1. Introduction
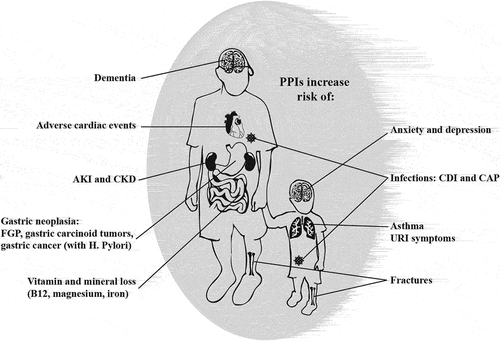
Since their introduction in 1980s, proton pump inhibitors (PPI) heralded a sweeping change in the management of acid-related disorders. Their efficacy and ease to obtain have led to common use of PPIs. Perhaps a direct consequence of decreasing stomach acidity, appreciation of the wide-ranging physiologic functions of low stomach pH increased; functions such asfacilitation of vitamin and mineral absorption and suppression of enteric infections. Incidental and recurrent Clostridioides difficile infections cause significant morbidity, but pneumonia and exacerbation of hepatic encephalopathy have also been reported with both short- and long-term PPI use. With chronic use, unopposed hypergastrinemia, gastric atrophy, and bacterial overgrowth have been associated with an increased incidence of gastric cancer [Citation1]. Additionally, idiosyncratic reactions unrelated to their pharmacotherapeutic profile have also been reported, notably interstitial nephritis (IN). Media attention and litigation followed hard to replicate and often conflicting PPI side effect evidence [Citation2] in nested case–control studies, retrospective observational studies (including studies based on secondary use of administrative health databases), and their meta-analysis. These studies show a spectrum of risks associated with PPI use and are beset by limitations inherent to the study population. Long-term PPI use, for example, is more common among older individuals with multiple confounding comorbidities and polypharmacy (directly relevant to C. difficile and IN risk, respectively). Limitations also exist in the study methodology, including cohort definition [Citation3] with respect to dose and duration of PPI use, compounded by difficulties in finding precisely matching controls not treated with PPIs.
With the results of these pharmacoepidemiology studies conflicting, prescribers and patients are left with the same question: Are the results of big data analysis a true risk signal or noise? We do not advocate that PPIs be used without consideration of consequences, rather that further prospective studies are needed to measure risk and provide mechanistic insights for adverse effects (AE). Until then, a rational approach to begin is to stratify the available AE data by the patients’ phenotype for cytochrome P450 (CYP) 2C19, the hepatic enzyme responsible for PPI drug metabolism.
2. Discussion
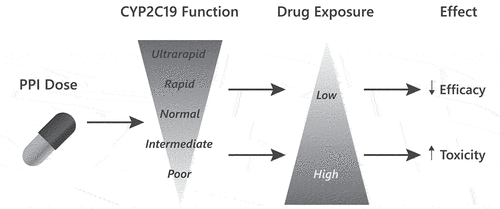
The majority of PPIs are metabolized into inactive compounds through the action of hepatic CYP2C19 (major) and CYP3A4 (minor) enzymes. Due to the genetic variants, CYP2C19 functional activity is highly variable across individuals, from poor metabolizers with no enzyme activity to ultrarapid metabolizers with two increased activity alleles (). CYP2C19 activity (i.e. phenotype) can be predicted by genotype and is an important determinant of the amount of drug exposure and therapeutic response achieved [Citation4]. For a given dose of PPI, individuals who more rapidly metabolize the drug have lower drug concentrations, leading to a higher risk of therapeutic failure. For individuals who more slowly metabolize the drug, the higher exposures achieved increase the likelihood of therapeutic response. However, recent data suggest that these individuals are also at higher risk for PPI toxicity (). Additionally, drug–drug interactions can affect phenotype, adding a level of complexity to determining phenotype [Citation5].
Table 1. CYP2C19 activity (i.e. phenotype) is based on the two copies of the CYP2C19 allele, one inherited from each parent.
2.1. Pediatrics
In a landmark, placebo-controlled, pediatric asthma trial of adjunct PPI treatment with 30 mg daily lansoprazole for 24 weeks (n = 280), investigators observed a higher incidence of upper respiratory infections, sore throat, and bronchitis in children in the treatment arm vs. placebo [Citation6]. However, the higher infection burden was only among children with the CYP2C19 intermediate metabolizer (IM) and poor metabolizer (PM) phenotype, not the normal metabolizer (NM) phenotype. From the same daily dose of lansoprazole, IMs and PMs had higher systemic lansoprazole concentrations than NMs, suggesting that slower CYP2C19 metabolism contributes to higher systemic PPI drug exposures and increases the risk of respiratory AE.
In a retrospective observational study leveraging a DNA biorepository study for 670 infants and toddlers treated with PPIs, investigators demonstrated that those with CYP2C19 rapid metabolizer (RM) and ultrarapid metabolizer (UM) phenotypes experienced fewer respiratory and gastrointestinal infections than those with the NM phenotype. This finding was consistently observed in both a univariate analysis and a multivariate analysis that adjusted for age, sex, PPI dose, and comorbidities that increase infection susceptibility [Citation7]. In contrast to the asthma trial [Citation6], no difference was observed between the combined PM/IM group (n = 183) vs. NMs (n = 267), potentially due to the modest sample size and limitations of retrospective data.
A prospective pragmatic trial found the incidence of respiratory infections to be 50% lower in children enrolled in a CYP2C19 genotype-guided arm of the trial, compared to the conventional dosing arm [Citation8]. Although the observed difference in AEs did not reach statistical significance (p = 0.07), likely due to small sample size (<30 children per arm), the study illustrated that AE risk during a 12-week treatment course with PPIs is likely related to CYP2C19 genotype.
2.2. Adults
Studies in adults are inconsistent with observations in children. A prospective study [Citation9] of 119 Japanese patients diagnosed with gastroesophageal reflux disease (GERD) and started on PPI evaluated treatment outcome difference between individuals with one copy (IM) or two copies (PM) of the CYP2C19 null function alleles *2 and *3. No differences in serious or non-serious AEs were identified in PM (n = 20) vs. IM (n = 26) vs. all other (n = 53) CYP2C19 phenotypes. In a large retrospective cohort [Citation10] of 3,326 Israeli patients treated with PPI with varying genotypes from PM to UM, the authors looked at hip, spine, and wrist fracture risk in the years following initiation of PPI and found no association between fracture risk and CYP2C19 phenotype. In fact, PMs had a lower risk of fracture, though underpowered and not statistically significant.
Another group [Citation11] identified a small cohort of primarily geriatric patients (n = 20) who developed IN while on PPI therapy with omeprazole. To test the hypothesis that these individuals would be primarily CYP2C19 PMs, patients were genotyped for CYP2C19 null function alleles to determine phenotype and also by measuring CYP2C19 activity using the CYP2C19 drug probe, proguanil. No association was found between IN and IM/PM status defined by genotype or the proguanil phenotype test. Contrary to the hypothesis, most subjects were NMs and significant CYP2C19 genotype–phenotype discordance was noted, leading the authors to conclude that advanced age is a risk for IN.
Investigations of less common AEs in adults are also conflicting. Studies evaluating the risk of visual disturbances [Citation12] found no connections with CYP2C19 metabolizer status; however, one group [Citation13] noted that chronic migraines were common with the CYP2C19 PM phenotype, specifically in men.
The abovementioned examples focus on PPIs other than rabeprazole, which is primarily metabolized through non-enzymatic reduction [Citation4] rather than CYP2C19 activity. Not surprisingly, in a study [Citation14] of 102 patients using rabeprazole as part of a Helicobacter pylori eradication protocol, no differences in AEs were found across the different metabolizer phenotypes. The AEs noted were diarrhea, headaches, rashes, and appetite loss – common and mild symptoms that are of less concern than fractures or kidney injury. Additionally, these AEs may have been unrelated to rabeprazole and instead related to other drugs used in the H. pylori treatment protocol (i.e. amoxicillin and clarithromycin).
3. Expert opinion
Inconsistent findings in pharmacoepidemiologic and non-pharmacoepidemiologic studies of PPI-associated AEs have led to patient and provider confusion regarding the safety profile of these commonly used medications. Even though previous publications have extensively discussed and advocated for CYP2C19 genotype-guided dosing, prescriber practice still relies heavily on standardized ‘one dose fits all’ dosing [Citation15,Citation16]. While large population studies provide the sample size needed to evaluate PPI-associated AEs, treating a large heterogeneous population as a single study group misses the highly confounding interindividual variability in CYP2C19 activity that contributes directly to the systemic exposure achieved from a given PPI dose. Examining CYP2C19 genotype alone may not be adequate, as CYP2C19 phenotype may also be affected by age. Based on the current evidence, certain age groups (pediatric and geriatric) are at increased risk of AEs. In pediatrics, AE risk of upper respiratory infections/symptoms appears to be, at least in part, related to CYP2C19 genotype and metabolizer status. In geriatrics, IN appears to be a rare and idiosyncratic AE, unrelated to CYP2C19 genetics, and possibly related to aging and co-medications. Stratifying patients based on age, as well as CYP2C19 genotype/phenotype, may elicit clearer evidence for the role of CYP2C19 activity in AEs.
In addition to patients with H. pylori and dyspeptic conditions, another population with high and increasing usage of PPIs are patients with eosinophilic esophagitis (EoE). This chronic, atopic condition is caused by exposure to food and/or aeroallergens. For unknown reasons, a subset of patients respond to PPI therapy. Since the only other therapies for EoE are topical steroids or diet eliminations – which can be challenging and carry side effects of poor growth, feeding difficulties, need for enteric tubes, and so on – timely identification of PPI responders is important. Patients are often on high PPI doses (e.g. 2 mg/kg) for prolonged periods of time, potentially risking higher AEs. Understanding the PPI drug dose→exposure→response relationship in EoE through examination of CYP2C19 metabolizer status () may help elucidate which patients are candidates for PPI therapy, and which are better served by other therapies. We recommend examining past and future PPI AE data through a pharmacogenetic lens, to delineate the depth of CYP2C19 contribution to PPI response and toxicity across age groups and diagnoses.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Author contributions
Drs. Shakhnovich, Van Driest, and Attard were involved in drafting this manuscript and revising it critically for important intellectual content. Dr. Chevalier was involved in drafting and revising and is the corresponding author.
Additional information
Funding
References
- Poly TN, Lin MC, Syed-Abdul S, et al. Proton pump inhibitor use and risk of gastric cancer: current evidence from epidemiological studies and critical appraisal. Cancers (Basel). 2022;14:13.
- Sheridan K. Heartburn and acid reflux drugs double risk of stomach cancer 2017 [Cited 2022 Aug 16]. Available from: https://www.newsweek.com/heartburn-and-acid-reflux-drugs-double-risk-stomach-cancer-699596.
- Yibirin M, De Oliveira D, Valera R, et al. Adverse effects associated with proton pump inhibitor use. Cureus. 2021;13(1):e12759.
- Hall M, Attard TM, Berry JG. Improving cohort definitions in research using hospital administrative databases-do we need guidelines? JAMA Pediatr. 2022;176(6):539–540.
- Lima JJ, Thomas CD, Barbarino J, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2C19 and proton pump inhibitor dosing. Clin Pharmacol Ther. 2021;109(6):1417–1423.
- Yucel E, Sancar M, Yucel A, et al. Adverse drug reactions due to drug-drug interactions with proton pump inhibitors: assessment of systematic reviews with AMSTAR method. Expert Opin Drug Saf. 2016;15(2):223–236.
- Lima JJ, Lang JE, Mougey EB, et al. Association of CYP2C19 polymorphisms and lansoprazole-associated respiratory adverse effects in children. J Pediatr. 2013;163(3):686–691.
- Bernal CJ, Aka I, Carroll RJ, et al. CYP2C19 phenotype and risk of proton pump inhibitor-associated infections. Pediatrics. 2019;144(6):6.
- Cicali EJ, Blake K, Gong Y, et al. Novel implementation of genotype-guided proton pump inhibitor medication therapy in children: a pilot, randomized, multisite pragmatic trial. Clin Transl Sci. 2019;12(2):172–179.
- Ohkusa T, Maekawa T, Arakawa T, et al. Effect of CYP2C19 polymorphism on the safety and efficacy of omeprazole in Japanese patients with recurrent reflux oesophagitis. Aliment Pharmacol Ther. 2005;21(11):1331–1339.
- Gronich N, Lavi I, Lejbkowicz F, et al. Association of CYP2C19 polymorphism with proton pump inhibitors effectiveness and with fractures in real-life: retrospective cohort study. Clin Pharmacol Ther. 2022;111(5):1084–1092.
- Helsby NA, Lo WY, Simpson IJ, et al. Omeprazole-induced acute interstitial nephritis is not related to CYP2C19 genotype or CYP2C19 phenotype. Br J Clin Pharmacol. 2010;69(5):516–519.
- Lutz M, Schwab M, Griese EU, et al. Visual disorders associated with omeprazole and their relation to CYP2C19 polymorphism. Pharmacogenetics. 2002;12(1):73–75.
- Pisanu C, Welander NZ, Rukh G, et al. Association between migraine prevalence, treatment with proton-pump inhibitors and CYP2C19 phenotypes in UK Biobank. Biomed Pharmacother. 2021;143:112234.
- Hokari K, Sugiyama T, Kato M, et al. Efficacy of triple therapy with rabeprazole for Helicobacter pylori infection and CYP2C19 genetic polymorphism. Aliment Pharmacol Ther. 2001;15(9):1479–1484.
- El Rouby N, Lima JJ, Johnson JA. Proton pump inhibitors: from CYP2C19 pharmacogenetics to precision medicine. Expert Opin Drug Metab Toxicol. 2018;14(4):447–460. Epub 20180412