury (DILI) in relation to oral doses and blood concentrations. Arch Toxicol. 2019 Jun;93(6):1609–1637.
- O’Brien PJ, Irwin W, Diaz D, et al. High concordance of drug-induced human hepatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening. Arch Toxicol. 2006 September 01;80(9):580–604.
- Xu JJ, Henstock PV, Dunn MC, et al., Cellular imaging predictions of clinical drug-induced liver injury. Toxicol Sci. 2008;105(1):97–105.
- Khetani SR, Kanchagar C, Ukairo O, et al. Use of micropatterned cocultures to detect compounds that cause drug-induced liver injury in humans. Toxicol Sci. 2013 Mar;132(1):107–117.
- Su S, Di Poto C, Roy R, et al. Long-term culture and characterization of patient-derived primary hepatocytes using conditional reprogramming. Exp Biol Med (Maywood). 2019 Aug;244(11):857–864.
- Chai J, Han L, Zhang J, et al. Conditional reprogramming inducing clinical cells proliferation: new research tools in tumor and inflammatory-related diseases. Curr Pharm Des. 2020;26(22):2657–2660.
- Gao X, Liu Y. A transcriptomic study suggesting human iPSC-derived hepatocytes potentially offer a better in vitro model of hepatotoxicity than most hepatoma cell lines. Cell Biol Toxicol. 2017 Aug;33(4):407–421.
- Aninat C, Piton A, Glaise D, et al. Expression of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab Dispos. 2006 Jan;34(1):75–83.
- Donato MT, Jover R, Gomez-Lechon MJ. Hepatic cell lines for drug hepatotoxicity testing: limitations and strategies to upgrade their metabolic competence by gene engineering. Curr Drug Metab. 2013 Nov;14(9):946–968.
- Gomez-Lechon MJ, Tolosa L, Donato MT. Upgrading HepG2 cells with adenoviral vectors that encode drug-metabolizing enzymes: application for drug hepatotoxicity testing. Expert Opin Drug Metab Toxicol. 2017 Feb;13(2):137–148.
- Donato MT, Hallifax D, Picazo L, et al. Metabolite formation kinetics and intrinsic clearance of phenacetin, tolbutamide, alprazolam, and midazolam in adenoviral cytochrome P450-transfected HepG2 cells and comparison with hepatocytes and in vivo. Drug Metab Dispos. 2010 Sep;38(9):1449–1455.
- Harada K, Kohara H, Yukawa T, et al. Cell-based high-throughput screening for the evaluation of reactive metabolite formation potential. Toxicol in vitro. 2021 Aug 01;74:105159.
- Jin M, Yi X, Liao W, et al. Advancements in stem cell-derived hepatocyte-like cell models for hepatotoxicity testing. Stem Cell Res Ther. 2021 Jan 25;12(1):84.
- Huang P, Zhang L, Gao Y, et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell. 2014 Mar 6;14(3):370–384.
- Xie B, Sun D, Du Y, et al. A two-step lineage reprogramming strategy to generate functionally competent human hepatocytes from fibroblasts. Cell Res. 2019 September 01;29(9):696–710.
- Fu GB, Huang WJ, Zeng M, et al. Expansion and differentiation of human hepatocyte-derived liver progenitor-like cells and their use for the study of hepatotropic pathogens. Cell Res. 2019 Jan;29(1):8–22.
- Tasnim F, Huang X, Lee CZW, et al. Recent advances in models of immune-mediated drug-induced liver injury. Front Toxicol. 2021;3 :605392.
- Ware BR, Berger DR, Khetani SR. Prediction of drug-induced liver injury in micropatterned co-cultures containing iPSC-derived human hepatocytes. Toxicol Sci. 2015 Jun;145(2):252–262.
- Cox CR, Lynch S, Goldring C, et al. Current perspective: 3D spheroid models utilizing human-based cells for investigating metabolism-dependent drug-induced liver injury. Front Med Technol. 2020;2:611913.
- Schyschka L, Sanchez JJ, Wang Z, et al. Hepatic 3D cultures but not 2D cultures preserve specific transporter activity for Acetaminophen-induced hepatotoxicity. Arch Toxicol. 2013 Aug;87(8):1581–1593.
- Basharat A, Rollison HE, Williams DP, et al. HepG2 (C3A) spheroids show higher sensitivity compared to HepaRG spheroids for drug-induced liver injury (DILI). Toxicol Appl Pharmacol. 2020 Dec 1;408:115279.
- Vorrink SU, Zhou Y, Ingelman-Sundberg M, et al. Prediction of drug-induced hepatotoxicity using long-term stable primary hepatic 3D spheroid cultures in chemically defined conditions. Toxicol Sci. 2018 Jun 1;163(2):655–665.
- Shinozawa T, Kimura M, Cai Y, et al. High-fidelity drug-induced liver injury screen using human pluripotent stem cell-derived organoids. Gastroenterology. 2021 Feb;160(3):831–846.
- Zhang CJ, O’Meara MJ, Meyer SR, et al. A multi-omics human liver organoid screening platform for DILI risk prediction. bioRxiv. 2021 Aug;457824.
- Xiao RR, Lv T, Tu X, et al. An integrated biomimetic array chip for establishment of collagen-based 3D primary human hepatocyte model for prediction of clinical drug-induced liver injury. Biotechnol Bioeng. 2021 Dec;118(12):4687–4698.
- Liu J, Li R, Xue R, et al. Liver extracellular matrices bioactivated hepatic spheroids as a model system for drug hepatotoxicity evaluations. Adv Biosyst. 2018;2(10):1800110.
- Bouwmeester MC, Bernal PN, Oosterhoff LA, et al. Bioprinting of human liver-derived epithelial organoids for toxicity studies. Macromol Biosci. 2021 Dec;21(12):e2100327.
- Martucci NJ, Morgan K, Anderson GW, et al. Nondestructive optical toxicity assays of 3D liver spheroids with optical coherence tomography. Adv Biosyst. 2018;2(3):1700212.
- Hiemstra S, Ramaiahgari SC, Wink S, et al. High-throughput confocal imaging of differentiated 3D liver-like spheroid cellular stress response reporters for identification of drug-induced liver injury liability. Arch Toxicol. 2019 Oct;93(10):2895–2911.
- Leite SB, Roosens T, El Taghdouini A, et al. Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials. 2016 Feb;78:1–10.
- Moradi E, Jalili-Firoozinezhad S, Solati-Hashjin M. Microfluidic organ-on-a-chip models of human liver tissue. Acta Biomater. 2020 Oct 15; 116:67–83.
- Ma C, Zhao L, Zhou EM, et al. On-Chip construction of liver lobule-like microtissue and its application for adverse drug reaction assay. Anal Chem. 2016 Feb 2;88(3):1719–1727.
- Rubiano A, Indapurkar A, Yokosawa R, et al. Characterizing the reproducibility in using a liver microphysiological system for assaying drug toxicity, metabolism and accumulation. Clin Transl Sci. 2020;14(3): 1049–1061.
- Bricks T, Paullier P, Legendre A, et al. Development of a new microfluidic platform integrating co-cultures of intestinal and liver cell lines. Toxicol In Vitro. 2014 Aug;28(5):885–895.
- Othman A, Ehnert S, Dropmann A, et al. Precision-cut liver slices as an alternative method for long-term hepatotoxicity studies. Arch Toxicol. 2020 Aug;94(8):2889–2891.
- Palma E, Doornebal EJ, Chokshi S. Precision-cut liver slices: a versatile tool to advance liver research. Hepatol Int. 2019 Jan;13(1):51–57.
- Hadi M, Westra IM, Starokozhko V, et al. Human precision-cut liver slices as an ex vivo model to study idiosyncratic drug-induced liver injury. Chem Res Toxicol. 2013 May 20;26(5):710–720.
- Oda S, Matsuo K, Nakajima A, et al. A novel cell-based assay for the evaluation of immune- and inflammatory-related gene expression as biomarkers for the risk assessment of drug-induced liver injury. Toxicol Lett. 2016 Jan 22;241:60–70.
- Kato R, Uetrecht J. Supernatant from hepatocyte cultures with drugs that cause idiosyncratic liver injury activates macrophage inflammasomes. Chem Res Toxicol. 2017 Jun 19; 30(6):1327–1332.
- Ogese MO, Faulkner L, Jenkins RE, et al. Characterization of drug-specific signaling between primary human hepatocytes and immune cells. Toxicol Sci. 2017 Jul 1;158(1):76–89.
- Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004 Aug;59(8):809–820.
- Whritenour J, Ko M, Zong Q, et al. Development of a modified lymphocyte transformation test for diagnosing drug-induced liver injury associated with an adaptive immune response. J Immunotoxicol. 2017 Jan 01;14(1):31–38.
- Li C, Ming Y, Hong W, et al. Comparison of hepatic transcriptome profiling between acute liver injury and acute liver failure induced by Acetaminophen in mice. Toxicol Lett. 2018 Feb;283:69–76.
- Knockaert L, Berson A, Ribault C, et al. Carbon tetrachloride-mediated lipid peroxidation induces early mitochondrial alterations in mouse liver. Lab Invest. 2012 Mar;92(3):396–410.
- Harrill AH, Watkins PB, Su S, et al. Mouse population-guided resequencing reveals that variants in CD44 contribute to acetaminophen-induced liver injury in humans. Genome Res. 2009 Sep;19(9):1507–1515.
- Church RJ, Wu H, Mosedale M, et al. A systems biology approach utilizing a mouse diversity panel identifies genetic differences influencing isoniazid-induced microvesicular steatosis. Toxicol Sci. 2014 Aug 1;140(2):481–492.
- Liu W, Zeng X, Liu Y, et al. The immunological mechanisms and immune-based biomarkers of drug-induced liver injury. Front Pharmacol. 2021;12:723940.
- Lu J, Jones AD, Harkema JR, et al. Amiodarone exposure during modest inflammation induces idiosyncrasy-like liver injury in rats: role of tumor necrosis factor-alpha. Toxicol Sci. 2012 Jan;125(1):126–133.
- Deng X, Stachlewitz RF, Liguori MJ, et al. Modest inflammation enhances diclofenac hepatotoxicity in rats: role of neutrophils and bacterial translocation. J Pharmacol Exp Ther. 2006 Dec;319(3):1191–1199.
- Uetrecht J. Mechanistic studies of idiosyncratic DILI: clinical implications. Front Pharmacol. 2019;10:837.
- Mak A, Uetrecht J. The combination of anti-CTLA-4 and PD1-/- mice unmasks the potential of isoniazid and nevirapine to cause liver injury. Chem Res Toxicol. 2015 Dec 21; 28(12):2287–2291.
- Cho T, Wang X, Yeung K, et al. Liver injury caused by green tea extract in PD-1(-/-) mice: an impaired immune tolerance model for idiosyncratic drug-induced liver injury. Chem Res Toxicol. 2021 Mar 15;34(3):849–856.
- Mak A, Kato R, Weston K, et al. Editor’s highlight: an impaired immune tolerance animal model distinguishes the potential of troglitazone/pioglitazone and tolcapone/entacapone to cause IDILI. Toxicol Sci. 2018 Feb 1;161(2):412–420.
- Shojaie L, Ali M, Iorga A, et al. Mechanisms of immune checkpoint inhibitor-mediated liver injury. Acta Pharm Sin B. 2021 Dec;11(12):3727–3739.
- Higuchi S, Yano A, Takai S, et al. Metabolic activation and inflammation reactions involved in carbamazepine-induced liver injury. Toxicol Sci. 2012 Nov;130(1):4–16.
- Yano A, Higuchi S, Tsuneyama K, et al. Involvement of immune-related factors in diclofenac-induced acute liver injury in mice. Toxicology. 2012 Mar 11;293(1–3):107–114.
- Wu K, Fan J, Huang X, et al. Hepatoprotective effects exerted by Poria Cocos polysaccharides against acetaminophen-induced liver injury in mice. Int J Biol Macromol. 2018 Jul 15;114:137–142.
- Li F, Qiu Y, Xia F, et al. Dual detoxification and inflammatory regulation by ceria nanozymes for drug-induced liver injury therapy. Nano Today. 2020 Dec 01;35:100925.
- Feng Y, Cui R, Li Z, et al. Methane alleviates acetaminophen-induced liver injury by inhibiting inflammation, oxidative stress, endoplasmic reticulum stress, and apoptosis through the Nrf2/HO-1/NQO1 signaling pathway. Oxid Med Cell Longev. 2019;2019:7067619.
- Hsiao CJ, Younis H, Boelsterli UA. Trovafloxacin, a fluoroquinolone antibiotic with hepatotoxic potential, causes mitochondrial peroxynitrite stress in a mouse model of underlying mitochondrial dysfunction. Chem Biol Interact. 2010 Oct 6; 188(1):204–213.
- Ong MM, Latchoumycandane C, Boelsterli UA. Troglitazone-induced hepatic necrosis in an animal model of silent genetic mitochondrial abnormalities. Toxicol Sci. 2007 May;97(1):205–213.
- Morita M, Akai S, Hosomi H, et al. Drug-induced hepatotoxicity test using gamma-glutamylcysteine synthetase knockdown rat. Toxicol Lett. 2009 Sep 10;189(2):159–165.
- Goda K, Muta K, Yasui Y, et al. Selenium and glutathione-depleted rats as a sensitive animal model to predict drug-induced liver injury in humans. Int J Mol Sci. 2019 Jun 27;20(13):3141.
- Uzi D, Barda L, Scaiewicz V, et al. CHOP is a critical regulator of Acetaminophen-induced hepatotoxicity. J Hepatol. 2013 Sep;59(3):495–503.
- Matsuo K, Sasaki E, Higuchi S, et al. Involvement of oxidative stress and immune- and inflammation-related factors in azathioprine-induced liver injury. Toxicol Lett. 2014 Jan 13;224(2):215–224.
- Hu Y, Wu M, Nishimura T, et al. Human pharmacogenetic analysis in chimeric mice with “humanized livers“. Pharmacogenet Genomics. 2013 Feb;23(2):78–83.
- Xu D, Nishimura T, Nishimura S, et al. Fialuridine induces acute liver failure in chimeric TK-NOG mice: a model for detecting hepatic drug toxicity prior to human testing. PLoS Med. 2014 Apr;11(4):e1001628.
- Kamimura H, Ito S, Nozawa K, et al. Formation of the accumulative human metabolite and human-specific glutathione conjugate of diclofenac in TK-NOG chimeric mice with humanized livers. Drug Metab Dispos. 2015 Mar;43(3):309–316.
- Xu D, Wu M, Nishimura S, et al. Chimeric TK-NOG mice: a predictive model for cholestatic human liver toxicity. J Pharmacol Exp Ther. 2015;352(2):274.
- Kohara H, Bajaj P, Yamanaka K, et al. High-throughput screening to evaluate inhibition of bile acid transporters using human hepatocytes isolated from chimeric mice. Toxicol Sci. 2020 Feb 1;173(2):347–361.
- Washburn ML, Bility MT, Zhang L, et al. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology. 2011 Apr;140(4):1334–1344.
- Guo J, Li Y, Shan Y, et al. Humanized mice reveal an essential role for human hepatocytes in the development of the liver immune system. Cell Death Dis. 2018 Jun 4;9(6):667.
- Weaver JL, Zadrozny LM, Gabrielson K, et al. BLT-immune humanized mice as a model for nivolumab-induced immune-mediated adverse events: comparison of the NOG and NOG-EXL strains. Toxicol Sci. 2019 May 1;169(1):194–208.
- Yan H, Semple KM, Gonzalez CM, et al. Bone marrow-liver-thymus (BLT) immune humanized mice as a model to predict cytokine release syndrome. Transl Res. 2019 Aug;210:43–56.
- Susukida T, Aoki S, Shirayanagi T, et al. HLA transgenic mice: application in reproducing idiosyncratic drug toxicity. Drug Metab Rev. 2020 Nov;52(4):540–567.
- Jiang W, Dai T, Xie S, et al. Roles of diclofenac and its metabolites in immune activation associated with acute hepatotoxicity in TgCYP3A4/hPXR-humanized mice. Int Immunopharmacol. 2020 Sep;86:106723.
- Segovia-Zafra A, Di Zeo-Sánchez DE, López-Gómez C, et al. Preclinical models of idiosyncratic drug-induced liver injury (iDILI): moving towards prediction. Acta Pharm Sin B. 2021 Dec 01;11(12):3685–3726.
- Vliegenthart AD, Tucker CS, Del Pozo J, et al. Zebrafish as model organisms for studying drug-induced liver injury. Br J Clin Pharmacol. 2014 Dec;78(6):1217–1227.
- Chng HT, Ho HK, Yap CW, et al. An investigation of the bioactivation potential and metabolism profile of Zebrafish versus human. J Biomol Screen. 2012 Aug;17(7):974–986.
- Hill A, Mesens N, Steemans M, et al. Comparisons between in vitro whole cell imaging and in vivo zebrafish-based approaches for identifying potential human hepatotoxicants earlier in pharmaceutical development. Drug Metab Rev. 2012 Feb;44(1):127–140.
- Mesens N, Crawford AD, Menke A, et al. Are zebrafish larvae suitable for assessing the hepatotoxicity potential of drug candidates? J Appl Toxicol. 2015 Sep;35(9):1017–1029.
- Higuchi A, Wakai E, Tada T, et al. Generation of a transgenic zebrafish line for in vivo assessment of hepatic apoptosis. Pharmaceuticals (Basel). 2021 Oct 31;14(11):1117.
- Nguyen XB, Kislyuk S, Pham DH, et al. Cell imaging counting as a novel ex vivo approach for investigating drug-induced hepatotoxicity in Zebrafish larvae. Int J Mol Sci. 2017 Feb 8;18(2):356.
- Dirven H, Vist GE, Bandhakavi S, et al. Performance of preclinical models in predicting drug-induced liver injury in humans: a systematic review. Sci Rep. 2021 Mar 18;11(1):6403.
- Ekins S, Mestres J, Testa B. In silico pharmacology for drug discovery: methods for virtual ligand screening and profiling. Br J Pharmacol. 2007 Sep;152(1):9–20.
- Zhu XW, Sedykh A, Liu SS. Hybrid in silico models for drug-induced liver injury using chemical descriptors and in vitro cell-imaging information. J Appl Toxicol. 2014 Mar;34(3):281–288.
- Greene N, Fisk L, Naven RT, et al. Developing structure-activity relationships for the prediction of hepatotoxicity. Chem Res Toxicol. 2010 Jul 19;23(7):1215–1222.
- Przybylak KR, Cronin MT. In silico models for drug-induced liver injury–current status. Expert Opin Drug Metab Toxicol. 2012 Feb;8(2):201–217.
- Pizzo F, Lombardo A, Manganaro A, et al. A new structure-activity relationship (SAR) model for predicting drug-induced liver injury, based on statistical and expert-based structural alerts. Front Pharmacol. 2016;7:442.
- Hewitt M, Przybylak K. In silico models for hepatotoxicity. Methods Mol Biol. 2016;1425:201–236.
- Rodgers AD, Zhu H, Fourches D, et al. Modeling liver-related adverse effects of drugs using knearest neighbor quantitative structure-activity relationship method. Chem Res Toxicol. 2010 Apr 19;23(4):724–732.
- Matthews EJ, Ursem CJ, Kruhlak NL, et al. Identification of structure-activity relationships for adverse effects of pharmaceuticals in humans: Part B. use of (Q)SAR systems for early detection of drug-induced hepatobiliary and urinary tract toxicities. Regul Toxicol Pharmacol. 2009 Jun 01;54(1):23–42.
- Ekins S, Williams AJ, Xu JJ. A predictive ligand-based bayesian model for human drug-induced liver injury. Drug Metab Dispos. 2010;38(12):2302.
- Li X, Chen Y, Song X, et al. The development and application of in silico models for drug induced liver injury. RSC Adv. 2018;8(15):8101–8111.
- Cruz-Monteagudo M, Cordeiro MN, Borges F. Computational chemistry approach for the early detection of drug-induced idiosyncratic liver toxicity. J Comput Chem. 2008 Mar;29(4):533–549.
- Low Y, Uehara T, Minowa Y, et al. Predicting drug-induced hepatotoxicity using QSAR and toxicogenomics approaches. Chem Res Toxicol. 2011 Aug 15;24(8):1251–1262.
- Vall A, Sabnis Y, Shi J, et al. The promise of AI for DILI prediction. Front Artif Intell. 2021;4:638410.
- Koido M, Kawakami E, Fukumura J, et al. Polygenic architecture informs potential vulnerability to drug-induced liver injury. Nat Med. 2020 Oct;26(10):1541–1548.
- Andrade RJ, Lucena MI, Fernández MC, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005 Aug;129(2):512–521.
- Chalasani N, Bonkovsky HL, Fontana R, et al. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015 Jun;148(7):1340–52.
- Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008 Dec;1356:1–4. .
- Bessone F, Hernandez N, Mendizabal M, et al. When the creation of a consortium provides useful answers: experience of the Latin American DILINetwork (LATINDILIN). Clin Liver Dis. 2019 Feb;13(2):51–57.
- Devarbhavi H, Joseph T, Sunil Kumar N, et al. The Indian network of drug-induced liver injury: etiology, clinical features, outcome and prognostic markers in 1288 patients. J Clin Exp Hepatol. 2021 May-Jun;11(3):288–298.
- Aiso M, Takikawa H, Tsuji K, et al. Analysis of 307 cases with drug-induced liver injury between 2010 and 2018 in Japan. Hepatol Res. 2019 Jan;49(1):105–110.
- Takikawa H, Murata Y, Horiike N, et al. Drug-induced liver injury in Japan: an analysis of 1676 cases between 1997 and 2006. Hepatol Res. 2009 May;39(5):427–431.
- Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012 Sep;107(9):1380–1387.
- A Prospective Cohort Study On Drug-Induced Liver Injury in China (DILI-P). CLINICALTRIALS.gov identifier: NCT02961413. Available from: https://clinicaltrials.gov/ct2/show/NCT02961413, Available from 24 April 2022
- Björnsson ES, Stephens C, Atallah E, et al. A new framework for advancing in drug-induced liver injury research. The Prospective European DILI Registry. Liver Int. 2022. Accepted Author Manuscript.
- Daly AK, Donaldson PT, Bhatnagar P, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009 Jul;41(7):816–819.
- Mallal S, Phillips E, Carosi G, et al. HLA-B*5701 screening for hypersensitivity to Abacavir. N Engl J Med. 2008 Feb 7;358(6):568–579.
- Donaldson PT, Daly AK, Henderson J, et al. Human leucocyte antigen class II genotype in susceptibility and resistance to co-amoxiclav-induced liver injury. J Hepatol. 2010 Dec;53(6):1049–1053.
- Lucena MI, Molokhia M, Shen Y, et al. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011 Jul;141(1):338–347.
- Stephens C, Lopez-Nevot MA, Ruiz-Cabello F, et al. HLA alleles influence the clinical signature of amoxicillin-clavulanate hepatotoxicity. PLoS One. 2013;8(7):e68111.
- Nicoletti P, Aithal GP, Bjornsson ES, et al. Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in HLA and other genes in a genome-wide association study. Gastroenterology. 2017 Apr;152(5):1078–1089.
- Cirulli ET, Nicoletti P, Abramson K, et al. A missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology. 2019 May;156(6):1707–1716.
- Cha HJ, M-J K, Ahn S-M, et al. Identification of classifier genes for hepatotoxicity prediction in non steroidal anti inflammatory drugs. Mol. Cell. Toxicol. 2010 Sep 01;6(3):247–253.
- Ware BR, McVay M, Sunada WY, et al. Exploring chronic drug effects on microengineered human liver cultures using global gene expression profiling. Toxicol Sci. 2017 Jun 1;157(2):387–398.
- Sato K, Meng F, Glaser S, et al. Exosomes in liver pathology. J Hepatol. 2016 Jul;65(1):213–221.
- Russo MW, Steuerwald N, Norton HJ, et al. Profiles of miRNAs in serum in severe acute drug induced liver injury and their prognostic significance. Liver Int. 2017 May;37(5):757–764.
- Moreno-Torres M, Quintas G, Castell JV. The potential role of metabolomics in drug-induced liver injury (DILI) assessment. Metabolites. 2022 Jun 19; 12(6):564.
- Ruiz-Aracama A, Peijnenburg A, Kleinjans J, et al. An untargeted multi-technique metabolomics approach to studying intracellular metabolites of HepG2 cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. BMC Genomics. 2011 May 20;12(12):251.
- Garcia-Canaveras JC, Castell JV, Donato MT, et al. A metabolomics cell-based approach for anticipating and investigating drug-induced liver injury. Sci Rep. 2016 Jun 6;6(1):27239.
- Ramirez T, Strigun A, Verlohner A, et al. Prediction of liver toxicity and mode of action using metabolomics in vitro in HepG2 cells. Arch Toxicol. 2018 Feb;92(2):893–906.
- Quintas G, Martinez-Sena T, Conde I, et al. Metabolomic analysis to discriminate drug-induced liver injury (DILI) phenotypes. Arch Toxicol. 2021 Sep;95(9):3049–3062.
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012 Jun 8; 336(6086):1268–1273.
- Olson H, Betton G, Robinson D, et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol. 2000 Aug;32(1):56–67.
- Scheer N, Wilson ID. A comparison between genetically humanized and chimeric liver humanized mouse models for studies in drug metabolism and toxicity. Drug Discov Today. 2016 Feb;21(2):250–263.
- Chen M, Suzuki A, Thakkar S, et al. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today. 2016 Apr;21(4):648–653.
- Vinken M, Hengstler JG. Characterization of hepatocyte-based in vitro systems for reliable toxicity testing.Arch Toxicol. 2018 October 01;92(10):2981–2986.
- Schadt S, Simon S, Kustermann S, et al. Minimizing Dili risk in drug discovery - A screening tool for drug candidates. Toxicol In Vitro. 2015 Dec 25;30(1 Pt B):429–437.
- Rusyn I, Arzuaga X, Cattley RC, et al. Key characteristics of human hepatotoxicants as a basis for identification and characterization of the causes of liver toxicity. Hepatology. 2021 Dec;74(6):3486–3496.
- Jain S, Norinder U, Escher SE, et al. Combining in vivo data with in silico predictions for modeling hepatic steatosis by using stratified bagging and conformal prediction. Chem Res Toxicol. 2021 Feb 15;34(2):656–668.
- Si-Tayeb K, Noto FK, Nagaoka M, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010 Jan;51(1):297–305.
- Takata A, Otsuka M, Kogiso T, et al. Direct differentiation of hepatic cells from human induced pluripotent stem cells using a limited number of cytokines. Hepatol Int. 2011 Dec;5(4):890–898.
- Nakamori D, Akamine H, Takayama K, et al. Direct conversion of human fibroblasts into hepatocyte-like cells by ATF5, PROX1, FOXA2, FOXA3, and HNF4A transduction. Sci Rep. 2017 Nov 30;7(1):16675.
- Ballester M, Bolonio M, Santamaria R, et al. Direct conversion of human fibroblast to hepatocytes using a single inducible polycistronic vector. Stem Cell Res Ther. 2019 Nov 4;10(1):317.
- Ju C, Reilly T. Role of immune reactions in drug-induced liver injury (DILI). Drug Metab Rev. 2012 Feb;44(1):107–115.
- Usui T, Faulkner L, Farrell J, et al. Application of in vitro T cell assay using human leukocyte antigen-typed healthy donors for the assessment of drug immunogenicity. Chem Res Toxicol. 2018 Mar 19;31(3):165–167.
