ABSTRACT
Introduction
Human epidermal growth factor receptor two (HER2) target therapies have drastically revolutionized the treatment of HER2-positive breast cancer. Starting with trastuzumab, early phase III trials have already highlighted its significant cardiotoxicity, which is also present, albeit to a lesser extent, in the new generation drugs. Also given the growing population of patients with cardiovascular diseases, it is vital to set up proper long-term follow-up to prevent morbidity related to the development of cardiotoxicity.
Areas covered
This review discusses the mechanisms of action underlying the cardiotoxicity of HER2 targeted therapies and the main clinical evidence on the toxicity of these drugs. In addition, the patterns of patient assessment prior to the initiation of therapy with HER2 targeted therapies are discussed, as well as the main evidence concerning the follow-up and management of cardiotoxicity.
Expert opinion
The mechanisms of cardiotoxicity of new HER2 drugs need further study and, likewise, methods to prevent, monitor and identify HER-2-induced cardiotoxicity need to be implemented. Although some studies highlight the validity of cardiac biomarkers as predictive factors for cardiotoxicity, their actual usefulness and timing is still debated. Further studies are needed to assess the effectiveness of possible pharmacological primary prevention.
1. Introduction
Breast cancer is the most common disease in women and has a high mortality rate, making it the second leading cause of death among women with cancer [Citation1]. Human epidermal growth factor receptor two (HER2) gene amplification is detected in 15–25% of breast cancer patients, with a higher prevalence in younger women and a higher percentage diagnosed at an advanced or metastatic clinical stage [Citation2]. Given its critical role in signaling networks that control proliferation, apoptosis, motility, and metastatic invasion, HER2 overexpression is connected to a more aggressive clinical phenotype and early metastases [Citation3]. The natural course of HER2-positive breast cancer was totally changed by the development of target therapies against HER2, starting with trastuzumab (TRZ), which greatly improved disease-free and overall survival [Citation4]. Despite the higher tolerability profile of TRZ therapy compared to chemotherapy, numerous studies have brought attention to the significant cardiotoxicity associated with the use of this drug. TRZ cardiotoxicity usually manifests as a decline in left ventricular ejection fraction (LVEF) and may result in the development of heart failure (HF) in about 0–4.1% of treated patients [Citation5–7]. The Food and Drug Administration (FDA) has approved several anti-HER2 medications for the treatment of breast cancer, including pertuzumab, trastuzumab emtansine (T-DM1), trastuzumab deruxtecan, and tucatinib. These medications have a lower cardiotoxic profile than TRZ and have further improved the prognosis of breast cancer patients. Given the increased survival due to the introduction of target drugs directed against HER2, it is vital to set up proper long-term follow-up to prevent morbidity related to the development of cardiotoxicity. This review aims to summarize the fundamental elements of cardiotoxicity care in patients with HER2-positive breast cancer as well as the primary characteristics of cardiotoxicity of the major HER2 target therapies.
2. HER2 signal pathways
Since the early 1980s results from in vivo and in vitro studies enlightened the role of HER2 signaling in the pathogenesis of breast cancer [Citation8,Citation9]. HER2 is a tyrosine kinase glycoprotein, a member of the human epidermal growth factor (EGF) receptors (EGFR or HER) family. HER family members (i.e. HER1 [ErbB1 or EGFR], HER2 [ErbB2], HER3 [ErbB3], and HER4 [ErbB4]) are important mediators of cell growth and proliferation. Pleiotropic actions of HER signaling network rely on the ability of HER receptors to combine in dimers, at the core of a complex signaling network. In the heart, several lines of evidence have emphasized the importance of HER2, both in fetal and adult life [Citation10–12]. Indeed, HER2 is involved in the response to stress conditions, as part of the neuregulin 1 (NRG-1)/ErbB2 signaling axis. Briefly, NRG-1, a protective growth factor released from cardiac endothelial cells, promotes dimerization of HER receptors, and activates downstream signaling pathways with a role in the maintenance of cardiomyocyte function and survival, including phosphoinositide 3 kinase (PI3K)/Akt and protein kinase/extracellular signal-regulated kinases (MAPK/ERK) [Citation12,Citation13].
3. Cardiotoxicity of anti HER-2 therapies
3.1. Trastuzumab
In 1998, the Food and Drug Administration firstly approved the first anti-HER2 monoclonal antibody, TRZ for the treatment of HER2 overexpressing breast cancers. TRZ acts by binding HER2 extracellular domain IV and uncoupling oncogenic HER2/HER3 ligand-independent interactions in HER2-overexpressing cancer cells. Interference with HER2/NRG-1 signaling plays a significant role in the development of cardiac adverse effects by hampering cell ability to activate signal transduction pathways of maintenance of cardiomyocytes integrity and function and, notably, cardioprotective responses against noxious stimuli. Data from both in vitro and in vivo studies have supported the role of impaired contractile and metabolic properties [Citation14], mitochondrial dysfunction [Citation15], and inhibition of autophagy [Citation16] in TRZ cardiotoxicity.
3.1.1. Metastatic setting
Since the medicine was first used in clinical practise more than 20 years ago, TRZ cardiotoxicity has been the subject of multiple clinical trials that have looked closely at this clinical phenomenon. The significant cardiac toxicity related to the drug’s administration was earlier shown in the first phase 3 trial conducted in patients with metastatic breast cancer receiving TRZ therapy [Citation4]. In particular, compared to patients who received chemotherapy alone (1% and 8%, respectively), patients who got a TRZ-containing treatment regimen (TRZ+ paclitaxel or TRZ + anthracyclines) had a greater incidence of cardiac dysfunction, ranging from 13% to 27% [Citation4]. In addition, the finding that patients receiving TRZ plus anthracyclines had a significantly higher incidence of HF than those receiving other treatment regimens (16% vs 1–3%) [Citation4] raised concerns about a possible synergism caused by the simultaneous administration of TRZ and anthracyclines. Due to the finding of unexpectedly high rates of cardiac dysfunction, subsequent studies looking into TRZ cardiotoxicity imposed stringent patient selection criteria, excluding those who were most likely to experience negative cardiac events. Therefore, the stricter exclusion criteria, modifications to chemotherapy regimens that prevented the simultaneous administration of TRZ and anthracyclines, likely account for the lower incidence of adverse cardiac events seen in subsequent studies on TRZ cardiotoxicity [Citation17]. Furthermore, although with less certainty, the implementation of cardiac monitoring protocols (absent in the first phase III study analyzed above) may also have contributed to a lower incidence of heart failure; early detection of damage caused by TRZ administration may allow early implementation of cardioprotective strategies, containing the evolution toward HF.
3.1.2. Adjuvant and neoadjuvant setting
In the multicentre randomized HERA trial [Citation7] conducted in women with early-stage invasive breast cancer, cardiotoxicity rates were significantly lower, with an incidence of HF and LVEF decline>10% of 0.6% and 7% in the TRZ-treated arm compared to 0.0% and 2.1% in the control arm. As mentioned above, a plausible explanation for a significantly lower incidence of cardiotoxicity compared to previous studies is related to the cardiological exclusion criteria including an LVEF<55%, history of HF, CAD with previous ischemic Q wave, uncontrolled hypertension and clinically significant valvular dysfunction. The use of these exclusion criteria suggests some deviation of the HERA trial from a real-world candidate population for TRZ therapy. Exclusion criteria also include a maximum cumulative dose of doxorubicin and epirubicin (360 mg and 720 per square meter of body-surface area) lower than the maximum recommended dose for this class of drugs [Citation18,Citation19]. In addition, most patients with previous exposure to anthracyclines had received epirubicin, which is less cardiotoxic than doxorubicin. Ultimately, the time elapsed between TRZ administration and the end of the anthracycline cycle was significantly longer than in other trials that showed higher rates of cardiotoxicity (mean 89 days vs mean 21 days in the NSABP B-31 trial) [Citation20].
The results of the research conducted by Naumann et al. [Citation21] support the hypothesis that a higher level of patient selection in the clinical studies contributed to a lower incidence of cardiotoxicity;
using a real-world cohort of patients receiving TRZ adjuvant treatment, the rates of cardiotoxicity were greater than anticipated (15.7%) and more common in elderly individuals and those who had previously received anthracycline treatment [Citation21].
A recent study using data from the ALTTO trial [Citation22] analyzed the incidence of cardiac events in a population of 4190 patients treated with adjuvant TRZ or TRZ +lapatinib with a median follow-up of 6.9 years. Cardiovascular events occurred in 9.3% of TRZ-treated patients; the majority of these were asymptomatic (73%) and occurred while patients were undergoing anti-HER-2 therapy [Citation22] (6.4% occurred during anti-HER2 treatment and 2.2% during follow-up, with a median time to onset of 6.6 months). A primarily reversible mode of toxicity was also indicated by the recovery rate of 83.8%, which was compatible with the absence of necrosis or ultrastructural changes to myocytes [Citation23].
Compared to anthracyclines, the administration of TRZ results in a distinct type of cardiotoxicity. Classified as a drug with type II cardiotoxicity, TRZ induces non-dose-dependent and mainly reversible cardiac damage, inducing myocardiocyte dysfunction rather than cardiomyocyte necrosis [Citation24]. Of note, in the study by Ewer et al. [Citation25], 25 patients out of 38 who had experienced a drop in LVEF following TRZ administration (previously treated with anthracyclines) were resubmitted to TRZ treatment together with cardioprotective therapy and 88% experienced stability in LVEF. These data suggest not only a reversibility of TRZ-induced damage, but also a temporal correlation between time since anthracyclines were administered and the extent of TRZ-induced cardiac damage. As mentioned above, the disruption of the HER2 signaling pathway by TRZ makes cardiomyocytes less susceptible to recovery from a previous insult [Citation26–28]. This accounts for the higher incidence of CV events in patients on previous anthracycline-containing therapeutic regimens [Citation29,Citation30], which, even at low-to-medium cumulative doses, are responsible for the development of primarily irreversible cardiomyocyte damage that culminates in fibrotic replacement of necrotic cardiomyocytes [Citation31]. The damage induced by the administration of anthracyclines is no longer bioptically visible after about 3 months of the onset of the insult [24]; it is therefore reasonable that the time elapsed between the last dose of anthracyclines and the administration of TRZ is relevant to the extent of the cardiotoxic damage induced by the disruption of the HER2 signaling pathway, making the cardiac damage all the more relevant the less time elapsed since the anthracyclines were administered [Citation29].
Given the role of anthracycline administration prior to TRZ administration as a risk factor for the development of cardiotoxicity, anthracycline-free treatment regimens were implemented in order to reduce the incidence of LVDS and HF.
Administration in node-negative patients of a therapy containing paclitaxel and TRZ without prior administration of anthracyclines [Citation32] showed a 3-year disease-free survival 98.7% and a low risk of cancer recurrence (2%) demonstrating the efficacy of this treatment regimen. The incidence of cardiotoxic events was low; only two patients developed HF (0.5%) and experienced a recovery of ejection fraction after discontinuation of TRZ. Furthermore, the incidence of a significant decline in LVEF was 3.2%, showing a cardiac safety profile of this treatment regimen [Citation32]. Of note, the protocol included echocardiographic evaluation of patients at 12 weeks, 6 months and one year from the start of the study protocol, suggesting the validity of a less frequent approach to assessing left ventricular function. Future studies are needed to evaluate the efficacy of an anthracyclines-free therapy in a larger and more differentiated patient population.
Treatment regimens with a shorter time of drug administration were assessed in order to lower the incidence of adverse cardiac events linked to the administration of TRZ.
The PHARE trial [Citation33] evaluated in the adjuvant setting the non-inferiority of treatment with TRZ lasting 6 months VS 12 months. Although the group receiving TRZ for 6 months showed a statistically significant lower incidence of cardiac adverse events (1.9% vs 5.7 p p<0.0001) the trial failed to demonstrate non-inferiority of disease-free survival in the group receiving TRZ for 6 months VS 12 months (2-year disease-free survival 91.1 vs 93.8, hazard ratio 1.28; prespecified non-inferiority hazard ratio margin 1.15) [Citation33].
In contrast, data obtained from the PERSEPHONE study [Citation34] for the evaluation of non-inferiority of TRZ treatment for 6 months VS 12 months confirmed the non-inferiority of 6 months VS 12 months treatment (4-year disease-free survival 89.4 vs 89.8, hazard ratio 1–07). The study also showed a statistically significant lower incidence of clinical cardiac dysfunction in the 6-month VS 12-month group (8% vs 11%, p = 0.00014) [Citation34]. In light of these data, further studies are needed to test the validity of this approach.
3.2. Pertuzumab
Pertuzumab is a recombinant humanized monoclonal antibody targeting HER2 extracellular domain II, which is involved in receptor dimerization [Citation35]. In cancer cells, pertuzumab induces antibody-dependent cytotoxic effects, similarly to TRZ. Furthermore, pertuzumab prevents HER2 from combining with HER3 and/or HER1 in heterotypic association, and hence, the oncogenic downstream signaling, a mechanism of action different and complementary from that of TRZ. The synergistic mechanisms of action of pertuzumab and TRZ are thought to provide a dual blockade of HER2 signaling, likely accounting for enhanced antitumor efficacy when the two drugs are combined [Citation36,Citation37].
3.2.1. Metastatic setting
Numerous studies have been conducted to determine the effectiveness and safety of a combination therapy approach due to the better HER-2 signaling cascade blocking profile provided by the administration of pertuzumab and TRZ together rather than separately [Citation38]. The phase III CLEOPATRA study, conducted in patients with HER2-positive metastatic breast cancer, provided the main evidence on the cardiovascular safety profile of TRZ and pertuzumab administration [Citation39]. Of the 808 trial participants, 402 received pertuzumab plus TRZ plus docetaxel as their first-line therapy, while the remaining 406 received placebo plus TRZ plus docetaxel [Citation40]. The most frequent cardiac event was LVD (8.3% versus 4.4% in the placebo and pertuzumab arms, respectively), and the total number of adverse cardiac effects was 14.5% in the pertuzumab arm and 16.4% in the placebo arm [Citation40], demonstrating a cardiovascular safety profile of pertuzumab plus TRZ comparable to that of TRZ administration alone. Furthermore, in the pertuzumab arm, 86.7% (placebo arm: 72%) of patients who experienced a reduction in LVEF of 10% points or more from baseline or to<50% showed a recovery of LVEF to≥50% [Citation40]. Of note, the different incidence profiles of LVD in the placebo group and the pertuzumab-treated group can be attributed to a different cardiotoxicity profile of pertuzumab, which demonstrated a higher incidence of other CV events, such as arrhythmic events and valvular dysfunction. In the pertuzumab and placebo groups, there were 1.8% and 1% cases of symptomatic LVD, respectively [Citation40].
3.2.2. Adjuvant and neoadjuvant setting
The APHINITY study [Citation41] investigated the efficacy of a treatment regimen containing TRZ and pertuzumab (in addition to standard chemotherapy) compared with TRZ + placebo. The 3-year rate of invasive-disease-free survival was higher in the pertuzumab recipient group (94.1%) than in the TRZ+placebo recipient group (93.2, hazard ratio 0.81). The frequency of cardiovascular adverse events was low in both study arms: primary cardiac events occurred in 17 patients (0.7%) in the pertuzumab group and in 8 patients (0.3%) in the placebo group. The incidence of NYHA class III and IV heart failure was 0.6% and 0.2% in the arm containing pertuzumab and placebo, respectively, confirming a cardiac safety profile relative to pertuzumab administration.
Four distinct treatment regimens were used in the phase II Neosphere research to examine the effectiveness and safety of neoadjuvant treatment with TRZ and pertuzumab [Citation42]. The acquired data revealed a cardiac safety profile, with a mean maximum reduction in LVEF of 4–5% and no appreciable changes when pertuzumab was added to TRZ [Citation42]. Probably due to the considerable cardiac comorbidities the patient had when enrolled in the trial, only one patient in the pertuzumab therapy group developed HF.
3.3. Trastuzumab emtansine (T-DM1)
T-DM1 is an antibody-drug conjugate approved in 2013 for the treatment of HER2-positive metastatic breast cancer. T-DM1 combines anti-HER2 monoclonal antibody (TRZ) with a cytotoxic agent emtansine, DM1, though a nonreducible thioether linker. DM1 acts by inhibiting the polymerization of tubulin dimers into mature microtubules, thus interrupting the cell cycle [Citation43,Citation44]. In HER-positive cancer cells, upon the binding of T-DM1 to target antigens, the complex is internalized by endocytosis and digested into the lysosomes. The released DM1-containing active metabolite results in apoptosis and cell death.
3.3.1. Metastatic setting
T-DM1 was compared to lapatinib plus capecitabine in the EMILIA phase III research, which involved 991 patients with metastatic breast cancer HER-2 positive, who had previously received treatment [Citation45]. T-DM1 not only demonstrated a cardiac safety profile, with only three patients experiencing a drop in LVEF to 40% and 1.7% experiencing a drop in LVEF 15% below the baseline value, but it also significantly extended progression-free survival and overall survival when compared to the administration of lapatinib+ capecitabine [Citation45].
3.3.2. Adjuvant and neoadjuvant setting
In the phase III MARIANNE study [Citation46], administration of T-DM1 alone demonstrated a lower incidence of LVEF decrease compared to administration of T-DM1 plus pertuzumab or TRZ plus taxane: administration of T-DM1 showed a 50% decrease in LVEF with a ≥ 15% decrease from baseline in 0. 8% of cases, whereas T-DM1 plus pertuzumab and TRZ plus taxane had higher incidence rates of 2.5% and 4.5%, respectively. A recent study by Noam Pondè et al. [Citation47] examined data from seven separate clinical studies and evaluated a population of 1961 individuals who had received T-DM1 therapy to investigate the cardiotoxicity of the drug. The total number of cardiac events encountered was 3.37% with recovery in 79% of cases after discontinuation of treatment [Citation47]. The most frequent cardiac event was a decrease in LVEF, with an incidence of 2.04% for a grade 1/2 decrease and 0.71% for a severe (grade 3/4) decrease or the development of HF [Citation47]. The temporary treatment discontinuation rate in patients in whom an adverse cardiac event was reported was significantly higher than the discontinuation rate of patients in whom only non-cardiac events were reported (56.1% vs. 27.8%). Furthermore, in the group of patients with reported adverse cardiac events in 57% of cases the CV event was indicated as being responsible for temporary discontinuation of treatment. In addition, treatment discontinuation had a similar ratio (28.8% in the group with reporting of adverse cardiac events and 10.3% in the group with only non-cardiac adverse events) and in 58% of the cases the cardiac event was indicated as responsible for discontinuation of treatment [Citation47]. These data would seem to suggest that the occurrence of cardiotoxicity has a significant impact in relation to treatment discontinuation. According to the National Cancer Institute-Common Terminology Criteria for adverse events (NCI-CTCAE) most events were classified as grade I and II (21.21% and 59.1%, respectively) while only 19.7% of events were classified as grade III and none as grade IV. These data therefore suggest that temporary discontinuation of treatment and permanent discontinuation are attributable to the strict discontinuation rules used in clinical trials, and therefore do not reflect what would be found outside the study protocol; in patients experiencing an adverse cardiac event, discontinuation of treatment should not be considered as a first choice and should be subject to a thorough cost-benefit analysis of discontinuation of life-saving treatment.
3.4. Trastuzumab deruxtecan
Trastuzumab deruxtecan is an immunoconjugate combining an antibody component and a topoisomerase I inhibitor, the exatecan derivative DXd, through a cleavable tetrapeptide linker. Trastuzumab deruxtecan combines with and inhibits human DNA topoisomerase I, resulting in the induction of DNA damage and activation of apoptosis pathways. Pharmacologically, Trastuzumab deruxtecan shows higher drug-to-antibody ratio as compared to T-DM1, about (8 vs. 3 to 4). Further, payload exhibits a higher membrane permeability and a shorter half-life, which enables to exert its effects on surrounding cells with lower systemic exposition, respectively [Citation48].
3.4.1. Metastatic setting
Evidence from the DESTINY-Breast04 trial showed that TRZ deruxtecan resulted in longer progression-free survival and overall survival as compared to physician’s choice of chemotherapy for the treatment of HER2-low unresectable or metastatic breast cancer, (i.e. immunohistochemical (IHC) score of 1+/IHC score of 2+ in absence of HER2 expression on in situ hybridization.) [Citation49]. This seemingly rely on the ability of Trastuzumab deruxtecan to act on HER2-low expressing cancer cell and to nearby cells, regardless the expression of the target antigen, through a bystander effect [Citation50]. Similar to T-DM1, in the studies conducted so far, the incidence of LVEF decrease appears to be low. In the phase II study DESTINY-Breast01 of 184 patients receiving trastuzumab deruxtecan therapy, 1.6% experienced a decrease in LVEF [Citation51]. A cardiac safety profile in relation to the administration of trastuzumab deruxtecan was also demonstrated by the phase II trial DESTINY-Gastric01, which reported no cardiac events [Citation52].
3.5. Tucatinib
Tucatinib is an orally administered small TKI approved in 2020 for the treatment HER2+ metastatic breast cancer. Tucatinib selectively targets HER2 and has proven antitumor efficacy in xenograft models of breast, gastric, colorectal, and esophageal cancers [Citation53]. Enhanced antitumor effects were achieved when tucatinib was combined with TRZ. Although data regarding tucatinib cardiotoxicity are still limited, clinical trials have reported lower rates of cardiotoxicity compared to other HER2; this is likely due to the high selectivity of tucatinib for the tyrosine kinase domain of HER2, while displaying a minor inhibitory activity against EGFR [Citation53].
4. Cardiovascular risk assessment before cancer therapy
4.1. History and clinical examination
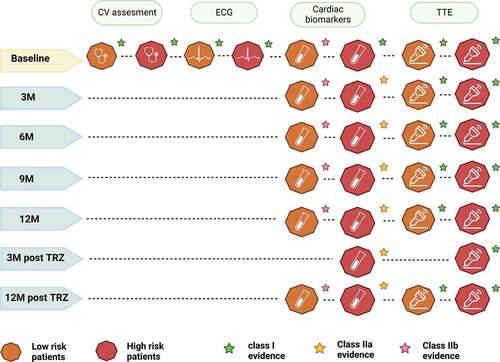
A thorough cardiovascular risk assessment is recommended in all patients before starting potentially cardiotoxic cancer therapy, together with a careful history and physical examination [Citation54]. The Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society has developed a useful algorithm for risk stratification of patients who are candidate to HER2 target therapy, assessing parameters such as previous cardiovascular disease, cardiac biomarkers, classical cardiovascular risk factors (e.g. age, hypertension, diabetes mellitus, smoking), and previous cardiotoxic treatment [Citation55] () [Citation21,Citation56–63]. Cardiological referral is recommended for patients at high or very high risk in order to perform the proper risk reduction measures. Patients in the moderate risk class should undergo management of cardiovascular risk factors and close cardiological monitoring. In the case of low-risk patients, a cardio oncological referral is not recommended, unless development of cancer therapy-related cardiovascular toxicity (CTR-CVT) [Citation54].
Table 1. Research examining the clinical risk factors for the induction of cardiomyopathy caused by trastuzumab.
4.2. Electrocardiogram
An ECG at baseline is recommended prior to the start of therapy to complete the cardiovascular risk assessment (). When ECG anomalies are found, a cardiologist referral is recommended for the patient; conversely, in the case of no anomalies, there is no indication for subsequent electrocardiographic evaluation during therapy.
4.3. Cardiac biomarkers
The timing for the assessment of cardiac biomarkers has not yet been precisely established and is still a matter of debate. In order to stratify the patient’s cardiovascular risk, a preliminary evaluation of biomarkers is advised, especially in individuals who are considered to be at high or very high risk [Citation64,Citation65]. Several studies have evaluated the efficacy of cardiac troponin in predicting the risk of developing cardiotoxicity in patients receiving TRZ therapy. In a cohort of 251 HER2-positive breast cancer patients, cardiac troponin (cTn) I levels were assessed before and after each cycle of TRZ, both in the adjuvant setting and in metastatic disease [Citation66]. The development of cardiotoxicity occurred in 72% of patients who had a troponin elevation and in only 7% of troponin-negative patients, making troponin elevation the strongest independent predictor of cardiotoxicity [Citation66].
In addition, increased baseline troponin I values were discovered in 19% of patients who had cardiotoxicity, and these values were indicative of a failure to regain cardiac function while receiving adequate heart failure treatment [Citation66]. Similarly, a research conducted on 452 patients found that baseline elevation of cTn I and T levels was linked to a higher risk of cardiotoxicity and lower LVEF values [Citation67]. Given the presence in both studies of a high proportion of patients previously treated with anthracyclines, the finding of elevated troponin values at baseline principally reflects previous anthracycline therapy. It is therefore still unclear whether the assessment of troponin at baseline can have the same predictive value for the development of cardiotoxicity in patients who have not previously received anthracycline therapy. Similarly, several studies have evaluated natriuretic peptide (Np) assessment at baseline for cardiovascular risk stratification and in predicting possible development of cardiotoxicity, but actually there is no indication for monitoring this biomarker during therapy with HER2-targeted therapies [Citation65,Citation68,Citation69]. Monitoring of Np and cTn during treatment should be considered every 2–3 cycles, and the third and 12th month after the end of therapy in high and very high risk patients with HER2 positive breast cancer. Evaluation of cardiac biomarkers is also possible in low or intermediate risk patients, although it is supported by lower levels of evidence [Citation64] ().
4.4. Cardiovascular imaging
Cardiovascular imaging plays a major role in patient risk stratification, in the detection of possible sub-clinical cardiovascular pathology, and in planning the future course of action in the patient who will undergo potentially cardiotoxic therapy. Among the available imaging techniques such as echocardiography, magnetic resonance imaging (MRI) and multi-gated acquisition (MUGA), the performance of a three-dimensional (3D) transthoracic echocardiography (TTE) is recommended, as it allows the acquisition of all the volumetric and functional information (e.g LVEF) necessary for the assessment of the patient prior to the start of therapy. If it is not possible to perform a TTE or the data obtained cannot be interpreted, MRI can be used as a second choice, although cost, the need for specialized staff and the lack of diffusion of this technique do not yet make it commonly used in clinical practice [Citation70]. MUGA is the test of third preference when neither TTE nor MRI can be conducted. This is mainly because the patient is exposed to a substantial amount of radiation during each session, which is particularly relevant in an oncological setting [Citation71]. Assessment of LVEF and global longitudinal strain (GLS) at baseline is recommended in all patients who will undergo HER2-targeted therapy both for cardiovascular risk stratification and to track changes in ventricular function during treatment [Citation72]. Borderline LVEF values (50–54%) or<50% at baseline correlate with an increased risk of developing cardiotoxicity from HER2 target therapy [Citation73]. Given the availability of multiple approaches to measure LVEF, is recommended to acquired LVEF value using the same technique in order to minimize inter-technique variability [Citation74].
Although LVEF monitoring is the current gold standard for monitoring for cardiotoxicity, there is still disagreement on its precision, repeatability, applicability, and timeliness in predicting cardiac dysfunction [Citation75,Citation76]. Assessment of GLS has been shown to have a higher and earlier predictive value of approximately 3 months for the development of cardiotoxicity than variation in LVEF [Citation77,Citation78]. The detection of altered GLS values would allow early cardioprotective therapy to be implemented, which would be useful in order to avoid possible discontinuation of anti-cancer treatment. Although further studies are still needed to implement the use of GLS in routine practice and to determine its precise timing of acquisition, the recent incorporation of GLS assessment into the cardio-oncology echocardiography protocol published by the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) is an important step toward its wider use [Citation70]. According to the most recent 2022 ESC Guidelines on cardio-oncology for the scheduling of echocardiographic acquisitions, patients receiving HER2 target treatments in both adjuvant and neoadjuvant settings should receive a TTE every three months and within a year after completing treatment () [Citation54]. In the first year of treatment for metastatic HER2+ disease, echocardiography is advised to be performed every three months [Citation54]; after that, if the patient is asymptomatic and there is no CV toxicity, surveillance can be decreased to every six months [Citation54].
Of note, a recent study by Dent and colleagues [Citation79] compared the effectiveness of a 3- versus 4-monthly cardiac monitoring protocol in a population of early breast cancer patients. The incidence rate of dysfunction between the two study arms was 16.3% in the 3-month monitoring arm and 12.4% in the 4-month monitoring arm, not reaching statistical significance (95% CI: 4.0 [IQR −5.9, 13.8]; p = 0.69). In view of the non-inferiority of cardiac monitoring at 4 months compared to 3 months, less frequent echocardiographic monitoring should be considered. Future studies are required to explore the precise timing of echocardiographic investigations in more detail, especially in relation to the introduction of anthracycline-free treatment regimens and a generally young early breast cancer population at low cardiovascular risk, where less frequent monitoring could be evaluated.
5. Management of HER2 target therapies cardiotoxicity
A decline in LVEF is the primary sign of cardiotoxicity connected to the use of HER2 target therapy. Although several studies have utilized a drop in LVEF of 15% or more from baseline or a value<50% as a cutoff for the development of cardiotoxicity [Citation80], historically a drop in LVEF of 10% was thought to be the cutoff for the onset of cancer therapy-related cardiac dysfunction (CTRCD) [Citation81]. Thus, a decrease in LVEF≥10% is regarded as probable cardiotoxic evidence, particularly if the value falls below 50%. The 2022 ESC Guidelines on cardio-oncology also implemented the GLS value in the definition of CTRCD, which is diagnosed if there is a relative decline in GLS>15% from baseline as well as new rise in cardiac biomarker [Citation54]. A multidisciplinary team should be involved in cases of cardiotoxicity related to HER2 target therapy in order to handle following therapeutic decisions most effectively [Citation82]. For patients who experience mild (LVEF>50%) or moderate (LVEF 40–49%) asymptomatic CTRCTD, there is no indication for discontinuation of treatment, whereas initiation of cardioprotective therapy with an ACE-i, beta-blocker or angiotensin receptor blockers is recommended [Citation83]. In patients with mild symptomatic CTRCD, a multidisciplinary team approach is recommended to continue or discontinue HER2 therapy [Citation83]. Temporary discontinuation of treatment is recommended in patients who develop symptomatic and asymptomatic severe (LVEF<40%) or symptomatic moderate CTRCD, and initiation of treatment for acute and chronic HF according to the ESC 2021 guidelines [Citation84] is recommended in cases of severe CTRCD.
6. Conclusions
The introduction of target therapies against HER2 has completely revolutionized the treatment of breast cancer patients, significantly increasing survival. Numerous studies have demonstrated the cardiotoxicity related to the administration of these drugs, highlighting the need for strategies to identify patients at increased risk of developing cardiovascular complications. Some studies have demonstrated a lower cardiotoxic profile of the newer HER2 target therapies such as T-DM1 and tucatinib; despite promising results, future studies are needed to comprehensively explore the cardiotoxic potential of these drugs.
The growing population of high-risk patients who are possible candidates for HER2 target therapies also makes it necessary to study treatment and follow-up strategies that would make the eventual administration of these drugs safe in this population. Beyond the cardiovascular assessment of the patient at baseline, useful tools for early detection of cardiotoxicity are cardiac biomarkers and imaging. Currently the biomarkers used in clinical practice are cTn and Np, which have been shown to be useful predictors of cardiotoxicity especially in patients previously treated with anthracyclines. The imaging techniques recommended by the ESC guidelines are three-dimensional ultrasound and secondarily MRI. Assessment of GLS could be a valid alternative to measuring LVEF, ensuring detection of early subclinical cardiac dysfunction, although future studies are needed to assess the efficacy of this technique. When CTRCD develops, secondary prevention protocols should be implemented by administering drugs (eg beta blocker, ACE inhibitor) where necessary, accompanied by more frequent patient follow-up.
7. Expert opinion
HER-2-targeted therapies have been a gold standard for the treatment of breast cancer and specific forms of gastric cancer for many years. As a new standard of care is anthracycline-free regimens for locally advanced HER2-positive breast cancer, robust data on cardiotoxicity in this specific population are awaited. Moreover, further studies are needed to better define the cardiotoxicity of new HER2 target therapies, such as T-DM1 and tucatinib. Today, methods to prevent, monitor and identify HER-2-induced cardiotoxicity need to be strengthened, especially in a new and increasingly represented high-risk patient population (including patients with preexisting cardiac dysfunction or a history of overt heart failure).
The use of primary pharmacological preventive strategies is still a rather debated topic and the data obtained from the studies conducted so far are rather heterogeneous. According to preliminary findings from the study by Guglin et al. [Citation85] anthracycline-exposed patients who are at high risk of experiencing cardiotoxicity from TRZ may benefit from treatment with lisinopril or carvedilol. Data from the PRADA study [Citation86] demonstrated a modest protective effect of Candesartan administration with a minor reduction in LVEF, whereas metoprolol did not reach statistical significance. On the contrary, a subsequent study [Citation87] demonstrated the non-efficacy of candesartan in preventing a decrease in LVEF in patients previously treated with anthracyclines. Future research is required for a more precise description of potential preventative treatments for TRZ cardiotoxicity due to the lack of studies on the effectiveness of preventive medication, particularly in individuals who have never received anthracycline treatment. Future studies including new HER2 target therapies such as trastuzumab deruxtecan, T-DM1 and tucatinib are also needed.
Clinical trials and retrospective analyses measure cardiac function using changes in LVEF with symptomatic HF as an endpoint. Although LVEF monitoring is the current gold standard of care for monitoring cardiotoxicity, there is a variety of evidence on the evaluation with more advanced echocardiographic techniques (speckle tracking imaging, 3D echocardiography) or cardiac MRI (mapping techniques).
Studies on the validity of using cardiac Hs-troponins as a predictor of cardiotoxicity affirm the usefulness of this biomarker mainly in patients who have previously undergone anthracycline therapy. The use of this biomarker in an anthracycline-naive setting is still a matter of debate as is the precise timing for its evaluation. As shown in the NeoALTTO study [Citation88], the evaluation of troponin I in patients treated with HER target therapies in the neoadjuvant setting did not prove predictive of the development of cardiotoxicity. Therefore, further studies are needed to establish the actual usefulness of this biomarker assay.
In addition, given the potential for early detection of cardiotoxicity from the assessment of cardiac biomarkers, future studies would be required to evaluate the potential efficacy of a combination with imaging techniques in order to obtain useful algorithms for an earlier detection of cardiotoxicity and a prompter prevention strategy.
Furthermore, several studies [Citation89,Citation90] are currently underway to evaluate the efficacy of other biomarkers, including myeloperoxidase (MPO), C-reactive protein (CRP) and growth differentiation factor-15 (GDF-15) to test their usefulness in patients undergoing cardiotoxic therapy. Given the preliminary encouraging results regarding their usefulness, further studies are needed to establish an appropriate timing for their measurement and possible intervention based on the values found.
Article highlights
Breast cancer is a clinical entity of major epidemiological importance
Target her 2 therapies, and in particular trastuzumab, are cardiotoxic, a relevant aspect in a growing population burdened with increased cardiovascular risk
Cardiotoxicity from HER2 targeted therapies is mainly characterized by asymptomatic decrease in LVEF and, to a lesser extent, with the development of heart failure
risk stratification is a vital aspect of the initial assessment of the patient candidate for HER2 targeted therapies
Cardiac biomarkers and trans-thoracic ultrasound are the main diagnostic tools for assessing cardiotoxicity from HER2 targeted therapies
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or mending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012: globocan 2012. Int J Cancer. 2015;136(5):E359–386. DOI:10.1002/ijc.29210
- Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 Status. JNCI. 2014 [[cited 2022 Dec 2]];106. InternetAvailable from: https://academic.oup.com/jnci/article-lookup/doi/10.1093/jnci/dju055
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–137.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. DOI:10.1056/NEJM200103153441101
- Joensuu H, Kellokumpu-Lehtinen P-L, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354(8):809–820. DOI:10.1056/NEJMoa053028
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. DOI:10.1056/NEJMoa052122
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-Positive Breast Cancer. N Engl J Med. 2005;353(16):1659–1672. DOI:10.1056/NEJMoa052306
- Muller WJ, Sinn E, Pattengale PK, et al. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54:105–115.
- Di Fiore PP, Pierce JH, Kraus MH, et al. Erb B-2 is a Potent Oncogene When Overexpressed in NIH/3T3 Cells. Science. 1987;237:178–182.
- Crone SA, Zhao Y-Y, Fan L, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8(5):459–465. DOI:10.1038/nm0502-459
- Özcelik C, Erdmann B, Pilz B, et al. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci, USA. 2002;99(13):8880–8885. DOI:10.1073/pnas.122249299
- Odiete O, Hill MF, Sawyer DB. Neuregulin in cardiovascular development and disease. Circ Res. 2012;111(10):1376–1385.
- Bersell K, Arab S, Haring B, et al. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270.
- Kitani T, Ong S-G, Lam CK, et al. Human-induced pluripotent stem cell model of trastuzumab-induced cardiac dysfunction in patients with breast cancer. Circulation. 2019;139:2451–2465.
- Grazette LP, Boecker W, Matsui T, et al. Inhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes. J Am Coll Cardiol. 2004;44:2231–2238.
- Mohan N, Shen Y, Endo Y, et al. Trastuzumab, but not pertuzumab, dysregulates HER2 signaling to mediate inhibition of autophagy and increase in reactive oxygen species production in human cardiomyocytes. Mol Cancer Ther. 2016;15:1321–1331.
- Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36.
- Labeling.Pfizer.com/[Internet]. (NY): pfizer; [cited 2023 Mar 23]. Available from: https://labeling.pfizer.com/ShowLabeling.aspx?id=12164
- Labeling.Pfizer.com/[Internet]. (NY): pfizer; [cited 2023 Mar 23]. Available from: https://labeling.pfizer.com/ShowLabeling.aspx?id=15025
- Perez EA, Romond EH, Suman VJ, et al. Updated results of the combined analysis of NCCTG N9831 and NSABP B-31 adjuvant chemotherapy with/without trastuzumab in patients with HER2-positive breast cancer. JCO. 2007;25(18_suppl):512. DOI:10.1200/jco.2007.25.18_suppl.512
- Naumann D, Rusius V, Margiotta C, et al. Factors predicting trastuzumab-related cardiotoxicity in a real-world population of women with HER2+ breast cancer. Anticancer Res. 2013;33(4):1717–1720.
- Eiger D, Pondé NF, Agbor-Tarh D, et al. Long-term cardiac outcomes of patients with HER2-positive breast cancer treated in the adjuvant lapatinib and/or trastuzumab treatment optimization trial. Br J Cancer. 2020;122(10):1453–1460. DOI:10.1038/s41416-020-0786-x
- Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol. 2015;12(9):547–558.
- JOURNAL SCAN. Journal scan. Oncol Times. 2005;27(17):14–16. doi: 10.1097/01.COT.0000294343.77525.55.
- Ewer MS, Vooletich MT, Durand J-B, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. JCO. 2005;23(31):7820–7826. DOI:10.1200/JCO.2005.13.300
- ElZarrad MK, Mukhopadhyay P, Mohan N, et al. Trastuzumab alters the expression of genes essential for cardiac function and induces ultrastructural changes of cardiomyocytes in mice. In: Peng T, editor. PLoS ONE. San Francisco, California, and Cambridge, United Kingdom: Plose One; 2013. p. e79543.
- Timolati F, Ott D, Pentassuglia L, et al. Neuregulin-1 beta attenuates doxorubicin-induced alterations of excitation–contraction coupling and reduces oxidative stress in adult rat cardiomyocytes. J Mol Cell Cardiol. 2006;41:845–854.
- Sawyer DB, Zuppinger C, Miller TA, et al. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1β and Anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105:1551–1554.
- Vejpongsa P, Yeh ETH. Prevention Of anthracycline-induced cardiotoxicity. J Am Coll Cardiol. 2014;64(9):938–945.
- Yu AF, Mukku RB, Verma S, et al. Cardiac safety of non-anthracycline trastuzumab-based therapy for HER2-positive breast cancer. Breast Cancer Res Treat. 2017;166(1):241–247. DOI:10.1007/s10549-017-4362-x
- Ewer MS, Ali MK, Mackay B, et al. A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving Adriamycin. JCO. 1984;2(2):112–117. DOI:10.1200/JCO.1984.2.2.112
- Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372(2):134–141. DOI:10.1056/NEJMoa1406281. .
- Pivot X, Romieu G, Debled M, et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14:741–748.
- Earl HM, Hiller L, Vallier A-L, et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet. 2019;393:2599–2612.
- Adams CW, Allison DE, Flagella K, et al. Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol Immunother. 2006;55(6):717–727. DOI:10.1007/s00262-005-0058-x
- Portera CC, Walshe JM, Rosing DR, et al. Cardiac Toxicity and efficacy of trastuzumab combined with pertuzumab in patients with trastuzumab-insensitive human epidermal growth factor receptor 2–positive metastatic breast Cancer. Clin Cancer Res. 2008;14:2710–2716.
- Scheuer W, Friess T, Burtscher H, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on her2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–9336.
- Nahta R, Hung M-C, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–2346.
- Swain SM, Baselga J, Kim S-B, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–734. DOI:10.1056/NEJMoa1413513
- Swain SM, Ewer MS, Cortés J, et al. Cardiac Tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with her2-positive metastatic breast cancer in Cleopatra: a randomized, double-blind, placebo-controlled phase III Study. Oncology. 2013;18:257–264.
- von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122–131. DOI:10.1056/NEJMoa1703643
- Gianni L, Pienkowski T, Y-H I, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. DOI:10.1016/S1470-2045(11)70336-9
- Krop I, Winer EP. Trastuzumab emtansine: a novel antibody–drug conjugate for HER2-Positive Breast Cancer. Clin Cancer Res. 2014;20:15–20.
- Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugate. Cancer Res. 2008;68:9280–9290.
- Verma S, Miles D, Gianni L, et al. Trastuzumab Emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. DOI:10.1056/NEJMoa1209124
- Perez EA, Barrios C, Eiermann W, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2–positive, advanced breast cancer: primary results from the phase III Marianne study. JCO. 2017;35(2):141–148. DOI:10.1200/JCO.2016.67.4887
- Pondé N, Ameye L, Lambertini M, et al. Trastuzumab emtansine (T-DM1)-associated cardiotoxicity: pooled analysis in advanced HER2-positive breast cancer. Eur J Cancer. 2020;126:65–73.
- Yin O, Xiong Y, Endo S, et al. Population Pharmacokinetics of Trastuzumab Deruxtecan in Patients with HER2‐Positive Breast Cancer and Other Solid Tumors. Clin Pharmacol Ther. 2021;109(5):1314–1325. DOI:10.1002/cpt.2096
- Modi S, Jacot W, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20. DOI:10.1056/NEJMoa2203690
- Rinnerthaler G, Gampenrieder S, Greil R. HER2 directed antibody-drug-conjugates beyond T-DM1 in breast cancer. IJMS. 2019;20(5):1115.
- Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–621. DOI:10.1056/NEJMoa1914510
- Shitara K, Bang Y-J, Iwasa S, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med. 2020;382(25):2419–2430. DOI:10.1056/NEJMoa2004413
- Kulukian A, Lee P, Taylor J, et al. Preclinical Activity of HER2-Selective Tyrosine Kinase Inhibitor Tucatinib as a Single Agent or in Combination with Trastuzumab or Docetaxel in Solid Tumor Models. Mol Cancer Ther. 2020;19:976–987.
- Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43:4229–4361.
- Ar L, Dent S, Stanway S, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22(11):1945–1960. DOI:10.1002/ejhf.1920
- Farolfi A, Melegari E, Aquilina M, et al. Trastuzumab-induced cardiotoxicity in early breast cancer patients: a retrospective study of possible risk and protective factors. Heart. 2013;99:634–639.
- Jawa Z, Perez RM, Garlie L, et al. Risk factors of trastuzumab-induced cardiotoxicity in breast cancer: a meta-analysis. Medicine. 2016;95:e5195.
- Guenancia C, Lefebvre A, Cardinale D, et al. Obesity as a risk factor for anthracyclines and trastuzumab cardiotoxicity in breast cancer: a systematic review and meta-analysis. JCO. 2016;34(26):3157–3165. DOI:10.1200/JCO.2016.67.4846
- Baron KB, Brown JR, Heiss BL, et al. Trastuzumab-induced cardiomyopathy: incidence and associated risk factors in an inner-city population. J Card Fail. 2014;20:555–559.
- Ezaz G, Long JB, Gross CP, et al. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. JAHA. 2014;3(1):e000472. DOI:10.1161/JAHA.113.000472
- Tang GH, Acuna SA, Sevick L, et al. Incidence and identification of risk factors for trastuzumab-induced cardiotoxicity in breast cancer patients: an audit of a single “real-world” setting. Med Oncol. 2017;34(9):154. DOI:10.1007/s12032-017-1018-y
- Gunaldi M, Duman BB, Afsar CU, et al. Risk factors for developing cardiotoxicity of trastuzumab in breast cancer patients: an observational single-centre study. J Oncol Pharm Pract. 2016;22(2):242–247. DOI:10.1177/1078155214567162
- Serrano C, Cortés J, De Mattos-Arruda L, et al. Trastuzumab-related cardiotoxicity in the elderly: a role for cardiovascular risk factors. Ann Oncol. 2012;23:897–902.
- Michel L, Mincu RI, Mahabadi AA, et al. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta‐analysis. Eur J Heart Fail. 2020;22(2):350–361. DOI:10.1002/ejhf.1631
- Demissei BG, Hubbard RA, Zhang L, et al. Changes in Cardiovascular Biomarkers with Breast Cancer Therapy and Associations with Cardiac Dysfunction. JAHA. 2020;9(2):e014708. DOI:10.1161/JAHA.119.014708
- Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-Induced Cardiotoxicity: clinical and Prognostic Implications of Troponin I Evaluation. JCO. 2010;28(25):3910–3916. DOI:10.1200/JCO.2009.27.3615.
- Zardavas D, Suter TM, Van Veldhuisen DJ, et al. Role of Troponins I and T and N -terminal prohormone of brain natriuretic peptide in monitoring cardiac safety of patients with early-stage human epidermal growth factor receptor 2–positive breast cancer receiving trastuzumab: a herceptin adjuvant study cardiac marker substudy. JCO. 2017;35:878–884.
- Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21(6):715–731. DOI:10.1002/ejhf.1494
- Feola M, Garrone O, Occelli M, et al. Cardiotoxicity after anthracycline chemotherapy in breast carcinoma: effects on left ventricular ejection fraction, troponin I and brain natriuretic peptide. Int J Cardiol. 2011;148:194–198.
- Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15:1063–1093.
- Fazel R, Krumholz HM, Wang Y, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361(9):849–857. DOI:10.1056/NEJMoa0901249
- Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195–1205. DOI:10.1016/S0140-6736(16)32616-2
- Perez EA, Suman VJ, Davidson NE, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the north central cancer treatment group n9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26(8):1231–1238. DOI:10.1200/JCO.2007.13.5467
- Thavendiranathan P, Grant AD, Negishi T, et al. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes. J Am Coll Cardiol. 2013;61(1):77–84. DOI:10.1016/j.jacc.2012.09.035
- Dang CT, Yu AF, Jones LW, et al. Cardiac surveillance guidelines for trastuzumab-containing therapy in early-stage breast cancer: getting to the heart of the matter. J Clin Oncol. 2016;34(10):1030–1033. DOI:10.1200/JCO.2015.64.5515
- Davis CC, Zelnak A, Eley JW, et al. Clinical utility of routine cardiac monitoring in breast cancer patients receiving trastuzumab. Ann Pharmacother. 2016;50(9):712–717. DOI:10.1177/1060028016654160
- Negishi K, Negishi T, Haluska BA, et al. Use of speckle strain to assess left ventricular responses to cardiotoxic chemotherapy and cardioprotection. Eur Heart J Cardiovasc Imaging. 2014;15(3):324–331. DOI:10.1093/ehjci/jet159
- Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5(5):596–603. DOI:10.1161/CIRCIMAGING.112.973321
- Dent S, Fergusson D, Aseyev O, et al. A randomized trial comparing 3- versus 4-monthly cardiac monitoring in patients receiving trastuzumab-based chemotherapy for early breast cancer. Current Oncol. 2021;28(6):5073–5083. DOI:10.3390/curroncol28060427. .
- Tan-Chiu E, Yothers G, Romond E, et al. Assessment Of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2–overexpressing breast cancer: nSABP B-31. JCO. 2005;23:7811–7819.
- Bird BRJH, Swain SM. Cardiac Toxicity in Breast Cancer Survivors: review of Potential Cardiac Problems. Clin Cancer Res. 2008;14:14–24.
- Fabiani I, Cipolla CM, Colombo N, et al. Cardioncological approach for trastuzumab therapy in breast cancer patients with cardiotoxicity: impact on adherence and clinical outcome. Front Pharmacol. 2020;11:1190.
- Curigliano G, Lenihan D, Fradley M, et al. Management of cardiac disease in cancer patients throughout oncological treatment: eSMO consensus recommendations. Ann Oncol. 2020;31:171–190.
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726.
- Guglin ME, Krischer J, Tamura R. “Lisinopril or carvedilol for prevention of trastuzumab induced cardiotoxicity.” 67th Annual American College of Cardiology Meeting. 2018. Orlando, FL: Orange County Convention Center.
- Gulati G, Heck SL, Ree AH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671–1680.
- Boekhout AH, Gietema JA, Milojkovic Kerklaan B, et al. Angiotensin II–Receptor inhibition with candesartan to prevent trastuzumab-related cardiotoxic effects in patients with early breast cancer: a randomized clinical trial. JAMA Oncol. 2016;2:1030.
- Ponde N, Bradbury I, Lambertini M, et al. Cardiac biomarkers for early detection and prediction of trastuzumab and/or lapatinib-induced cardiotoxicity in patients with HER2-positive early-stage breast cancer: a NeoALTTO sub-study (BIG 1-06). Breast Cancer Res Treat. 2018;168:631–638.
- Ky B, Putt M, Sawaya H, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–816.
- Putt M, Hahn VS, Januzzi JL, et al. Longitudinal changes in multiple biomarkers are associated with cardiotoxicity in breast cancer patients treated with doxorubicin, taxanes, and trastuzumab. Clin Chem. 2015;61:1164–1172.