ABSTRACT
Introduction
Paracetamol is one of the most used medicines worldwide and is the most common important poisoning in high-income countries. In overdose, paracetamol causes dose-dependent hepatotoxicity. Acetylcysteine is an effective antidote, however despite its use hepatotoxicity and many deaths still occur.
Areas covered
This review summarizes paracetamol overdose and toxicity (including mechanisms, risk factors, risk assessment, and treatment). In addition, we summarize the epidemiology of paracetamol overdose worldwide. A literature search on PubMed for poisoning epidemiology and mortality from 1 January 2017 to 26 October 2022 was performed to estimate rates of paracetamol overdose, liver injury, and deaths worldwide.
Expert opinion
Paracetamol is widely available and yet is substantially more toxic than other analgesics available without prescription. Where data were available, we estimate that paracetamol is involved in 6% of poisonings, 56% of severe acute liver injury and acute liver failure, and 7% of drug-induced liver injury. These estimates are limited by lack of available data from many countries, particularly in Asia, South America, and Africa. Harm reduction from paracetamol is possible through better identification of high-risk overdoses, and better treatment regimens. Large overdoses and those involving modified-release paracetamol are high-risk and can be targeted through legislative change.
1. Introduction
Paracetamol (acetaminophen) is one of the most widely used medicines worldwide and is readily available without prescription in most countries [Citation1]. It is listed on the World Health Organisation’s (WHO) Essential Medicines List [Citation2]. It is recommended as a first-line treatment for most cases of pain and fever and is safe to use in children as young as one-month old as well as women who are pregnant [Citation3]. It comes in a variety of forms and strengths including oral tablets, capsules, and liquid formulations as well as rectal suppositories.
Compared to other analgesics accessible without a prescription, paracetamol has a relatively narrow therapeutic index. Toxicity can occur following dosing errors, accidental exploratory ingestions in children, and deliberate self-harm overdose. Paracetamol can cause severe hepatotoxicity with as little as 10 g (or 200 mg/kg for patients under 50 kg) in an acute overdose. Repeated supratherapeutic ingestion can cause toxicity in doses only slightly above the maximum daily therapeutic dose [Citation4]. There is an effective antidote to paracetamol (N-acetylcysteine or NAC); however, despite this, paracetamol toxicity is still the leading cause of acute liver failure (ALF) in most high-income countries [Citation5–10]. Severe cases may require liver transplantation or result in death [Citation9,Citation11].
This review explores recent estimates in rates of overdose and hepatotoxicity from paracetamol, including mechanism, diagnosis, and treatments. We explore global patterns of paracetamol poisoning epidemiology. For the purposes of this review, we define poisoning as contact, whether intentional or accidental, with a substance which may cause harm.
1.1. Literature search
We performed two separate literature searches for this review.
We attempted to analyze available data to determine the number of paracetamol poisoning exposures worldwide (Section 5). When data on paracetamol specifically were not available, we used data on analgesics. To do this we used three methods (). The first was a PubMed search for overall poisoning epidemiology literature limited to the last 5 years and manually screened for relevant studies containing data on analgesics and/or paracetamol (see Supplementary Methods). The second was a search of available annual reports from poisons information center websites using the 2021 Poisons Centre Directory available from the WHO. Lastly, using the WHO’s 2021 directory we emailed each PIC for their most recent annual report or data on paracetamol exposures. From this, we were able to include 88 relevant publications, 17 annual reports or statistical information from websites and data from 10 e-mail responses (, Supplementary Table S1). For additional details on methodology, see supplementary methods.
The rest of the review covers a wide range of topics on clinical toxicology of paracetamol. For this, we conducted searches on PubMed and Embase from inception to December 2022. We used keyword searches and focused on specific points, including risk assessment, risk factors, management, and legislative change/restrictions. We also looked for systematic reviews relating to paracetamol and conducted citation chaining.
2. Discovery and use
Paracetamol is an aniline derivative. Acetanilide was the first aniline derivative to be used as an antipyretic and analgesic in 1886 [Citation1,Citation12]. However, it was found to have frequent toxic effects (particularly methaemoglobinemia) [Citation13] and so exploration of further aniline derivatives commenced [Citation1]. This led to the discovery of phenacetin and paracetamol in the late 1800s [Citation14,Citation15]. Phenacetin was deemed safer and was used frequently for around 50 years [Citation1,Citation14]. However, in the late 1940s it was discovered that paracetamol was the less toxic major metabolite of phenacetin and acetanilide, and that it did not cause methemoglobinemia [Citation1,Citation16]. This combined with a low risk of nephrotoxicity resulted in paracetamol becoming available commercially in the 1950s [Citation1,Citation14]. Further safety concerns about aspirin in the 1970s led to paracetamol’s current first-line use as an antipyretic and analgesic [Citation1,Citation14].
The mechanism of action of paracetamol is still not entirely understood. Antipyretic effects are thought to be due to the inhibition of prostaglandin production [Citation17]. However, it does not display any anti-inflammatory effects (cf. non-steroidal anti-inflammatory drugs (NSAIDs)), suggesting that it only acts centrally rather than peripherally [Citation17].
Analgesic effects may also be due to interference with descending serotonergic pain pathways through their activation [Citation17]. As such these analgesic effects can be inhibited by serotonin antagonists [Citation17,Citation18]. Paracetamol is generally regarded as a first-line analgesic and is preferred over NSAIDs in certain patient groups. Despite this, a recent systematic review found there was sufficient evidence for the use of paracetamol in only 4 of 44 painful conditions and evidence it was not effective for some common indications [Citation19].
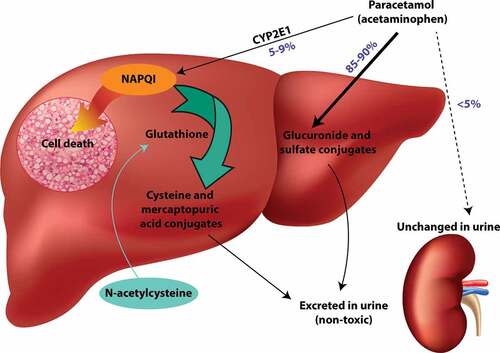
In therapeutic use, paracetamol is rapidly absorbed reaching therapeutic levels after 30 min and peak plasma concentrations within 2 h [Citation20]. Immediate release paracetamol can be taken every 4–6 h and modified/extended release (MR/ER) every 8 h to maintain therapeutic concentrations [Citation3]. Most paracetamol is metabolized by glucuronidation and sulfation and these metabolites are then renally excreted () [Citation1,Citation10,Citation21]. The remaining small amounts are converted into N-acetyl-p-benzoquinoneimine (NAPQI), a toxic metabolite, or excreted unchanged in the urine [Citation1,Citation10,Citation21].
Figure 2. Toxic mechanism of paracetamol and mechanism of action of acetylcysteine. Paracetamol is primarily detoxified by glucuronide and sulfate conjugates which are then excreted in the urine. A small percentage is metabolized by CYP2E1 to the reactive intermediate NAPQI. Under normal conditions, NAPQI can be detoxified by reaction with glutathione to form cysteine and mercaptopuric acid conjugates. If glutathione is depleted (e.g. in paracetamol overdose), NAPQI binds to cell macromolecules causing hepatocyte cell death. The antidote acetylcysteine replenishes cysteine, which is the rate-limiting factor for glutathione synthesis.
3. Toxicity
3.1. Mechanism and manifestations of toxicity
The main toxic effect of paracetamol is hepatoxicity. Paracetamol is a ‘pro-poison’ that exerts its toxic effect through the toxic reactive metabolite, NAPQI. This metabolite is formed by cytochrome P−450 (CYP) enzymes, primarily CYP2E1 and CYP3A4. NAPQI is formed in small amounts in therapeutic doses where it is readily detoxified by conjugation with glutathione [Citation1,Citation10,Citation21]. In overdose, there may be insufficient glutathione to detoxify NAPQI, causing it to bind to cellular proteins (adduct formation) () [Citation10,Citation21]. NAPQI primarily binds to cysteine residues but can potentially also damage proteins at methionine, tryptophan, and tyrosine residues [Citation22]. The mitochondria are a key target for NAPQI adduct formation. Formation of reactive oxygen species causes oxidative stress and leads to activation of c-jun N-terminal kinase (JNK) [Citation23]. The JNK enzyme translocates to the mitochondria, leading to mitochondrial dysfunction, cessation of ATP formation, and mitochondrial membrane rupture [Citation23]. This leads to cellular necrosis. The role of mitochondria in paracetamol hepatotoxicity has been reviewed extensively [Citation24–27]. Severe liver injury leads to loss of hepatic synthetic function and coagulopathy and hypoglycemia. Loss of hepatic metabolic functions leads to encephalopathy and lactic acidosis [Citation20,Citation28]. The clinical manifestations of hepatotoxicity are delayed, with peak serum transaminase levels occurring two to three days after the overdose [Citation10,Citation28]. Approximately 12–13% of acute overdoses result in hepatotoxicity even with treatment [Citation29], with 2–5% progressing to liver failure [Citation30] and 0.2–0.5% resulting in death [Citation30].
Acute kidney injury can also occur, even in the absence of liver failure, and may be delayed [Citation31,Citation32]. Nephrotoxicity may be direct due to tubular necrosis [Citation31–34] from renal NAPQI production or indicate hepatorenal syndrome [Citation32,Citation33].
Paracetamol is also a direct mitochondrial toxin and at very high concentrations can result in central nervous system (CNS) depression. Coma may occur in the absence of hepatotoxicity or other drugs causing CNS depression and may lead to delayed diagnosis and treatment of paracetamol overdose [Citation35–37].
3.2. Risk factors for toxicity
Various factors can increase the risk for toxicity, predominantly by affecting paracetamol’s metabolic pathways.
3.2.1. Massive overdoses
Ingestion of ‘massive’ overdoses (>30 g) increases production of the reactive metabolite NAPQI (). This will be exacerbated when the major metabolic pathways become saturated [Citation10,Citation20]. Sulfation saturates at lower concentrations than glucuronidation [Citation38], which saturates with massive overdose. The risk of hepatotoxicity is between 13%−24% in patients who ingest a massive overdose [Citation39–41]. Hepatotoxicity may occur despite early acetylcysteine treatment within 8 h of the ingestion [Citation39–41].
3.2.2. Delayed presentation
Symptoms of hepatotoxicity may be delayed in onset for up to 48 h after ingestion [Citation28]. They include nausea, vomiting and abdominal pain, or tenderness, and may prompt an individual to seek care [Citation10]. Acetylcysteine therapy should be initiated within 8 h of ingestion to be most effective (described below, Section 4.2) [Citation42]. Therefore, late presenters are at greater risk and often already have severe hepatotoxicity [Citation20,Citation29].
3.2.3. Enzyme inducers
Medications which induce the activity of the cytochrome P450 (CYP) enzyme CYP2E1, such as carbamazepine or isoniazid, can theoretically increase the production of NAPQI and contribute to toxicity [Citation43].
3.2.4. Glutathione depletion
Detoxification of NAPQI is dependent on glutathione levels. Individuals who are fasting, malnourished, or anorexic are at increased risk for toxicity due to lower glutathione levels [Citation44]. Hepatotoxicity may occur despite ingestions or concentrations below the usual toxic thresholds [Citation45].
3.2.5. Alcohol consumption
Chronic alcohol consumption may be associated with an increased risk of hepatotoxicity following paracetamol overdose [Citation45]. This may be due to the CYP2E1 induction [Citation43,Citation46,Citation47] or poor nutrition and lower glutathione stores [Citation46]. Caution is given against regular paracetamol use in this setting. Chronic alcohol users are a population more likely to take overdoses, and who often present late, which could be responsible for poorer outcomes [Citation48]. People who chronically consume large quantities of alcohol have also been reported to take larger paracetamol overdoses [Citation49]. Thus, this association likely reflects both behavioral factors and metabolic factors.
In contrast, acute ingestion of alcohol at the time of paracetamol overdose is protective against the development of toxicity [Citation50]. Ethanol is also a substrate for the CYP enzymes responsible for NAPQI generation and competes with paracetamol for CYP2E1, reducing the amount of NAPQI being produced [Citation43].
3.2.6. Ingestion of modified release (MR) and extended release (ER) paracetamol
Sustained release formulations of paracetamol include MR and ER paracetamol. MR paracetamol is available in several countries, including Australia and New Zealand. MR paracetamol contains 665 mg paracetamol, 31% immediate release, and 69% slow release [Citation51,Citation52]. ER paracetamol is available on the US market, and contains 650 mg paracetamol, 50% immediate release, and 50% slow release [Citation53]. Both MR and ER formulations are designed to provide pain relief for up to 8 h.
Overdose with MR paracetamol has been associated with very prolonged absorption and multiple peaks [Citation54] in paracetamol concentrations may occur after overdose and patients often require prolonged treatment due to continuously elevated concentrations [Citation51,Citation54,Citation55]. There may be formation of pharmacobezoars [Citation51,Citation56] which makes absorption even more erratic and this may be seen in massive overdoses of immediate release paracetamol as well [Citation39,Citation57]. There is a higher risk of hepatotoxicity even with early treatment, likely due to the generally higher doses and a mismatch between the timing of acetylcysteine and high paracetamol concentrations.
A retrospective review found that in Australia there was an increasing rate of paracetamol overdose with the MR formulation and that they often involved a larger number of tablets [Citation58]. In addition, deaths from this formulation accounted for a third of the total cases recorded between 2009 and 2017 [Citation58].
For ER paracetamol, early data suggested similar risk between immediate release and ER paracetamol overdose [Citation59]. However, there have since been reports of delayed peaks and delayed nomogram line crossing (where the initial level was below the treatment line, and subsequent levels are above the line) [Citation55]. While it is generally agreed that the Rumack-Matthew nomogram (described below, Section 3.3.1) cannot be safely used for risk assessment in MR and ER overdose [Citation60], it is unclear whether the documented risks for MR paracetamol extend to the ER formulation. MR paracetamol has a longer Tmax than ER paracetamol [Citation60]. In addition, MR and ER products are formulated differently. MR tablets use a hydroxypropylmethylcellulose coating [Citation53], and ER tablets are a bilayered tablet where one side contains immediate release and the other slow release paracetamol (in a matrix formulation) [Citation59]. More studies are needed to ascertain the risks of ER paracetamol compared to immediate release and MR formulations. In the absence of these, it is prudent to assume that the risk is higher for all sustained release paracetamol preparations.
3.2.7. Repeated supratherapeutic ingestion and staggered overdoses
Repeated supratherapeutic ingestions occur when patients exceed the recommended doses of paracetamol over a prolonged period for therapeutic purposes. Dental pain appears to be associated with repeated supratherapeutic ingestions, when compared to other types of pain [Citation61]. Repeated supratherapeutic ingestion carries an increased risk of hepatotoxicity when compared to acute single overdose. In a single-center study from the US, hepatotoxicity occurred in 52% of patients with accidental (repeated supratherapeutic) overdose, compared to 14% of those with deliberate overdose [Citation62]. This was despite the accidental group ingesting smaller overdoses [Citation62]. The accidental cases presented later and were more likely to have chronic alcohol use disorder, likely contributing to the increased morbidity [Citation62]. Other studies have compared repeated supratherapeutic and deliberate paracetamol overdose and similarly found therapeutic misadventure to be a risk factor for hepatotoxicity and death [Citation63,Citation64]. Those that develop significant hepatotoxicity typically have elevated aminotransferases on presentation [Citation65,Citation66].
Repeated overdoses can also occur with self-harm intent (often termed ‘staggered’ overdoses). Similar to those with therapeutic intent, these exposures have increased risk of hepatotoxicity when compared to acute self-harm overdoses [Citation67]. This is likely due to delayed presentation [Citation67].
3.2.8. Other risk factors
While the above factors are the best-established risk factors for toxicity following paracetamol overdose, other potential risk factors are emerging. A large retrospective study in the US found significantly increased risk of acute liver injury in people infected with hepatitis C virus, and people with nonalcoholic fatty liver disease (NAFLD) [Citation44]. Hepatitis C virus infection was also associated with higher risk of progression to severe liver disease and mortality [Citation44].
NAFLD has also been identified as a risk factor for hepatic injury following paracetamol overdose [Citation44]. The increased risk from NAFLD has been estimated as similar to that from alcoholic liver disease [Citation68]. This risk is likely related to NAFLD, rather than obesity itself, as some studies have shown no increased risk of paracetamol hepatotoxicity in obese individuals compared to non-obese individuals [Citation69,Citation70]. It has been postulated that the risk of paracetamol hepatotoxicity in an obese individual will depend on the presence of NAFLD and the balance of protective factors (e.g. higher glucuronidation, increased volume of distribution, slower absorption, and reduced CYP3A4 activity) and negative factors (increased CYP2E1 activity, lower glutathione stores) [Citation71].
Genetic polymorphisms in enzymes responsible for paracetamol metabolism have been identified as having the potential to increase risk of hepatotoxicity. Heruth et al [Citation72] have reviewed 147 single nucleotide polymorphisms potentially associated with paracetamol hepatotoxicity. Further studies are needed to ascertain the clinical relevance of these polymorphisms [Citation72]. It is possible that genetic variability could explain elevations in alanine aminotransferase (ALT) in healthy adults administered paracetamol at the maximum therapeutic dose of 4 g per day, and the rare cases of acute liver injury at therapeutic doses [Citation72]. In a genetic study of subjects taking therapeutic paracetamol, 20 genes implicated in paracetamol metabolism and drug-induced liver injury (DILI) development were assayed [Citation73]. The only gene associated with maximum ALT was SULT1E1, which produces the sulfotransferase family 1E member 1 enzyme [Citation73].
3.3. Risk assessment of paracetamol overdose
Biomarkers help determine risk of toxicity, need for treatment, and treatment effectiveness. The usual tests used to guide the management of paracetamol overdose are: serum paracetamol concentration, liver function tests (particularly alanine and aspartate aminotransferases, ALT & AST), and international normalized ratio (INR).
3.3.1. Paracetamol concentrations and the Rumack-Matthew nomogram
Paracetamol concentration is plotted against time from ingestion on the Rumack-Matthew nomogram [Citation74]. This nomogram is used to help in estimating the risk of liver toxicity, and thus whether treatment with acetylcysteine is indicated (see Section 4.2) [Citation75]. Concentrations lying above the line indicate that patients require acetylcysteine and concentrations more than double the line indicate an even greater risk [Citation74]. Some guidelines indicate an increased dose of acetylcysteine in these cases [Citation74]. The Rumack-Matthew nomogram is not useful to predict risk for late presenters (>24 h) or repeated overdoses [Citation74,Citation75].
While the Rumack-Matthew nomogram is used in most countries, the United Kingdom (UK) now uses a nomogram with a lower line (half of the original treatment threshold) [Citation76–78]. This new nomogram was introduced in 2012 due to an isolated fatal case when the paracetamol concentration was below the nomogram line and no treatment was given [Citation77,Citation78]. The lower nomogram line has greatly increased the number of patients receiving acetylcysteine, increasing healthcare costs [Citation77,Citation79]. These patients may be unnecessarily exposed to acetylcysteine and potential adverse reactions [Citation76,Citation77]. Further, various studies have shown that patients with lower paracetamol levels have a greater rate of experiencing adverse effects when treated with NAC [Citation77,Citation80].
3.3.2. Alanine and aspartate aminotransferase (ALT & AST)
ALT and AST are hepatic enzymes that are released into the blood following hepatic cellular damage and are used to diagnose or exclude acute liver injury [Citation42,Citation76]. Therapeutic doses of paracetamol may cause small rises in hepatic enzymes with prolonged treatment [Citation81]. However, aminotransferases return to normal ranges with continued treatment. After an acute overdose ALT/AST may increase after 8 h [Citation42], however they can remain within the normal range for up to 24 h before hepatotoxicity becomes evident [Citation82].
The rate of ALT/AST rise appears important, with initial aminotransferase levels the highest in the patients who developed the most severe toxicity [Citation83]. Between presentation and aminotransferase peak, ALT and AST typically rise in a 1:1 ratio [Citation84]. ALT is generally slower to fall than AST, and an AST:ALT ratio < 0.4 indicates that aminotransferases have peaked and are falling [Citation84]. If the AST:ALT ratio is > 2 at presentation, this may herald worse outcomes, and also has been associated with chronic excessive ethanol use [Citation84]. Increased severity of hepatotoxicity is also associated with a slower fall in AST and in the AST:ALT ratio [Citation84].
Paracetamol-induced liver injury causes marked elevations in aminotransferases, in comparison to most other causes of liver injury. Paracetamol-induced hepatotoxicity is generally defined as either AST or ALT > 1000 IU/L. In patients with severe toxicity, aminotransferase concentrations > 10,000 IU/L are possible; however, once hepatotoxicity is diagnosed, prognosis is better indicated by other markers e.g. INR, pH, and lactate (see Section 3.3.5).
ALT/AST is often measured at presentation and periodically to indicate the need for continued therapy. It is worth noting that ALT/AST is not specific for liver injury and may also lack sensitivity in early liver injury [Citation42,Citation85,Citation86].
3.3.3. The paracetamol-aminotransferase multiplication product
The multiplication product of ALT and paracetamol concentration has been proposed as a risk-stratification tool for predicting hepatotoxicity following paracetamol overdose [Citation87]. One benefit is that this method can be used when exact time of ingestion is unknown, and for staggered and supratherapeutic overdoses. The diagnostic accuracy of this product has been confirmed, with an initial multiplication product of > 10,000 mg/L x IU/L being strongly associated with hepatotoxicity [Citation88]. Patients with a product of <1500 mg/L x IU/L were very unlikely to develop hepatotoxicity [Citation88]. This tool has also been shown to be accurate for MR paracetamol overdose [Citation89]. A recent systematic review has concluded that the paracetamol-aminotransferase multiplication product can complement the Rumack-Matthew nomogram, especially for delayed presentations and staggered ingestions [Citation90].
3.3.4. International normalized ratio (INR)
INR is used to determine the severity of hepatotoxicity as the liver is responsible for the production of many clotting factors [Citation42]. However, paracetamol overdose and acetylcysteine can both directly cause a mildly prolonged INR and thus the test is only useful in established hepatotoxicity [Citation91]. INR levels are then used to indicate severity, prognosis, and the duration of treatment with acetylcysteine [Citation74].
3.3.5. Liver unit referral criteria
These investigations guide treatment with acetylcysteine and stratify risk. Guidelines vary internationally, however generally ALT >1000 U/L is considered an indicator of severe liver injury, and INR > 3 indicates acute liver failure (ALF) and warrants liver unit referral [Citation74]. Other criteria that warrant referral to a specialized liver unit include: oliguria or creatinine > 200 micromol/L, acidosis (pH < 7.3), or lactate >3 mmol/L, hypoglycemia, thrombocytopenia, hypotension, and encephalopathy (see Section 4.7) [Citation74].
3.3.6. Novel biomarkers
There are many other potential biomarkers to predict the development of liver toxicity and guide treatment [Citation42,Citation75,Citation76,Citation86]. Ideal biomarkers would detect liver damage earlier than ALT and INR and also predict severity and prognosis. Promising candidates include mitochondrial damage and necrosis markers and paracetamol protein adducts which measure the toxic metabolite NAPQI bound to cellular proteins [Citation42,Citation75,Citation76,Citation82]. Paracetamol-protein adducts have been shown to be marginally superior to traditional biomarkers at predicting acute liver injury and can diagnose paracetamol-induced hepatotoxicity in the absence of a history of ingestion [Citation38,Citation82,Citation92,Citation93].
4. Treatment of paracetamol overdose
4.1. Charcoal
Activated charcoal binds paracetamol and thus may reduce the amount of paracetamol absorbed from the gastrointestinal tract [Citation94]. It may be useful in acute paracetamol overdoses that present to primary care facilities within 2 h of ingestion (or 4 h for ‘massive’ overdoses or exposures to MR/ER preparations) [Citation74]. However, it is not useful in patients who ingest liquid formulations due to the rapid absorption [Citation74]. It is also not recommended after repeated supratherapeutic ingestions [Citation74]. Charcoal may reduce peak paracetamol concentrations, the need for NAC and decrease the risk of hepatotoxicity [Citation39,Citation95].
4.2. N-acetylcysteine (NAC)
NAC is the mainstay of paracetamol overdose management. NAC restores glutathione by replenishing cysteine, the rate-limiting factor for glutathione synthesis [Citation96,Citation97]. This allows for the detoxification of NAPQI and prevents hepatotoxicity in most cases [Citation98]. The risk of developing hepatotoxicity is substantially reduced when NAC is given within 8 h of ingestion [Citation21].
Before the availability of NAC treatment, morbidity and mortality following paracetamol overdose was considerable; many developed acute liver injury and 3–5% died [Citation99,Citation100]. Since acetylcysteine has become standard treatment, deaths are rare, less than 1% in most cohorts [Citation94].
Various regimens for NAC have been used. NAC was originally delivered orally until the development of an intravenous formulation [Citation101]. Both routes are effective, although the intravenous formulation is now more commonly used, and has advantages when there are factors that may affect oral absorption including vomiting, gastrointestinal conditions or evidence of hepatic failure [Citation29]. Several intravenous regimens have been the subject of clinical trials and the ‘3-bag regimen’ was most common for many years [Citation74,Citation94,Citation101]. The use of simplified ‘2-bag’ regimens reduces adverse drug reactions but has comparable efficacy [Citation74,Citation102–104]. The simplest modification is a 200 mg/kg bag delivered over 4 h, followed by a 100 mg/kg bag delivered over 16 h [Citation74]. The two or three bag regimens can also be combined into one infusion bag given with variable rates, which may reduce the risk of gaps in treatment [Citation105].
Abbreviated NAC regimens are sometimes used in the treatment of repeated supratherapeutic ingestion. In Australia, an 8-h NAC regimen is used in repeated supratherapeutic ingestions where ALT remains static and paracetamol concentrations remain low [Citation74]. A retrospective study indicated that this is likely safe (there were no cases of re-presentation with hepatotoxicity) and reduces hospital length of stay [Citation106]. In the US, the Rocky Mountain Poison and Drug Center protocol treats repeated supratherapeutic ingestion patients with 12 h of NAC, with treatment only being continued if ALT/AST is rising or the paracetamol concentration is elevated [Citation107].
There is increasing recognition that dosing NAC based on body weight may not be sufficient to prevent injury in patients taking high doses of paracetamol. This has led to the practice of increased NAC dosing in high-risk patients. For example, doubling the dose of NAC given in the second/third bag when paracetamol concentrations are more than double the nomogram line reduces the risk of hepatotoxicity [Citation39,Citation74]. Optimal NAC dosing regimens and future directions have been recently reviewed [Citation108].
Biomarkers including hepatic enzymes, INR, and paracetamol concentrations are used to guide the duration of NAC [Citation74]. Extended NAC infusions should be given in high-risk patients (e.g. patients with persistently elevated paracetamol concentrations or established hepatotoxicity) [Citation74].
4.3. Methionine
Prior to the active use of acetylcysteine, other treatments such as methionine were used [Citation94,Citation99,Citation109]. Similar to NAC, methionine is an antidote used to increase glutathione and treat or prevent hepatotoxicity [Citation109]. Although primarily administered orally, it has been administered intravenously [Citation99,Citation109,Citation110]. Methionine may cause gastrointestinal, neurological, or cardiovascular side effects which limit its acceptability [Citation109,Citation110] and so it is no longer routinely recommended. Although initially regarded by the WHO as having a similar efficacy and safety profile as acetylcysteine [Citation94], methionine is no longer on the most recent WHO Essential Medicines List [Citation2].
4.4. Cimetidine
Cimetidine is a histamine−2 receptor antagonist and an inhibitor of CYP2E1 [Citation111]. Based on this inhibition, it has been proposed as a potential treatment for paracetamol overdose [Citation111]. There are many animal studies showing that cimetidine reduced hepatotoxicity from paracetamol, summarized by Mullins et al [Citation111]. However, disappointingly, clinical effectiveness in humans has not been demonstrated, albeit these have been small studies with few high-risk patients [Citation111].
4.5. Fomepizole
Fomepizole is best known as a treatment for toxic alcohol poisoning, where it inhibits alcohol dehydrogenase [Citation112]. Fomepizole has recently been proposed as a treatment to prevent hepatotoxicity following paracetamol overdose. The mechanism is unrelated to alcohol dehydrogenase but is thought to be a result of two functions [Citation112]. Fomepizole inhibits CYP2E1, reducing conversion of paracetamol into NAPQI [Citation112]. Secondly, it can also prevent the activation of the JNK enzyme and resultant mitochondrial dysfunction [Citation112,Citation113].
The evidence for use of fomepizole in this context is primarily from animal models [Citation113] and small case series [Citation114,Citation115]. Interpretation of results from observational case series has been controversial [Citation112,Citation116,Citation117]. Some have advocated for the use of fomepizole as an adjunct to NAC in massive overdoses [Citation114]. Others have called for the need for large comparative studies to generate evidence before fomepizole is considered a recommended treatment [Citation116]. If the promising early results are replicated in robust clinical trials, fomepizole could fill an un-met need in this space, particularly for patients where NAC is less effective (massive overdose, delayed presentation).
4.6. Calmangafodipir
Manganese superoxide dismutase is a mitochondrial protein that scavenges free radicals and can prevent mitochondrial injury. Toxic doses of paracetamol inhibit manganese superoxide dismutase in animal studies [Citation118]. Restoring or mimicking manganese superoxide dismutase activity could be used as a treatment for paracetamol overdose. Calmangafodipir is a superoxide dismutase mimetic [Citation119]. It has been successfully used in Phase 2 trials for chemotherapy induced peripheral neuropathy mediated by oxidative stress. In a small study where calmangafodipir was used as an adjunct to NAC in paracetamol overdose, no safety issues were identified [Citation119]. That trial also found that calmangafodipir may reduce liver injury biomarkers, however the sample size and design was not powered to study efficacy, and most patients enrolled were low-risk for hepatotoxicity [Citation119]. Further studies designed to assess efficacy are needed. Fomepizole, calmangafodipir, and other potential therapeutic targets for paracetamol hepatotoxicity have been recently reviewed [Citation120].
4.7. Liver transplant
In patients with severe liver failure and a grave prognosis, orthotopic liver transplant is considered [Citation121]. Kings College Criteria are widely used to predict patients who are likely to die without a liver transplant [Citation121,Citation122]. There is some controversy surrounding the benefits of liver transplantation following paracetamol overdose, with a systematic review suggesting that transplant may confer little long-term survival benefit [Citation121,Citation123].
4.8. Lack of global treatment guidelines
Clinical practice guidelines allow for consolidation of available materials to guide areas such as diagnosis, treatment recommendations and monitoring. They are generally easy to navigate and provide clear instructions on the course of management for the suspected condition. Their use is invaluable in a variety of conditions with potentially severe outcomes, including paracetamol poisoning. However, other than the development of the Australian and New Zealand guidelines, those providing guidance on the management of this condition are not readily available or freely accessible [Citation74]. Many countries may have established national or regional guidelines, although it is possible that they are only accessible through subscription-based online resources or via contact with a Poisons Information Centre (PIC). However, the development of consistent global guidelines is difficult as the treatment of paracetamol poisoning is not a ‘one size fits all’ approach. Various countries have adopted alternate NAC regimens or even lower cut-off limits to start treatment based on the Rumack-Matthew nomogram [Citation101]. Denmark even forgoes the use of this nomogram and treats all patients with a full course of NAC regardless of paracetamol concentrations [Citation80].
5. Epidemiology of paracetamol overdose
5.1. Methodology for estimating the impact of paracetamol overdose globally
The search strategy conducted for this section of the review is described above (Section 1.1) and in further detail in Supplementary Methods.
The data is presented as yearly counts per 100,000 population where a catchment population was able to be determined and/or as a percentage of all poisoning exposures. Where possible, we calculated case fatality rates by dividing the number of cause-specific poisoning fatalities by the number of exposures.
Weighted averages were calculated using meta-analysis to account for heterogeneity between the data sources. Meta-analysis was performed in R 4.0.4. For full details, including definitions of analgesics, DILI, and ALF/ALI, see Supplementary Methods.
5.2. Data availability by region
There were a total of 115 data sources containing information about analgesic and/or paracetamol poisoning. Countries in Europe, North America, and Asia were the greatest sources of contribution (Supplementary Figure S1). The available data is not proportional to population [Citation124], and many continents provided little data. Some publications included data from multiple countries however these were often countries within the same geographical area. Within countries, some data sets contain information from certain regions only. In addition, data sources available differed by country. As such in the figures, the data points for each country have been color coded according to the type of data source: poisons information centers, a single hospital/treatment center and multiple hospitals/treatment centers or large administrative data sets.
5.3. Overall and intentional exposures
5.3.1. Rates as a percentage
Where data were available, we found a weighted mean of 9.7% (range 0.7–40.2%) of poisoning cases were attributable to analgesics (Supplementary Figure S2) [Citation125–157]. Denmark had the highest proportion of poisonings attributable to analgesics at 40.2%, noting that this was a proportion of drug poisonings only rather than all poisonings [Citation133]. The lowest rate was reported by a German Poisons Information Centre [Citation151].
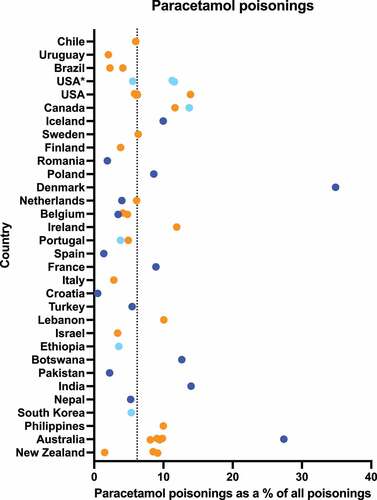
Paracetamol accounted for an average of 6.2% (range 0.5–34.9%) of poisonings where data were available () [Citation125–131,Citation133,Citation135,Citation136,Citation138,Citation140,Citation142–144,Citation148,Citation150,Citation156–183] (including additional data from e-mail responses, Supplementary Table S1). It accounted for over a quarter of all drug poisonings in Denmark [Citation133] and of all poisonings in a cohort of > 17 000 patients in Australia [Citation159]. Croatia had very few paracetamol poisonings (<1%) [Citation162].
Figure 3. Percentage of poisonings relating to paracetamol. Dark blue points represent data from a single hospital/treatment center, light blue points represent data from multiple hospitals/treatment centers or large administrative databases, while orange points represent poisons information center data. Dotted line shows weighted mean across sources 6.2%.
Intensive care unit (ICU) poisonings represent a severe subset of poisonings and were not pooled with overall rates. Analgesic poisonings account for 8.0% of ICU poisonings in France [Citation184] and 12.4% in Germany [Citation185]. An Iranian source reported that paracetamol accounted for only 0.7% of ICU poisonings [Citation186].
For sources reporting intentional exposures only, analgesic poisonings accounted for 16.3% (range 6.2–29.2%) (Supplementary Figure S3) [Citation135,Citation148,Citation187–192].
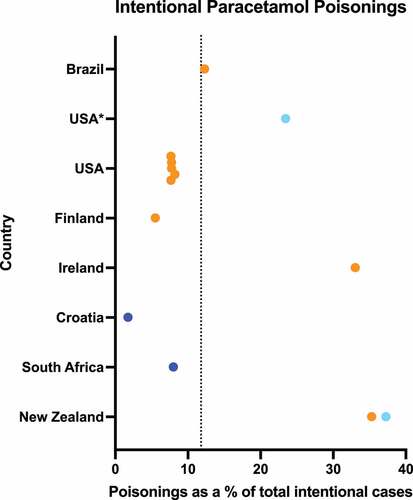
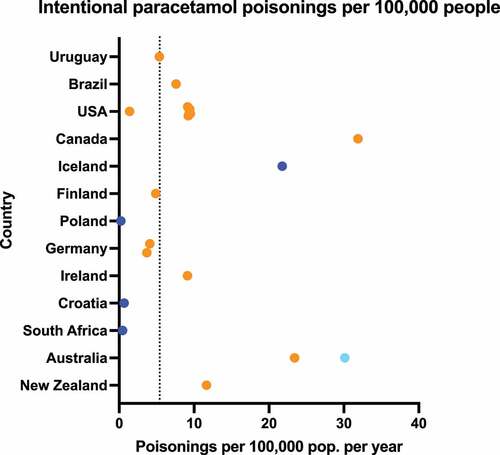
Intentional paracetamol poisonings accounted for 11.8% (range 1.8–37.3%) of all intentional poisonings, highest in New Zealand and lowest in Croatia () [Citation125–128,Citation144,Citation148,Citation157,Citation162,Citation193,Citation194] (including additional data from e-mail responses, Supplementary Table S1).
Figure 4. Percentage of poisonings caused by intentional ingestion of paracetamol. Dark blue points represent data from a single hospital/treatment center, light blue points represent data from multiple hospitals/treatment centers or large administrative databases, while orange points represent poisons information center data. Dotted line shows weighted mean across all sources: 11.8%.
5.3.2. Rates per 100,000 population per year
We found a weighted mean of 11.5 (range 0.1–88.9) analgesic poisonings per 100,000 population per year, lowest in China and Botswana (Supplementary Figure S4) [Citation125–128,Citation130–134,Citation136,Citation139,Citation145–157]. On average intentional ingestions of analgesics contributed to 4.9 (range 0.4–26.0) poisonings per 100,000 population per year, with data only available for four countries (Supplementary Figure S5) [Citation148,Citation188,Citation192,Citation195].
Paracetamol was involved in an average of 7.4 (range 0.1–63.6) poisonings per 100,000 population per year (see ) [Citation58,Citation125–128,Citation130,Citation131,Citation133,Citation136,Citation148,Citation150,Citation156–160,Citation162,Citation163,Citation166,Citation168,Citation169,Citation171,Citation172,Citation174,Citation176–182,Citation196–200] (including additional data from e-mail responses, Supplementary Table S1). The lowest rate was reported in a data source reporting on females only, in Ethiopia [Citation163]. Australia had the highest rate reported at 63.6 per 100,000 population per year [Citation182]. Focusing on intentional exposures, there were 5.4 (range 0.2–31.9) intentional paracetamol poisonings per 100,000 population per year () [Citation58,Citation125–128,Citation148,Citation157,Citation162,Citation194,Citation196,Citation197,Citation200] (including additional data from e-mail responses, Supplementary Table S1).
Figure 5. Paracetamol poisonings per 100,000 population per year. Dark blue points represent data from a single hospital/treatment center, light blue points represent data from multiple hospitals/treatment centers or large administrative databases, while orange points represent poisons information center data. Dotted line shows weighted mean across all sources: 7.4/100,000.
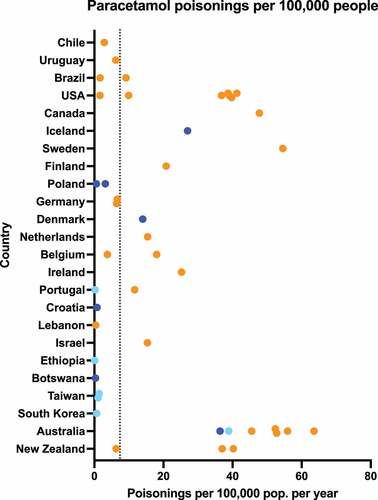
Figure 6. Intentional paracetamol poisonings per 100,000 population per year. Dark blue points represent data from a single hospital/treatment center, light blue points represent data from multiple hospitals/treatment centers or large administrative databases, while orange points represent poisons information center data. Dotted line shows weighted mean across all sources: 5.4/100,000.
5.4. Rates of hepatotoxicity and liver failure
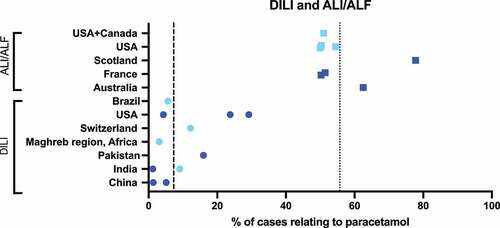
Overall, paracetamol accounted for a weighted mean of 55.7% (range 50.0–77.8%) of severe acute liver injury (ALI) and/or ALF [Citation201–208] and 7.3% (range 1.2–29.2%) of drug-induced liver injury (DILI) [Citation209–219] (see ). The country with the highest percentage of DILI cases caused by paracetamol was the U.S.A at almost 30% [Citation217] followed by Pakistan at 16% [Citation215]. For sources examining causes of ALI/ALF, Scotland and Australia had the highest number of cases relating to paracetamol at approximately 78% [Citation204] and 63% [Citation201], respectively.
Figure 7. Proportion of patients who develop ALI/ALF or DILI after paracetamol use. Dark blue points represent data from a single hospital/treatment center and light blue points represent data from multiple hospitals/treatment centers or large administrative databases. Dotted (ALI/ALF) and dashed (DILI) lines show weighted mean across all sources: ALI/ALF = 55.7%, DILI = 7.3%.
5.5. Fatalities
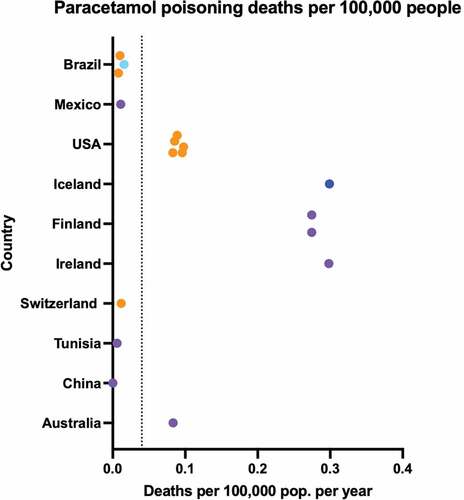
Where data were available, analgesics contributed to 0.1 (range 0.0003–0.7) deaths per 100,000 population per year (Supplementary Figure S6) [Citation125–128,Citation157,Citation195,Citation220–224]. When data sources including opioids were removed, this dropped to 0.04/100,000. Sources reporting on paracetamol only found similar results, with a weighted mean of 0.04 (range 0.0001–0.3) deaths per 100,000 population per year attributable to paracetamol () [Citation58,Citation125–128,Citation148,Citation153,Citation157,Citation160,Citation196,Citation221,Citation222,Citation224–228].
Figure 8. Deaths attributable to paracetamol poisonings per 100,000 population per year. Dark blue points represent data from a single hospital/treatment center, light blue points represent data from multiple hospitals/treatment centers or large administrative databases, orange points represent poisons information center data, purple points represent coronial/forensic databases. Dotted line shows weighted mean across all sources: 0.04/100,000.
The case fatality rate for analgesic poisonings averaged 0.7% (range 0.1–2.5%) (Supplementary Figure S7) [Citation125–128,Citation141,Citation157,Citation195]. A German ICU data source reported a case fatality rate of 4.1% but this was not pooled with other data sources due to the bias toward more severe poisonings in the ICU [Citation185]. The case fatality rate for paracetamol poisonings was 0.4% (range 0.1–2.3%) (Supplementary Figure S8) [Citation58,Citation125–128,Citation135,Citation143,Citation144,Citation148,Citation157,Citation160,Citation196,Citation229,Citation230].
5.6. Limitations of available data
There were several limitations impacting the amount and type of data that was collected. By conducting a literature search through PubMed only, some relevant studies may have been missed. Although this data may have been published in annual reports instead, not all countries have a PIC and there is also a clear lack of representation of low- and middle-income countries in the available data. Using the WHO’s poisons center directory, e-mails were sent out to 280 different PICs yet there were only 11 direct responses and 66 e-mails that were undeliverable. Over 50 websites were inaccessible and although there were some PICs with an annual report, many did not report on the data required for this analysis. Many websites were a direct link to the hospital that the PIC worked out of rather than the PIC itself and many did not contain any annual reports. Data presented in languages other than English may have been interpreted incorrectly and as such some data may not be accurate.
Operational models of PICs vary worldwide. Some provide advice to healthcare practitioners only, whereas others also include members of the public. Some act as toxicology services within hospitals, while others provide advice over the phone or via a hybrid model with online databases (such as the UK, New Zealand, and the Netherlands). This could result in variability in rates recorded. Attempts were made to identify catchment populations to allow calculation of population rates, however this was not available for much of the literature resulting in the use of rates as a percentage where available. Some data sources only provided rates without providing raw data or catchment populations [Citation195,Citation196] and as such were excluded from the calculation of the weighted means. Additionally, some countries had data from certain regions only. It is unlikely that particular regions are representative of a whole country, which may reduce the accuracy of these results.
Death data were from a range of data sources including poison centers where outcome data is sought (e.g. U.S.A), single hospitals, and coronial/forensic/national death statistics data. These data sources each have their limitations, for example poisons center and hospital datasets do not capture out-of-hospital deaths, and some patients will be lost to poisons center follow-up. The datasets using coronial/forensic data may over-estimate cases by counting those where paracetamol was detected in multi-drug overdose, but hepatotoxicity did not occur. There was no clear pattern between data source and estimate (), indicating that variability may be due to country-specific differences as well as data capture.
Many publications contained exclusions such as age or sex, and we excluded pediatric only data sources as the rates are likely to be very different to whole-of-population rates where intentional overdose predominates. Some sources were limited to drug poisonings only which is likely to result in the variety of estimates seen. Definitions for analgesics were not consistent throughout the literature. We attempted to remove data relating to opioids however sometimes this was not possible as data was already pooled by the original study authors. Poisons center enquiries were not well defined and may include exposures in addition to calls regarding general information as well as recalls for the same exposure, for this reason we only included poisons center data on exposures.
6. Paracetamol availability and its impacts
6.1. Non pharmacy sales, pharmacy sales, and pack size restrictions
Paracetamol is available without a prescription in most countries. It is one of the most common over-the-counter (OTC) analgesics used in suicidal overdoses [Citation231]. A study in Ireland found that paracetamol was used in over half of all overdoses using OTC drugs [Citation232]. The volumes that can be purchased and the availability of non-pharmacy sales differ substantially globally (). A study of 21 European countries found that over half of the participating countries including France, Italy, and Switzerland did not have paracetamol available for purchase outside of pharmacies and that the rate of paracetamol-related poisoning enquiries was subsequently lower in most of these countries [Citation233]. Countries such as the UK, Denmark, and Ireland allow for sales of paracetamol outside of pharmacies however the pack sizes are limited to 5–8 g [Citation233]. The introduction of paracetamol sales from non-pharmacy outlets in Sweden in 2009 was associated with an increase in the rate of paracetamol poisonings [Citation234], however restrictions were reintroduced in 2015 [Citation233].
Table 1. Restrictions on pack sizes of over-the-counter (OTC) pharmacy and non-pharmacy sales of immediate release paracetamol.
Over two-thirds of European countries have paracetamol pack size restrictions within pharmacies, ranging from 8 to 30 g [Citation233]. The number of paracetamol-related poisoning enquiries was higher in some of these countries [Citation233].
In the U.S.A and Canada, paracetamol is available outside of pharmacies in very large packs [Citation30]. Paracetamol was involved in almost half of suicides/attempted suicides using OTC analgesics in the U.S.A [Citation235].
In Singapore, paracetamol can be sold outside of pharmacies without restriction [Citation30]. Within Australia, and similarly in New Zealand, products containing up to 20 tablets of 500 mg of immediate-release paracetamol can be sold outside of pharmacies [Citation236,Citation237]. There are no legal limits on the maximum amount (number of packs) of paracetamol that can be purchased outside of pharmacies. In Australia and New Zealand, within pharmacies customers may purchase packs of up to 100 tablets of immediate-release paracetamol without any intervention by a pharmacist [Citation236,Citation237].
6.2. MR/ER Paracetamol
MR paracetamol presents a significant challenge in overdose and is more dangerous than immediate-release paracetamol. It can cause hepatotoxicity despite early treatment with NAC, and treatment with charcoal and increased NAC are not effective in preventing hepatotoxicity even in those who present early.
After a decision made by the European Medicines Agency, countries in the European Union such as the UK (formerly), Denmark, and Ireland do not supply any MR paracetamol, even on prescription [Citation30,Citation238]. On the other hand, countries such as the United States and Singapore have no restrictions on ER paracetamol and allow this formulation to be sold outside of pharmacies as well [Citation30].
In Australia, the MR formulation was originally available in pharmacies without consultation with a pharmacist, similar to the immediate-release paracetamol. In 2020, overdose concerns led to it being up-scheduled such that supply required a pharmacist’s approval (similar changes also occurred in New Zealand) [Citation236, Citation239].
6.3. Impact of regulatory changes
Worldwide there have been few studies on changes aimed at reducing the occurrence of paracetamol overdose and its complications. Some countries have implemented pack size restrictions and/or moved to restrict access to paracetamol outside of pharmacies.
6.3.1. The United Kingdom
In the UK in 1998 legislation was introduced to reduce the pack sizes of available paracetamol both within and outside of pharmacies [Citation240]. Customers would be allowed to purchase up to 8 g of paracetamol in general stores and up to 16 g within pharmacies but without assistance; any greater amount required discussion with a pharmacist [Citation240]. In the year following the intervention, there was a significant reduction in both the total number of paracetamol overdoses and the number of severe paracetamol overdoses at the Royal Free Hospital [Citation241]. Benzodiazepine overdoses were used as a control, and remained stable [Citation241]. A study that combined Office for National Statistics data with information from six liver units and monitoring systems in general hospitals found consistent results [Citation242]. Suicidal deaths reduced by 22% in the first year, which was sustained for the following two years [Citation242]. Liver unit admissions and liver transplants were reduced by ~ 30% and large overdoses were reduced by 20% [Citation242]. There was a simultaneous increase in ibuprofen overdoses but little-to-no effect on suicides [Citation242].
Some studies of this intervention have had neutral or negative findings. A study using national deaths data and hospital admissions data found declining mortality and hospital admissions from paracetamol between 1993 and 2002 [Citation243]. However, the authors concluded that the contribution of the 1998 intervention was unclear [Citation243]. An interrupted time-series analysis found a decrease in paracetamol poisoning mortality associated with the intervention [Citation244]. Fatal poisoning by some other agents (including paracetamol compounds not affected by the legislation) also decreased [Citation244]. The authors concluded that the reduction seen might have been part of a broader trend [Citation244].
A Scottish study of hospitalizations and deaths from paracetamol found no reduction in paracetamol overdose frequency or mortality following the 1998 intervention [Citation245].
A systematic review examining the effects of this intervention found that there was a significantly reduced amount of paracetamol being purchased and a reduction in the number of admissions to liver units and subsequent transplants [Citation240]. There was also a significant decrease in the total number of deaths caused by paracetamol, albeit with still over 100 deaths per year [Citation246].
Retailer adherence to the legislation has been questioned. A ‘mystery shopper’ study in South London in 2004 tested whether amounts in excess of the 1998 legislation could be purchased from non-pharmacy and pharmacy outlets [Citation247]. Researchers were able to purchase > 32 tablets in four of eight pharmacies, and > 16 tablets in 13 of 16 other retailers [Citation247]. The same study surveyed patients at a London hospital who took paracetamol overdoses. Approximately half of patients ingesting > 16 paracetamol tablets purchased paracetamol for the purpose of taking an overdose [Citation247]. Of these 35 people, 15 went to multiple different stores, whereas 16 were able to purchase multiple packs from one store [Citation247].
Importantly, while maximum pack sizes were mandated, limits to number of packs that could be purchased were not implemented by the UK Medicines and Healthcare Products Regulatory Agency (MHRA). Thus, stores selling more than one pack in the above study were contravening the spirit of the 1998 legislation but were not breaking the law [Citation248]. In 2009, the MHRA introduced guidelines stating ‘no retailer should sell more than two packets of paracetamol (500 mg) in one transaction, retailers should be discouraged from promoting the sale of more than one pack at a time and that it is illegal to sell more than 100 tablets of paracetamol (500 mg) … in one transaction’ [Citation248]. Despite this, a more recent mystery shopper study of non-pharmacy outlets found that 58% of retailers sold more than the recommended amount, and 23% sold more than the legal amount [Citation248].
Taken together, evidence from the UK suggests that changes in paracetamol availability can reduce severe self-poisoning and deaths, but these changes are more modest than initially hoped [Citation249]. Lack of enforcement of guidelines and laws likely reduced the impact of this legislation. Legal limits to the number of packs that can be purchased, and active enforcement should be considered by countries considering similar legislation.
6.3.2. Denmark
Denmark undertook two interventions [Citation250]. The first in 2011 restricted sales to persons aged 18 years and over while the second in 2013 restricted pack sizes to 20 tablets for OTC purchases within pharmacies [Citation250]. After the age restriction, there was a reduction in the number of poisonings in those aged 10–17 years but no change in total non-opioid analgesic poisonings [Citation250]. This restriction may have also played a part in reducing the number of cases of self-harm seen in adolescents in Denmark [Citation251]. The introduction of the pack size restriction saw a decrease in the number of total poisonings and their severity [Citation250].
6.3.3. North America
In 2014, the U.S.A attempted to reduce unintentional overdose, and therefore hepatotoxicity, by reducing the quantity of acetaminophen when combined with opioids to 325 mg per tablet [Citation252]. However, PIC data from before and after the change identified no significant variation to the rates of treatment for poisoning or hepatotoxicity [Citation252]. Canada attempted to reduce the risk of accidental overdose with paracetamol by updating labeling standards on two occasions (2009 and 2016); however, no significant changes occurred [Citation253].
6.3.4. Australia
In response to rising rates of overdose with MR paracetamol in Australia, and the increased risk of toxicity with MR paracetamol, Australia up-scheduled MR paracetamol from ‘Pharmacy Only’ to ‘Pharmacist Only’ in 2020 [Citation239]. This meant that MR paracetamol could only be purchased from behind the pharmacists’ counter, and a pharmacist had to be involved in the sale. Early evaluation of this intervention indicates that it was not associated with a reduction in poisonings with MR paracetamol [Citation254].[Citation253]
7. Conclusions
Paracetamol is one of the most widely used medicines in the world. This is despite a recent systematic review indicating lack of efficacy for many conditions for which it is considered first line. It is widely available without prescription, however access varies greatly internationally. Paracetamol is one of the most used substances in overdose in many parts of the world, and the leading cause of acute liver injury in many countries. Where data were available, we estimate it is used in 6% of poisonings, implicated in 56% of cases of severe acute liver injury and acute liver failure and 7% of drug-induced liver injury. Approximately 0.4% of paracetamol overdose cases are fatal. Acetylcysteine is an effective antidote, and yet hepatotoxicity and death still occur. The three main factors making hepatotoxicity more likely are (i) larger overdoses, (ii) overdose with MR/ER paracetamol, and (iii) delays to treatment. Recent research has focused on changes to acetylcysteine regimens to reduce adverse reactions and improve efficacy for high-risk overdoses, as well as the exploration of novel biomarkers and metabolic treatments. In addition, public health interventions such as pack size restrictions and age limits for purchasing paracetamol have been evaluated and been shown as an effective way at reducing harm from paracetamol overdose.
8. Expert opinion
If paracetamol was discovered today, drug regulators would likely enforce tighter restrictions than those currently in place due to the risk of hepatotoxicity. Despite widespread use and availability, paracetamol has a narrow safety margin compared to other over-the-counter analgesics. For example, the toxic dose of paracetamol is 10 times a therapeutic dose, whereas the toxic dose of ibuprofen is 60 times a therapeutic dose. Worldwide accessibility of paracetamol varies substantially. Broadly, continental Europe has strict rules: many countries do not allow non-pharmacy sales, and sales within pharmacies are typically limited to small packs. The UK reduced available pack sizes in 1998 in response to rising rates of overdose and deaths. In the U.S.A., there appear to be no limits on pack size (1000 tablet packs can be purchased).
The magnitude of the paracetamol problem is unknown in many countries. Despite our best efforts to capture published studies and gray literature, our estimates of the burden of paracetamol poisoning globally may not be internationally representative, and many countries had no data available. This is somewhat surprising, as there is an ICD−10 code for paracetamol overdose, so this information should be collected in most countries. Disappointingly, the global burden of disease studies reports specifically only on carbon monoxide poisoning with all other poisonings combined. Improving global estimates of disease burden from poisoning is vital.
Paracetamol has long been considered the most important common poisoning in high-income countries and is increasingly being used in overdose in low- and middle-income countries [Citation255]. Acetylcysteine is an effective antidote: without it it is estimated that 3–5% of overdoses would be lethal [Citation99,Citation100]. Despite its long-term use, controversies in the management of overdose remain. We found few publicly accessible treatment guidelines, however we know treatment differs substantially globally. This includes the UK lowering of the treatment line in the Rumack-Matthew nomogram, and some countries treating all overdoses, regardless of serum concentrations. Acetylcysteine regimens differ, with new regimens being recently introduced in many countries to reduce adverse effects relating to the large loading dose.
Changing use patterns, including increasing ‘massive’ overdoses and use of modified-release paracetamol present treatment challenges. Recent innovations in N-acetylcysteine dosing regimens, new antidotes, and increased recognition of the role of activated charcoal may improve outcomes for patients. The identification of novel biomarkers has the potential to improve early identification of hepatotoxicity and better target treatments. This is an area of ongoing active research.
Emerging potential antidotes that target mitochondrial effects of NAPQI include fomepizole and calmangafodipir. If early clinical results are confirmed by robustly designed trials, these antidotes could fill a much-needed gap for scenarios where acetylcysteine is less effective. This includes people who present late and with established hepatotoxicity. The clinical toxicology field should learn from the experience with hydroxychloroquine and COVID−19, where early studies (animal experiments, small case series) showed promising results, which were later refuted by a robust platform trial. Given the frequency of paracetamol overdose, well designed, large clinical trials of these antidotes should be possible before they are considered routine care. In 10 years, the face of paracetamol management may look completely different if new biomarkers and new treatments become commonplace.
Continued innovation to improve outcomes has never been more important. Many countries are facing an epidemic of self-poisoning, particularly among adolescent females. This has been observed in the U.S.A, Canada, Australia, Egypt, and Norway, and is likely happening un-documented in many other countries. Paracetamol is the agent taken most frequently by young females, who have higher rates of OTC medicines use in overdose compared to adults (likely due to reduced access to prescription medicines). In 2022, Australia’s national drug regulator launched an enquiry into paracetamol overdoses in young people in response to surging rates.
The drivers behind surging rates of youth self-harm are complex. However, there are simple solutions to reduce harm from paracetamol overdose. Any intervention should be aimed at the most modifiable risk factors for hepatotoxicity: overdose size and overdose with MR/ER paracetamol. Self-harm overdoses tend to be impulsive, many people contemplate their overdose for less than 5 min. People typically take what is available to them in the home, and often consume every tablet they have access to. Reducing the paracetamol ‘tablet burden’ in homes by reducing pack sizes available without prescription is expected to impact overdose size and thus harm. This played out in the UK, with pack size restrictions reducing large overdoses, liver transplants and deaths without harm from methods substitution. However, the UK intervention was not as effective as legislators hoped, and paracetamol poisoning remains a concern. This sub-optimal effect is possibly due to some retailers breaching the spirit of the legislation, as indicated by mystery shopper studies. Other countries considering pack size restrictions should limit the number of packs that can be sold in one transaction. Admittedly, this may be more difficult to legislate and enforce than simple pack size limits.
In addition, reducing the casual use of MR/ER paracetamol would minimize its use in overdoses by vulnerable people. Recent data from Australia indicate that making MR paracetamol only available following consultation with a pharmacist did not curb poisonings. Previous Australian experience with codeine has indicated that more restrictive rescheduling to prescription only had a much stronger effect on poisonings than ‘Pharmacist Only’ rescheduling. The European Medicines Agency suspended marketing of modified-release paracetamol due to an unacceptable safety profile in overdose. The sole therapeutic benefit of MR paracetamol is a slight simplification in dosage regimen (three doses/day rather than four). Restrictions could be considered elsewhere in the world.
The relative lack of studies evaluating paracetamol poisoning interventions indicates that legislative measures are often implemented without evaluation plans. For example, we were unable to find any published data on the impact of the EMA suspending MR paracetamol on paracetamol hepatotoxicity and death. Prospective evaluation of any legislation aimed to reduce harm from poisoning is essential for targeting future interventions and informing responses in other jurisdictions.
It is important to keep this in perspective. Tonnes of paracetamol are consumed worldwide every day, mostly without harm. Paracetamol has a very good safety profile in therapeutic use. It has minimal adverse reactions if taken appropriately, a short list of clinically significant drug interactions, and can be taken by young infants, pregnant women, and people who cannot tolerate NSAIDs. Despite recent efficacy concerns, there are many people for whom paracetamol will remain first-line treatment for pain. Public health measures and continued clinical innovations are needed to ensure an acceptable risk-benefit profile.
Article highlights
Paracetamol (acetaminophen) is one of the most used medicines worldwide. It is safe if taken at therapeutic doses, however is commonly taken in overdose, where it can cause hepatotoxicity.
Risk factors for toxicity include: very large overdoses, overdose with modified release paracetamol, delays to treatment, treatment with enzyme inducers, malnutrition, and chronic alcohol use.
Acetylcysteine is an effective antidote. It replenishes glutathione stores, allowing detoxification of paracetamol’s toxic reactive metabolite. Recently, many changes to acetylcysteine treatment regimens have emerged to better treat high-risk overdoses and minimize adverse reactions. Other emerging potential treatments include fomepizole and calmangafodipir.
Epidemiological data on paracetamol poisoning are limited or non-existent in many parts of the world. Based on available data, we estimate that paracetamol is involved in 6% of global poisonings, 56% of severe acute liver injury and acute liver failure, and 7% of drug-induced liver injury. We estimate that 0.4% of paracetamol poisoning cases are fatal.
Access to paracetamol differs widely internationally, some countries allow unlimited non-pharmacy sales while some only allow sales in pharmacies. Available pack sizes also vary substantially.
Some countries have introduced effective interventions aimed at reducing self-harm poisonings with paracetamol. This includes pack size restrictions in the UK and Denmark, and Denmark placing an age limit on sales.
Declaration of interest
A Chidiac is the recipient of a PhD stipend from Reckitt Benckiser as part of an untied educational grant awarded to R. Cairns. R. Cairns has also recieved conference speaker fees/honoraria from Reckitt Benckiser and The Pharmacy Guild of Australia. These funders had no role in the preparation of this review. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
One reviewer receives research funding on the safety of acetaminophen from the US sponsor (J&J), there are no personal financial relationships. The remaining reviewers have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download MS Word (1.8 MB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17425255.2023.2223959
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Brune K, Renner B, Tiegs G. Acetaminophen/Paracetamol: a history of errors, failures and false decisions. Eur J Pain. 2015;19(7):953–965. doi: 10.1002/ejp.621
- WHO model list of essential medicines - 22nd list, 2021. Geneva (Switzerland): World Health Organization; 2021.
- Paracetamol [Internet]. Adelaide (Australia): Australian Medicines Handbook Pty Ltd; 2022 [cited 2022 Aug 16]. Available from: https://amhonline-amh-net-au/chapters/analgesics/drugs-pain-relief/non-opioid-analgesics/paracetamol
- Acheampong P, Thomas SH. Determinants of hepatotoxicity after repeated supratherapeutic paracetamol ingestion: systematic review of reported cases. Br J Clin Pharmacol. 2016;82(4):923–931. doi: 10.1111/bcp.13028
- Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42(6):1364–1372. doi: 10.1002/hep.20948
- Schiødt FV, Atillasoy E, Shakil AO, et al. Etiology and outcome for 295 patients with acute liver failure in the united states. Liver Transpl Surg. 1999;5(1):29–34. doi: 10.1002/lt.500050102
- Bernal W, Wendon J. Acute liver failure; clinical features and management. Eur J Ganstroenterol Hepatol. 1999;11(9):977–984. doi: 10.1097/00042737-199909000-00005
- Wei G, Bergquist A, Broomé U, et al. Acute liver failure in Sweden: etiology and outcome. J Intern Med. 2007;262(3):393–401. doi: 10.1111/j.1365-2796.2007.01818.x
- Ostapowicz G, Fontana RJ, Schiødt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137(12):947–954. doi: 10.7326/0003-4819-137-12-200212170-00007
- Bunchorntavakul C, Reddy KR. Acetaminophen-related hepatotoxicity. Clin Liver Dis. 2013;17(4):587–607. doi: 10.1016/j.cld.2013.07.005
- Gulmez SE, Larrey D, Pageaux GP, et al. Liver transplant associated with paracetamol overdose: results from the seven-country SALT study. Br J Clin Pharmacol. 2015;80(3):599–606. doi: 10.1111/bcp.12635
- Cahn A, Hepp P. Das Antifebrin, ein neues Fiebermittel. Centralbl Klin Med. 1886;7:561–564. German.
- Mallet C, Eschalier A. Landmark papers in pain: seminal papers in pain with expert commentaries. Oxford (United Kingdom): Oxford University Press. Chapter 4, The rediscovery of paracetamol;2018. p. 12–16. doi: 10.1093/med/9780198834359.003.0004
- Aronoff DM. Aspirin and Reyes syndrome: discovery of aspirin and paracetamol. Drug Saf. 2002;25(10):751. doi: 10.2165/00002018-200225100-00007
- von Mering J. Beiträge zur Kenntnis der Antipyretica. Ther Monatsch. 1893;7:577–587. German.
- Brodie BB, Axelrod J. The fate of acetophenetidin (phenacetin) in man and methods for the estimation of acetophenetidin and its metabolites in biological material. J Pharmacol Exp Ther. 1949;97(1):58–67.
- Anderson BJ. Paracetamol (Acetaminophen): mechanisms of action. Pediatr Anesth. 2008;18(10):915–921. doi: 10.1111/j.1460-9592.2008.02764.x
- Graham GG, Davies MJ, Day RO, et al. The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology. 2013;21(3):201–232. doi: 10.1007/s10787-013-0172-x
- Abdel Shaheed C, Ferreira GE, Dmitritchenko A, et al. The efficacy and safety of paracetamol for pain relief: an overview of systematic reviews. Med J Aust. 2021;214(7):324–331. doi: 10.5694/mja2.50992
- Agrawal S, Khazaeni B. Acetaminophen Toxicity. Florida (United States of America): StatPearls Publishing; 2022.
- Hodgman MJ, Garrard AR. A review of acetaminophen poisoning. Crit Care Clin. 2012;28(4):499–516. doi: 10.1016/j.ccc.2012.07.006
- Leeming MG, Gamon LF, Wille U, et al. What are the potential sites of protein arylation by N-Acetyl-p-benzoquinone Imine (NAPQI)? Chem Res Toxicol. 2015;28(11):2224–2233. doi: 10.1021/acs.chemrestox.5b00373
- Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. 2010;196:369–405.
- Ramachandran A, Jaeschke H. A mitochondrial journey through acetaminophen hepatotoxicity. Food Chem Toxicol. 2020;140:111282. doi: 10.1016/j.fct.2020.111282
- Ramachandran A, Jaeschke H. Acetaminophen hepatotoxicity: a mitochondrial perspective. Advances in Pharmacology Volume 85. Academic Press;2019; p. 195–219. doi: 10.1016/bs.apha.2019.01.007
- Moles A, Torres S, Baulies A, et al. Mitochondrial–Lysosomal axis in acetaminophen hepatotoxicity. Front Pharmacol. 2018;9:453. doi: 10.3389/fphar.2018.00453
- Jaeschke H, Duan L, Nguyen NT, et al. Mitochondrial damage and biogenesis in acetaminophen-induced liver injury. Liver Res. 2019;3(3–4):150–156. doi: 10.1016/j.livres.2019.10.002
- Saccomano SJ. Acute acetaminophen toxicity in adults. Nurse Pract. 2019;44(11):42–47. doi: 10.1097/01.NPR.0000586020.15798.c6
- Green JL, Heard KJ, Reynolds KM, et al. Oral and intravenous acetylcysteine for treatment of acetaminophen toxicity: a systematic review and meta-analysis. West J Emerg Med. 2013;14(3):218–226. doi: 10.5811/westjem.2012.4.6885
- Buckley N, Calear A, Cairns R, et al. Independent expert report on the risks of intentional self-poisoning with paracetamol. Canberra (Australia): Department of Health and Aged Care (Therapeutic Goods Administration); 2022.
- Waring WS, Jamie H, Leggett GE. Delayed onset of acute renal failure after significant paracetamol overdose: a case series. Hum Exp Toxicol. 2010;29(1):63–68. doi: 10.1177/0960327109350799
- Eguia L, Materson BJ. Acetaminophen-related acute renal failure without fulminant liver failure. Pharmacotherapy. 1997;17(2):363–370.
- Loh CS, Ponampalam R. Nephrotoxicity associated with acute paracetamol overdose: a case report and review of the literature. Hong Kong J Of Emerg Med. 2006;13(2):105–110. doi: 10.1177/102490790601300202
- Hart SGE, Beierschmitt WP, Wyand DS, et al. Acetaminophen nephrotoxicity in CD-1 Mice: i. evidence of a role for in situ activation in selective covalent binding and toxicity. Toxicol Appl Pharmacol. 1994;126(2):267–275. doi: 10.1006/taap.1994.1116
- Flanagan RJ, Mant TGK. Coma and metabolic acidosis early in severe acute paracetamol poisoning. Hum Toxicol. 1986;5(3):179–182. doi: 10.1177/096032718600500305
- Roth B, Woo O, Blanc P. Early metabolic acidosis and coma after acetaminophen ingestion. Ann Emerg Med. 1999;33(4):452–456. doi: 10.1016/S0196-0644(99)70312-4
- Wiegand TJ, Margaretten M, Olson KR. Massive acetaminophen ingestion with early metabolic acidosis and coma: treatment with IV NAC and continuous venovenous hemodiafiltration. Clin Toxicol (Phila). 2010;48(2):156–159. doi: 10.3109/15563650903524142
- Vliegenthart ADB, Kimmitt RA, Seymour JH, et al. Circulating acetaminophen metabolites are toxicokinetic biomarkers of acute liver injury. Clin Pharmacol Ther. 2017;101(4):531–540. doi: 10.1002/cpt.541
- Chiew AL, Isbister GK, Kirby KA, et al. Massive paracetamol overdose: an observational study of the effect of activated charcoal and increased acetylcysteine dose (ATOM-2). Clin Toxicol (Phila). 2017;55(10):1055–1065. doi: 10.1080/15563650.2017.1334915.
- Downs JW, Cumpston KL, Kershner EK, et al. Clinical outcome of massive acetaminophen overdose treated with standard-dose N-acetylcysteine. Clin Toxicol (Phila). 2021;59(10):932–936. doi: 10.1080/15563650.2021.1887493
- Lewis JC, Lim M, Lai L, et al. Evaluation of N-acetylcysteine dose for the treatment of massive acetaminophen ingestion. Clin Toxicol (Phila). 2022;60(4):507–513. doi: 10.1080/15563650.2021.1984503
- Gloor Y, Schvartz D, Samer CF. Old problem, new solutions: biomarker discovery for acetaminophen liver toxicity. Expert Opin Drug Metab Toxicol. 2019;15(8):659–669. doi: 10.1080/17425255.2019.1642323
- The Flockhart Cytochrome P450 Drug-Drug Interaction Table [Internet]. Indiana (United States of America): Division of Clinical Pharmacology, Indiana University School of Medicine; [updated 2021, cited 2022 Oct 4]. Available from: https://drug-interactions.medicine.iu.edu/MainTable.aspx
- Nguyen GC, Sam J, Thuluvath PJ. Hepatitis C is a predictor of acute liver injury among hospitalizations for acetaminophen overdose in the United States: a nationwide analysis. Hepatology. 2008;48(4):1336–1341. doi: 10.1002/hep.22536
- Whitcomb DC, Block GD. Association of Acetaminophen Hepatotoxicity with Fasting and Ethanol Use. JAMA. 1994;272(23):1845–1850. doi: 10.1001/jama.1994.03520230055038
- Seeff LB, Cuccherini BA, Zimmerman HJ, et al. Acetaminophen Hepatotoxicity in Alcoholics. Ann Intern Med. 1986;104(3):399–404. doi: 10.7326/0003-4819-104-3-399
- Peterson FJ, Holloway DE, Erickson RR, et al. Ethanol induction of acetaminophen toxicity and metabolism. Life Sci. 1980;27(18):1705–1711. doi: 10.1016/0024-3205(80)90646-3
- Prescott LF. Paracetamol, alcohol and the liver. Br J Clin Pharmacol. 2000;49(4):291–301. doi: 10.1046/j.1365-2125.2000.00167.x
- Bray GP, Mowat C, Muir DF, et al. The Effect of Chronic Alcohol Intake on Prognosis and Outcome in Paracetamol Overdose. Hum Exp Toxicol. 1991;10(6):435–438. doi: 10.1177/096032719101000612
- Waring WS, Stephen AF, Malkowska AM, et al. Acute Ethanol Coingestion Confers a Lower Risk of Hepatotoxicity after Deliberate Acetaminophen Overdose. Acad Emerg Med. 2008;15(1):54–58. doi: 10.1111/j.1553-2712.2007.00019.x
- Graudins A, Chiew A, Chan B. Overdose with modified-release paracetamol results in delayed and prolonged absorption of paracetamol. Intern Med J. 2010;40(1):72–76. doi: 10.1111/j.1445-5994.2009.02096.x
- Li J, Chiew AL, Isbister GK, et al. Population pharmacokinetics of immediate-release and modified-release paracetamol and its major metabolites in a supratherapeutic dosing study. Clin Toxicol (Phila). 2022;60(1):25–32. doi: 10.1080/15563650.2021.1928163
- Spyker DA, Dart RC, Yip L, et al. Population pharmacokinetic analysis of acetaminophen overdose with immediate release, extended release and modified release formulations. Clin Toxicol (Phila). 2022;60(10):1113–1121. doi: 10.1080/15563650.2022.2114361
- Chiew AL, Isbister GK, Page CB, et al. Modified release paracetamol overdose: a prospective observational study (ATOM-3). Clin Toxicol (Phila). 2018;56(9):810–819. doi: 10.1080/15563650.2018.1439950.
- Michienzi A, Tobarran N, Hieger MA. Extended Release Acetaminophen Overdose with Delayed Peak Concentrations. Am J Ther. 2022;29(6):e655–e656. doi: 10.1097/MJT.0000000000001329
- Hoegberg LCG, Refsgaard F, Pedersen SH, et al. Potential pharmacobezoar formation of large size extended-release tablets and their dissolution – an in vitro study. Clin Toxicol (Phila). 2019;57(4):271–281. doi: 10.1080/15563650.2018.1513138
- Li YK, Lam KF, Wong CLW, et al. In vitro study of pharmacobezoar formation in simulated acetaminophen overdose. Clin Toxicol (Phila). 2020;58(9):900–906. doi: 10.1080/15563650.2019.1705971
- Cairns R, Brown JA, Wylie CE, et al. Paracetamol poisoning‐related hospital admissions and deaths in Australia, 2004–2017. Med J Aust. 2019;211(5):218–223. doi: 10.5694/mja2.50296
- Dart RC, Green JL, Bogdan GM. The Safety Profile of Sustained Release Paracetamol During Therapeutic Use and Following Overdose. Drug Saf. 2005;28(11):1045–1056. doi: 10.2165/00002018-200528110-00005
- Chiew AL, Buckley NA. Comment on: “population pharmacokinetic analysis of acetaminophen overdose with immediate release, extended release and modified release formulations”. Clin Toxicol (Phila). 2023;61(2):139–140. doi: 10.1080/15563650.2022.2135519
- Vogel J, Heard KJ, Carlson C, et al. Dental pain as a risk factor for accidental acetaminophen overdose: a case-control study. Am J Emerg Med. 2011;29(9):1125–1129. doi: 10.1016/j.ajem.2010.08.006
- Schiødt FV, Rochling FA, Casey DL, et al. Acetaminophen Toxicity in an Urban County Hospital. New Engl J Med. 1997;337(16):1112–1118. doi: 10.1056/NEJM199710163371602
- Gyamlani GG, Parikh CR. Acetaminophen toxicity: suicidal vs accidental. Crit Care. 2002;6(2):155–159. doi: 10.1186/cc1475
- Myers RP, Shaheen AAM, Li B, et al. Impact of Liver Disease, Alcohol Abuse, and Unintentional Ingestions on the Outcomes of Acetaminophen Overdose. Clin Gastroenterol Hepatol. 2008;6(8):918–925. doi: 10.1016/j.cgh.2008.02.053
- Egan H, Isbister GK, Robinson J, et al. Retrospective evaluation of repeated supratherapeutic ingestion (RSTI) of paracetamol. Clin Toxicol (Phila). 2019;57(8):703–711. doi: 10.1080/15563650.2018.1547829
- Alhelail MA, Hoppe JA, Rhyee SH, et al. Clinical course of repeated supratherapeutic ingestion of acetaminophen. Clin Toxicol (Phila). 2011;49(2):108–112. doi: 10.3109/15563650.2011.554839
- Craig DGN, Bates CM, Davidson JS, et al. Staggered overdose pattern and delay to hospital presentation are associated with adverse outcomes following paracetamol-induced hepatotoxicity. Br J Clin Pharmacol. 2012;73(2):285–294. doi: 10.1111/j.1365-2125.2011.04067.x
- Myers RP, Shaheen AAM. Hepatitis C, alcohol abuse, and unintentional overdoses are risk factors for acetaminophen-related hepatotoxicity. Hepatology. 2009;49(4):1399–1400. doi: 10.1002/hep.22798
- Rutherford A, Davern T, Hay JE, et al. Influence of High Body Mass Index on Outcome in Acute Liver Failure. Clin Gastroenterol Hepatol. 2006;4(12):1544–1549. doi: 10.1016/j.cgh.2006.07.014
- Radosevich JJ, Patanwala AE, Erstad BL. Hepatotoxicity in Obese versus Nonobese Patients with Acetaminophen Poisoning Who are Treated with Intravenous N-Acetylcysteine. Am J Ther. 2016;23(3):e714–e719. doi: 10.1097/01.mjt.0000434043.62372.00
- Michaut A, Moreau C, Robin MA, et al. Acetaminophen-induced liver injury in obesity and nonalcoholic fatty liver disease. Liver Int. 2014;34(7):e171–e179. doi: 10.1111/liv.12514
- Heruth DP, Shortt K, Zhang N, et al. Genetic Association of Single Nucleotide Polymorphisms with Acetaminophen-Induced Hepatotoxicity. J Pharmacol Exp Ther. 2018;367(1):95–100. doi: 10.1124/jpet.118.248583
- Monte AA, Arriaga Mackenzie I, Pattee J, et al. Genetic variants associated with ALT elevation from therapeutic acetaminophen. Clin Toxicol (Phila). 2022;60(11):1198–1204. doi: 10.1080/15563650.2022.2117053
- Chiew AL, Reith D, Pomerleau A, et al. Updated guidelines for the management of paracetamol poisoning in Australia and New Zealand. Med J Aust. 2020;212(4):175–183. doi: 10.5694/mja2.50428
- Wong A, Graudins A. Risk prediction of hepatotoxicity in paracetamol poisoning. Clin Toxicol (Phila). 2017;55(8):879–892. doi: 10.1080/15563650.2017.1317349
- Dear JW, Antoine DJ. Stratification of paracetamol overdose patients using new toxicity biomarkers: current candidates and future challenges. Expert Rev Clin Pharmacol. 2014;7(2):181–189. doi: 10.1586/17512433.2014.880650
- Bateman DN, Carroll R, Pettie J, et al. Effect of the UK’s revised paracetamol poisoning management guidelines on admissions, adverse reactions and costs of treatment. Br J Clin Pharmacol. 2014;78(3):610–618. doi: 10.1111/bcp.12362
- Gosselin S, Hoffman RS, Juurlink DN, et al. Treating acetaminophen overdose: thresholds, costs and uncertainties. Clin Toxicol (Phila). 2013;51(3):130–133. doi: 10.3109/15563650.2013.775292
- Bateman DN, Dear JW, Carroll R, et al. Impact of reducing the threshold for acetylcysteine treatment in acute paracetamol poisoning: the recent United Kingdom experience. Clin Toxicol (Phila). 2014;52(8):868–872. doi: 10.3109/15563650.2014.954125
- Schmidt LE, Dalhoff K. Risk factors in the development of adverse reactions to N-acetylcysteine in patients with paracetamol poisoning. Br J Clin Pharm. 2001;51(1):87–91. doi: 10.1046/j.1365-2125.2001.01305.x
- Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase Elevations in Healthy Adults Receiving 4 Grams of Acetaminophen Daily: a Randomized Controlled Trial. JAMA. 2006;296(1):87–93. doi: 10.1001/jama.296.1.87
- Chiew AL, James LP, Isbister GK, et al. Early acetaminophen-protein adducts predict hepatotoxicity following overdose (ATOM-5). J Hepatol. 2020;72(3):450–462. doi: 10.1016/j.jhep.2019.10.030
- Green TJ, Sivilotti MLA, Langmann C, et al. When do the aminotransferases rise after acute acetaminophen overdose? Clin Toxicol (Phila). 2010;48(8):787–792. doi: 10.3109/15563650.2010.523828
- Curtis RM, Sivilotti MLA. A descriptive analysis of aspartate and alanine aminotransferase rise and fall following acetaminophen overdose. Clin Toxicol (Phila). 2015;53(9):849–855. doi: 10.3109/15563650.2015.1077968
- Lindena J, Sommerfeld U, Höpfel C, et al. Catalytic Enzyme Activity Concentration in Tissues of Man, Dog, Rabbit, Guinea Pig, Rat and Mouse. Approach to a Quantitative Diagnostic Enzymology, III. Communication. J Clin Chem Clin Biochem. 1986;24(1):35–47. doi: 10.1515/cclm.1986.24.1.35
- Antoine DJ, Dear JW, Lewis PS, et al. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58(2):777–787. doi: 10.1002/hep.26294.
- Sivilotti MLA, Green TJ, Langmann C, et al. Multiplying the serum aminotransferase by the acetaminophen concentration to predict toxicity following overdose. Clin Toxicol (Phila). 2010;48(8):793–799. doi: 10.3109/15563650.2010.523829
- Wong A, Sivilotti MLA, Dargan PI, et al. External validation of the paracetamol-aminotransferase multiplication product to predict hepatotoxicity from paracetamol overdose. Clin Toxicol (Phila). 2015;53(8):807–814. doi: 10.3109/15563650.2015.1066507.
- Wong A, Sivilotti MLA, Graudins A. Accuracy of the paracetamol-aminotransferase multiplication product to predict hepatotoxicity in modified-release paracetamol overdose. Clin Toxicol (Phila). 2017;55(5):346–351. doi: 10.1080/15563650.2017.1290253
- Yau CE, Chen H, Lim BPY, et al. Performance of the paracetamol-aminotransferase multiplication product in risk stratification after paracetamol (acetaminophen) poisoning: a systematic review and meta-analysis. Clin Toxicol (Phila). 2023;61(1):1–11. doi: 10.1080/15563650.2022.2152350
- Owens KH, Medlicott NJ, Zacharias M, et al. Population pharmacokinetic–pharmacodynamic modelling to describe the effects of paracetamol and N-acetylcysteine on the international normalized ratio. Clin Exp Pharmacol Physiol. 2015;42(1):102–108. doi: 10.1111/1440-1681.12327
- Davern TJ II, James LP, Hinson JA, et al. Measurement of serum acetaminophen–protein adducts in patients with acute liver failure. Gastroenterology. 2006;130(3):687–694. doi: 10.1053/j.gastro.2006.01.033
- James LP, Letzig L, Simpson PM, et al. Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab Dispos. 2009;37(8):1779–1784. doi: 10.1124/dmd.108.026195
- Chiew AL, Gluud C, Brok J, et al. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst Rev. 2018;(2):CD003328. doi: 10.1002/14651858.CD003328.pub3
- Buckley NA, Buckley N, Whyte IM, et al. Activated charcoal reduces the need for N-Acetylcysteine treatment after acetaminophen (paracetamol) overdose. J Toxicol Clin Toxicol. 1999;37(6):753–757. doi: 10.1081/CLT-100102452
- Lauterburg BH, Corcoran GB, Mitchell JR. Mechanism of Action of N-Acetylcysteine in the Protection Against the Hepatotoxicity of Acetaminophen in Rats in vivo. J Clin Invest. 1983;71(4):980–991. doi: 10.1172/JCI110853
- Tenório MCdS, Graciliano NG, Moura FA, et al. N-Acetylcysteine (NAC): impacts on human health. Antioxidants. 2021;10(6):967. doi: 10.3390/antiox10060967
- Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N‐acetylcysteine. Hepatology. 2010;51(1):246–254. doi: 10.1002/hep.23267
- Prescott LF, Illingworth RN, Critchley JA, et al. Intravenous N-acetylcystine: the treatment of choice for paracetamol poisoning. Br Med J. 1979;2(6198):1097–1100. doi: 10.1136/bmj.2.6198.1097
- Clark R, Borirakchanyavat V, Davidson AR, et al. Hepatic damage and death from overdose of paracetamol. Lancet. 1973;301(7794):66–70. doi: 10.1016/S0140-6736(73)90466-2
- Bateman DN, Dear JW. Acetylcysteine in paracetamol poisoning: a perspective of 45 years of use. Toxicol Res (Camb). 2019;8(4):489–498. doi: 10.1039/C9TX00002J
- Wong A, Graudins A. Simplification of the standard three-bag intravenous acetylcysteine regimen for paracetamol poisoning results in a lower incidence of adverse drug reactions. Clin Toxicol (Phila). 2016;54(2):115–119. doi: 10.3109/15563650.2015.1115055
- McNulty R, Lim JME, Chandru P, et al. Fewer adverse effects with a modified two-bag acetylcysteine protocol in paracetamol overdose. Clin Toxicol (Phila). 2018;56(7):618–621. doi: 10.1080/15563650.2017.1408812
- Pettie JM, Caparrotta TM, Hunter RW, et al. Safety and Efficacy of the SNAP 12-hour Acetylcysteine Regimen for the Treatment of Paracetamol Overdose. EClinicalMedicine. 2019;11:11–17.
- Johnson MT, McCammon CA, Mullins ME, et al. Evaluation of a Simplified N-Acetylcysteine Dosing Regimen for the Treatment of Acetaminophen Toxicity. Ann Pharmacother. 2011;45(6):713–720. doi: 10.1345/aph.1P613
- Wong A, Gunja N, McNulty R, et al. Analysis of an 8-hour acetylcysteine infusion protocol for repeated supratherapeutic ingestion (RSTI) of paracetamol. Clin Toxicol (Phila). 2018;56(3):199–203. doi: 10.1080/15563650.2017.1359620
- Daly FFS, O’Malley GF, Heard K, et al. Prospective evaluation of repeated supratherapeutic acetaminophen (paracetamol) ingestion. Ann Emerg Med. 2004;44(4):393–398. doi: 10.1016/j.annemergmed.2004.05.005
- Janković SM. Acetaminophen toxicity and overdose: current understanding and future directions for NAC dosing regimens. Expert Opin Drug Metab Toxicol. 2022;18(11):745–753. doi: 10.1080/17425255.2022.2151893
- Prescott LF, Sutherland GR, Park J, et al. Cysteamine, Methionine, and Penicillamine in the Treatment of Paracetamol Poisoning. Lancet. 1976;308(7977):109–113. doi: 10.1016/S0140-6736(76)92842-7
- Vale JA, Meredith TJ, Goulding R. Treatment of Acetaminophen Poisoning: the Use of Oral Methionine. Arch Intern Med. 1981;141(3):394–396. doi: 10.1001/archinte.1981.00340030126023
- Mullins ME, Yeager LH, Freeman WE. Metabolic and mitochondrial treatments for severe paracetamol poisoning: a systematic review. Clin Toxicol (Phila). 2020;58(12):1284–1296. doi: 10.1080/15563650.2020.1798979
- Dear JW. Fomepizole should not be used more liberally in paracetamol overdose. Br J Clin Pharmacol. 2023;89(2):599–601. doi: 10.1111/bcp.15596
- Akakpo JY, Ramachandran A, Duan L, et al. Delayed Treatment with 4-Methylpyrazole Protects Against Acetaminophen Hepatotoxicity in Mice by Inhibition of c-Jun n-Terminal Kinase. Toxicol Sci. 2019;170(1):57–68. doi: 10.1093/toxsci/kfz077
- Link SL, Rampon G, Osmon S, et al. Fomepizole as an adjunct in acetylcysteine treated acetaminophen overdose patients: a case series. Clin Toxicol (Phila). 2022;60(4):472–477. doi: 10.1080/15563650.2021.1996591
- Filip AB, Berg SE, Mullins ME, et al. Fomepizole as an adjunctive therapy for acetaminophen poisoning: cases reported to the toxicology investigators consortium (ToxIC) database 2015–2020. Clin Toxicol (Phila). 2022;60(9):1006–1011. doi: 10.1080/15563650.2022.2070071.
- Bateman DN, Dear JW, Eddleston M, et al. Comment on Fomepizole as an adjunct in acetylcysteine treated acetaminophen overdose patients: a case series. Clin Toxicol (Phila). 2022;60(5):666–667. doi: 10.1080/15563650.2021.2009849
- Link SL, Rampon G, Osmon S, et al. Reply to Comments on Fomepizole as an adjunct in acetylcysteine treated acetaminophen overdose patients: a case series. Clin Toxicol (Phila). 2022;60(5):668–669. doi: 10.1080/15563650.2021.2016799
- Agarwal R, MacMillan-Crow LA, Rafferty TM, et al. Acetaminophen-Induced Hepatotoxicity in Mice Occurs with Inhibition of Activity and Nitration of Mitochondrial Manganese Superoxide Dismutase. J Pharmacol Exp Ther. 2011;337(1):110–118. doi: 10.1124/jpet.110.176321
- Morrison EE, Oatey K, Gallagher B, et al. Principal results of a randomised open label exploratory, safety and tolerability study with calmangafodipir in patients treated with a 12 h regimen of N-acetylcysteine for paracetamol overdose (POP trial). EBioMedicine. 2019;46:423–430. doi: 10.1016/j.ebiom.2019.07.013
- Akakpo JY, Ramachandran A, Jaeschke H. Novel strategies for the treatment of acetaminophen hepatotoxicity. Expert Opin Drug Metab Toxicol. 2020;16(11):1039–1050. doi: 10.1080/17425255.2020.1817896
- Ding GKA, Buckley NA. Evidence and consequences of spectrum bias in studies of criteria for liver transplant in paracetamol hepatotoxicity. QJM. 2008;101(9):723–729. doi: 10.1093/qjmed/hcn077
- O’Grady JG, Alexander GJM, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97(2):439–445. doi: 10.1016/0016-5085(89)90081-4
- O’Grady J. Timing and benefit of liver transplantation in acute liver failure. J Hepatol. 2014;60(3):663–670. doi: 10.1016/j.jhep.2013.10.024
- 7 continents [Internet]. Worldometer; [cited 2023 Jan 5]. Available from: https://www.worldometers.info/geography/7-continents/
- Gummin DD, Mowry JB, Spyker DA, et al. 2016 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th Annual Report. Clin Toxicol (Phila). 2017;55(10):1072–1254. doi: 10.1080/15563650.2017.1388087
- Gummin DD, Mowry JB, Spyker DA, et al. 2017 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila). 2018;56(12):1213–1415. doi: 10.1080/15563650.2018.1533727
- Gummin DD, Mowry JB, Spyker DA, et al. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report. Clin Toxicol (Phila). 2019;57(12):1220–1413. doi: 10.1080/15563650.2019.1677022
- Gummin DD, Mowry JB, Beuhler MC, et al. 2019 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 37th Annual Report. Clin Toxicol (Phila). 2020;58(12):1360–1541. doi: 10.1080/15563650.2020.1834219
- Descamps AMK, Vandijck DM, Buylaert WA, et al. Characteristics and costs in adults with acute poisoning admitted to the emergency department of a university hospital in Belgium. PLoS ONE. 2019;14(10):e0223479. doi: 10.1371/journal.pone.0223479
- Descamps AK, Vandijck D, Buylaert W, et al. Hospital referrals of patients with acute poisoning by the Belgian Poison Centre: analysis of characteristics, associated factors, compliance and costs. Emerg Med J. 2021;38(7):511–519. doi: 10.1136/emermed-2019-209202
- Mbongwe B, Moinami J, Masupe T, et al. Nature and sources of poisoning in patients admitted to a referral hospital in Gaborone, Botswana; findings and implications. Hosp Pract. 2020;48(2):100–107. doi: 10.1080/21548331.2020.1739415
- Zhang Y, Yu B, Wang N, et al. Acute poisoning in Shenyang, China: a retrospective and descriptive study from 2012 to 2016. BMJ Open. 2018;8(8):e021881. doi: 10.1136/bmjopen-2018-021881
- Andersen CU, Nielsen LP, Møller JM, et al. Acute drug poisonings leading to hospitalization. Basic Clin Pharmacol Toxicol. 2022;130(2):328–336. doi: 10.1111/bcpt.13688
- Afandiyev I, Kobidze T, Kereselidze M, et al. СРАВНИТЕЛЬНАЯ СТРУКТУРА ОСТРЫХ ОТРАВЛЕНИЙ В ДВУХ СТОЛИЧНЫХ ГОРОДАХ РЕГИОНА ЮЖНОГО КАВКАЗА [The comparative structure of acute poisonings in two capital cities of the South Caucasus region]. Georgian Med News. 2019;287(2):105–110. Russian.
- Gudjonsdottir GA, Thordardottir AM, Kristinsson J. Framskyggn rannsókn á eitrunum sem komu til meðferðar á bráðamóttökum Landspítala 2012 [A prospective study on acute poisonings presenting to the Emergency Department at Landspitali University Hospital in Iceland 2012]. Laeknabladid. 2017;103(6):275–280. Icelandic. doi: 10.17992/lbl.2017.06.140
- Bentur Y, Lurie Y, Cahana A, et al. Poisoning in Israel: annual Report of the Israel Poison Information Center, 2017. Isr Med Assoc J. 2019;21(3):175–182.
- Yehya A, Albals D, Issa R, et al. Retrospective assessment of acute poisoning incidents by pharmaceutical agents in Jordan: data from Pharmacy One™ Poison Call Center, 2014 to 2018-Part II. Pharmacol Res Perspect. 2020;8(2):e00583. doi: 10.1002/prp2.583
- Hondebrink L, Rietjens SJ, Donker DW, et al. A quarter of admitted poisoned patients have a mild poisoning and require no treatment: an observational study. Eur J Intern Med. 2019;66:41–47. doi: 10.1016/j.ejim.2019.05.012
- Liakoni E, Berger F, Klukowska-Rötzler J, et al. Characteristics of emergency department presentations requiring consultation of the national Poisons Information Centre. Swiss Med Wkly. 2019;149(5152):w20164. doi: 10.4414/smw.2019.20164
- Sungur S, Bilge U, Acar N, et al. Retrospective evaluation of adult poisoning cases admitted to emergency department of a University Hospital in Turkey. Niger J Clin Pract. 2018;21(8):1023–1028. doi: 10.4103/njcp.njcp_291_17
- Friedman N, Shoshani-Levy M, Brent J, et al. Fatalities in poisoned patients managed by medical toxicologists. Clin Toxicol (Phila). 2020;58(7):688–691. doi: 10.1080/15563650.2019.1672877
- Beauchamp GA, Carey JL, Adams T, et al. Sex Differences in Poisonings Among Older Adults: an Analysis of the Toxicology Investigators Consortium (ToxIC) Registry, 2010 to 2016. Clin Ther. 2018;40(8):1366–1374.e8. doi: 10.1016/j.clinthera.2018.06.012
- Farrugia LA, Rhyee SH, Calello DP, et al. The Toxicology Investigators Consortium Case Registry-the 2016 Experience. J Med Toxicol. 2017;13(3):203–226. doi: 10.1007/s13181-017-0627-3
- Spyres MB, Farrugia LA, Kang AM, et al. The Toxicology Investigators Consortium Case Registry-the 2019 Annual Report. J Med Toxicol. 2020;16(4):361–387. doi: 10.1007/s13181-020-00810-7
- Leonard JB, Minhaj FS, Paterson E, et al. Exposures in pregnant patients reported to United States Poison Centers. Clin Toxicol (Phila). 2022;60(3):356–361. doi: 10.1080/15563650.2021.1968420
- Babić Ž, Turk R. Izvješće Centra za kontrolu otrovanja za 2021 [Report of the Poison Control Centre for 2021]. Arh Hig Rada Toksikol. 2022;73(1): 88–93. Croatian.
- Tygerberg Poison Information Centre Annual Report 2018. Cape Town (South Africa): Tygerberg Poison Information Centre.
- Sebben VC, Lessa CAS. Relatório Anual 2020 [2020 Annual Report]. Porto Alegre (Brazil): Atendimentos do Centro de Informação Toxicológica do Rio Grande do Sul – CIT/RS; 2022. Portugese
- Pan-Canadian Poison Centres 2020 Annual Report. Canada: Canadian Association of Poison Centres and Clinical Toxicology.
- Bekaert E, De Cock P, De Leener K, et al. Jaarrapport 2021 [Annual Report 2021]. Brussels (Belgium): Antigifcentrum – Centre Antipoisons; 2022. Dutch.
- Anhang zum EU Jahresbericht der VIZ 2014 [Annex to the EU Annual Report of the VIZ 2014]. Freiburg (Germany): Vergiftungs-Informations-Zentrale Freiburg; 2015. German.
- Jahresbericht 2021 [Annual Report 2021]. Göttingen (Germany): Giftinformationszentrum-Nord der Länder Bremen, Hamburg, Niedersachsen und Schleswig-Holstein (GIZ-Nord); 2022. German.
- Anhang zum Jahresbericht 2021 [Appendix to Annual Report 2021]. Zürich (Switzerland): Tox Info Suisse; 2022. German.
- Jahresbericht 2021 [Annual Report 2021]. Bonn (Germany): Informationszentrale gegen Vergiftungen des Landes Nordrhein-Westfalen. German.
- Boletim CIAV - Centro de Informação Antivenenos Dados Estatísticos 2021 [CIAV Bulletin – Antivenom Information Center Statistical Data 2021]. Lisbon (Portugal): CIAV - Centro de Informação Antivenenos. Portugese.
- Annual Report 2018 Victorian Poisons Information Centre. Heidelberg (Australia): Victorian Poisons Information Centre.
- Gummin DD, Mowry JB, Beuhler MC, et al. 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clin Toxicol (Phila). 2021;59(12):1282–1501. doi: 10.1080/15563650.2021.1989785
- Huynh A, Cairns R, Brown JA, et al. Patterns of poisoning exposure at different ages: the 2015 annual report of the Australian Poisons Information Centres. Med J Aust. 2018;209(2):74–79. doi: 10.5694/mja17.01063
- Pillans PI, Page CB, Ilango S, et al. Self-poisoning by older Australians: a cohort study. Med J Aust. 2017;206(4):164–169. doi: 10.5694/mja16.00484
- Magalhães AFA, Caldas ED. Two health information systems to characterize poisoning in Brazil-a descriptive study. J Public Health. 2019;41(1):203–211. doi: 10.1093/pubmed/fdy008
- Kaur J, McFaull SR, Bang F. Trends in emergency department visits for acetaminophen-related poisonings: 2011-2019. Health Promot Chronic Dis Prev Can. 2020;40(4):130–133. doi: 10.24095/hpcdp.40.4.05
- Klobučar I, Potočnjak I, Dumančić J, et al. Acute poisonings in Croatia: differences in epidemiology, associated comorbidities and final outcomes - a single-centre 15-year follow-up. Clin Toxicol (Phila). 2019;57(3):181–188. doi: 10.1080/15563650.2018.1506129
- Eyasu M, Dida T, Worku Y, et al. Acute poisonings during pregnancy and in other non-pregnant women in emergency departments of four government hospitals, Addis Ababa, Ethiopia: 2010-2015. Trop Med Int Health. 2017;22(10):1350–1360. doi: 10.1111/tmi.12940
- Salles J, Calonge J, Franchitto N, et al. Factors associated with hospitalization after self-poisoning in France: special focus on the impact of alcohol use disorder. BMC Psychiatry. 2018;18(1):287. doi: 10.1186/s12888-018-1854-0
- Nair SJ, Sujatha C, Chettiar KPS, et al. Toxico-epidemiology of acute poisoning; an exploratory study from a tertiary care hospital in South India along with global comparisons and solutions. J Forensic Leg Med. 2021;83:102247. doi: 10.1016/j.jflm.2021.102247
- Hitti E, El Zahran T, Hamade H, et al. Toxicological exposures reported to a telephonic consultation service at a tertiary care hospital in Lebanon. Clin Toxicol (Phila). 2020;58(9):886–892. doi: 10.1080/15563650.2019.1709643
- Thapa S, Dawadi BR, Upreti AR. Acute Poisoning among Patients Presenting to the Emergency Department of a Tertiary Care Center: a Descriptive Cross-sectional Study. JNMA J Nepal Med Assoc. 2020;58(227):470–473. doi: 10.31729/jnma.4997
- Kumpula EK, Shieffelbien LM, Pomerleau AC. Enquiries to the New Zealand National Poisons Centre in 2018. Emerg Med Australas. 2021;33(1):45–51. doi: 10.1111/1742-6723.13563
- Kumpula EK, Richards R, Norris P, et al. A retrospective analysis of calls to the New Zealand National Poisons Centre regarding Pacific patients. N Z Med J. 2021;134(1528):26–34.
- Khan M, Khurram M, Raza S. Gender based differences in patients of poisoning managed at a Medical Unit. J Pak Med Assoc. 2019;69(7):1025–1028.
- Garczewski B, Wiśniewski M, Waldman W, et al. ZATRUCIA PARACETAMOLEM LECZONE W POMORSKIM CENTRUM TOKSYKOLOGII W LATACH 2010–2015 [Acetaminophen poisonings hospitalized at the Pomeranian Center of Toxicology in 2010-2015]. Med Pr. 2019;70(6):733–738. Polish. doi: 10.13075/mp.5893.00885
- Scripcaru G, Mateus C, Nunes C. Adverse drug events-Analysis of a decade. A Portuguese case-study, from 2004 to 2013 using hospital database. PLoS ONE. 2017;12(6):e0178626. doi: 10.1371/journal.pone.0178626
- Lionte C, Sorodoc V, Jaba E, et al. Development and validation of a risk-prediction nomogram for in-hospital mortality in adults poisoned with drugs and nonpharmaceutical agents: an observational study. Medicine. 2017;96(12):e6404. doi: 10.1097/MD.0000000000006404
- Jung KY, Kim T, Hwang SY, et al. Availability of drug at convenient stores is not associated with an increased incidence of their poisoning. Pharmacoepidemiol Drug Saf. 2019;28(4):536–543. doi: 10.1002/pds.4760
- Supervía Caparrós A, Pallàs Villaronga O, Clemente Rodríguez C, et al. Características diferenciales de las intoxicaciones en los pacientes ancianos atendidos en un servicio de urgencias [Characteristics of emergency poisoning cases in elderly versus younger patients]. Emergencias. 2017;29(5):335–338. Spanish.
- Bowman C, Thornton S, Oller L, et al. Utilization of a poison control center by critical access hospitals-one state’s experience. Clin Toxicol (Phila). 2021;59(11):1015–1022. doi: 10.1080/15563650.2021.1903485
- Exposisiones Humanas A Produtos Farmaceuticos/Medicamentos. Datos de mayor incidencia de reportes a CITUC, durante el primer semestre del 2018 [Human Exposures to Pharmaceutical Products/Medications. Data with the highest incidence of reports to CITUC, during the first semester of 2018]. Centro de Información Toxicológica de la Pontificia Universidad Católica de Chile. http://cituc.uc.cl/infografias/. Spanish.
- Nugteren-van Lonkhuyzen JJ, van Velzen AG, Mulder-Spijkerboer HN, et al. Acute vergiftigingen bij mens en dier - NVIC Jaaroverzicht 2021. [Acute poisonings in humans and animals - NVIC Annual Review 2021]. Utrecht (Netherlands): Nationaal Vergiftigingen Informatie Centrum;2022. (NVIC Rapport 01/2022). Dutch.
- Swedish Poisons Information Centre Annual Report 2021. Solna (Sweden): Giftinformationscentralen (Swedish Poisons Information Centre).
- CIAV – Centro de Informação Antivenenos Dados Estatísticos 2021 [CIAV – Antivenom Information Center Statistical Data 2021]. Lisbon (Portugal): CIAV - Centro de Informação Antivenenos. Portugese.
- Queensland Poisons Information Centre Annual Report 2019. South Brisbane (Australia): Queensland Poisons Information Centre.
- Western Australian Poisons Information Centre Annual Report 2019. Perth (Australia): Western Australian Poisons Information Centre; 2020.
- 2021 Top 10 Agents (In-Patients + Telephone Referrals) [Internet]. Philippines: National Poison Management and Control Center; 2022 [cited 2023 Jan 5]. Available from: https://www.facebook.com/photo/?fbid=464531358795251&set=a.354201129828275
- Mainoli B, Gonçalves N, Ferreira JJ, et al. Potential drug-drug interactions in acute poisonings managed in the intensive care unit: occurrence, risk factors and relationship to patient severity on admission. Basic Clin Pharmacol Toxicol. 2022;130(2):337–345. doi: 10.1111/bcpt.13698
- Siedler S, Trageser HB, Grensemann J, et al. Akute Intoxikationen auf der Intensivstation: eine 10-Jahres-Analyse [Acute intoxications in the intensive care unit: a 10-year analysis]. Med Klin Intensivmed Notfmed. 2022;117(2):129–136. German. doi: 10.1007/s00063-021-00839-8
- Mehrpour O, Akbari A, Jahani F, et al. Epidemiological and clinical profiles of acute poisoning in patients admitted to the intensive care unit in eastern Iran (2010 to 2017). BMC Emerg Med. 2018;18(1):30. doi: 10.1186/s12873-018-0181-6
- Gondim APS, Nogueira RR, Lima JGB, et al. Suicide attempts by exposure to toxic agents registered in a Toxicological Information and Assistance Center in Fortaleza, Ceará, Brazil, 2013. Epidemiol Serv Saude. 2017;26(1):109–119. doi: 10.5123/S1679-49742017000100012
- Song SJ, Park GJ, Lee JH, et al. The Characteristics of Elderly Individuals Who Attempted Suicide by Poisoning: a Nationwide Cross-sectional Study in Korea. J Korean Med Sci. 2020;35(35):e286. doi: 10.3346/jkms.2020.35.e286
- Sein Anand Ł, Sein Anand J. Self-poisonings before and during the initial year of the COVID-19 pandemic in northern Poland. Int J Occup Med Environ Health. 2022;35(5):527–535. doi: 10.13075/ijomeh.1896.01838
- Knipe D, Silva T, Aroos A, et al. Hospital presentations for self-poisoning during COVID-19 in Sri Lanka: an interrupted time-series analysis. Lancet Psychiatry. 2021;8(10):892–900. doi: 10.1016/S2215-0366(21)00242-X
- Beauchamp GA, Fishbein J, Makar GA, et al. Sex differences in patients with suicidal intent that are managed by toxicologists: an analysis of the Toxicology Investigators’ Consortium (ToxIC) Registry. Am J Emerg Med. 2020;38(2):333–338. doi: 10.1016/j.ajem.2019.158450
- Canner JK, Giuliano K, Selvarajah S, et al. Emergency department visits for attempted suicide and self harm in the USA: 2006-2013. Epidemiol Psychiatr Sci. 2018;27(1):94–102. doi: 10.1017/S2045796016000871
- Kumpula EK, Lambie B, Quigley P, et al. Prescribers aware: a cross-sectional study from New Zealand emergency departments on the substances used in intentional self-poisoning and their sources. J Prim Health Care. 2020;12(3):235–243. doi: 10.1071/HC20017
- Laher AE, Motara F, Gihwala R, et al. The profile of patients presenting with intentional self-poisoning to the Charlotte Maxeke Johannesburg Academic Hospital emergency department, South Africa. S Afr Med J. 2022;112(5):347–351. doi: 10.7196/SAMJ.2022.v112i5.16366
- Kumpula EK, Nada-Raja S, Norris P, et al. A descriptive study of intentional self-poisoning from New Zealand national registry data. Aust N Z J Public Health. 2017;41(5):535–540. doi: 10.1111/1753-6405.12702
- Friðriksdóttir ÞA, Jónsdóttir F, Snook CP, et al. Paracetamol poisoning: a population-based study from Iceland. Scand J Gastroenterol. 2021;56(7):832–839. doi: 10.1080/00365521.2021.1921254
- Popiolek I, Hydzik P, Jagielski P, et al. Risk Factors for Hepatotoxicity Due to Paracetamol Overdose in Adults. Medicina (Kaunas). 2021;57(8):752. doi: 10.3390/medicina57080752
- Huang HS, Ho CH, Weng SF, et al. Long-term mortality of acetaminophen poisoning: a nationwide population-based cohort study with 10-year follow-up in Taiwan. Scand J Trauma Resusc Emerg Med. 2018;26(1):5. doi: 10.1186/s13049-017-0468-8
- Chung YT, Chou CY, Tsai WC, et al. Acetaminophen Poisoning May Increase Coronary Artery Disease Risk: a Nationwide Cohort Study. Cardiovasc Toxicol. 2018;18(4):386–391. doi: 10.1007/s12012-017-9442-y
- Mehrpour O, Saeedi F, Hoyte C. Decision tree outcome prediction of acute acetaminophen exposure in the United States: a study of 30,000 cases from the National Poison Data System. Basic Clin Pharmacol Toxicol. 2022;130(1):191–199. doi: 10.1111/bcpt.13674
- Nash E, Sabih AH, Chetwood J, et al. Drug-induced liver injury in Australia, 2009-2020: the increasing proportion of non-paracetamol cases linked with herbal and dietary supplements. Med J Aust. 2021;215(6):261–268. doi: 10.5694/mja2.51173
- Mallet M, Haq M, Tripon S, et al. Elevated procalcitonin is associated with bacterial infection during acute liver failure only when unrelated to acetaminophen intoxication. Eur J Gastroenterol Hepatol. 2017;29(7):811–816. doi: 10.1097/MEG.0000000000000862
- Louvet A, Ntandja Wandji LC, Lemaître E, et al. Acute Liver Injury with Therapeutic Doses of Acetaminophen: a Prospective Study. Hepatology. 2021;73(5):1945–1955. doi: 10.1002/hep.31678
- Moore JK, MacKinnon AC, Man TY, et al. Patients with the worst outcomes after paracetamol (acetaminophen)-induced liver failure have an early monocytopenia. Aliment Pharmacol Ther. 2017;45(3):443–454. doi: 10.1111/apt.13878
- Spivak I, Arora J, Meinzer C, et al. Low Serum Hepcidin is Associated with Reduced Short-Term Survival in Adults with Acute Liver Failure. Hepatology. 2019;69(5):2136–2149. doi: 10.1002/hep.30486
- Fontana RJ, Engle RE, Gottfried M, et al. Role of Hepatitis E Virus Infection in North American Patients with Severe Acute Liver Injury. Clin Transl Gastroenterol. 2020;11(11):e00273. doi: 10.14309/ctg.0000000000000273
- Koch DG, Speiser JL, Durkalski V, et al. The Natural History of Severe Acute Liver Injury. Am J Gastroenterol. 2017;112(9):1389–1396. doi: 10.1038/ajg.2017.98
- Stravitz RT, Fontana RJ, Meinzer C, et al. Coagulopathy, Bleeding Events, and Outcome According to Rotational Thromboelastometry in Patients with Acute Liver Injury/Failure. Hepatology. 2021;74(2):937–949. doi: 10.1002/hep.31767
- Santos G, Figueira ERR, D’Albuquerque LAC, et al. Evaluation of drug-induced liver injury as etiology for acute liver failure in Brazil. Ann Hepatol. 2021;23:100310. doi: 10.1016/j.aohep.2021.100310
- Liu Y, Zhan SP, Song L, et al. Drug-Induced Liver Injury: clinical and Etiologic Features at a Large Tertiary Teaching Hospital in China. Med Sci Monit. 2020;26:e919435. doi: 10.12659/MSM.919435
- Zhang C, Wu Y, Yuan S, et al. Characteristics of Drug-Induced Liver Injury in Northeast China: disease Spectrum and Drug Types. Dig Dis Sci. 2020;65(11):3360–3368. doi: 10.1007/s10620-019-06030-6
- Das S, Behera SK, Xavier AS, et al. Agreement Among Different Scales for Causality Assessment in Drug-Induced Liver Injury. Clin Drug Investig. 2018;38(3):211–218. doi: 10.1007/s40261-017-0601-5
- Rathi C, Pipaliya N, Patel R, et al. Drug Induced Liver Injury at a Tertiary Hospital in India: etiology, Clinical Features and Predictors of Mortality. Ann Hepatol. 2017;16(3):442–450. doi: 10.5604/01.3001.0009.8600
- Bourhia M, Ullah R, Alqahtani AS, et al. Evidence of drug-induced hepatotoxicity in the Maghrebian population. Drug Chem Toxicol. 2022;45(3):985–989. doi: 10.1080/01480545.2020.1797088
- Abid A, Subhani F, Kayani F, et al. Drug induced liver injury is associated with high mortality—A study from a tertiary care hospital in Pakistan. PLoS ONE. 2020;15(4):e0231398. doi: 10.1371/journal.pone.0231398
- Ortland I, Mirjalili M, Kullak-Ublick GA, et al. Drug-induced liver injury in Switzerland: an analysis of drug-related hepatic disorders in the WHO pharmacovigilance database VigiBase™ from 2010 to 2020. Swiss Med Wkly. 2021;151(1920):w20503. doi: 10.4414/smw.2021.20503
- Yeboah-Korang A, Louissaint J, Tsung I, et al. Utility of a Computerized ICD-10 Algorithm to Identify Idiosyncratic Drug-Induced Liver Injury Cases in the Electronic Medical Record. Drug Saf. 2020;43(4):371–377. doi: 10.1007/s40264-019-00903-5
- Yeboah-Korang A, Memon A, Patel N, et al. Impact of Prior Drug Allergies on the Risk, Clinical Features, and Outcomes of Idiosyncratic Drug-Induced Liver Injury in Adults. Dig Dis Sci. 2022;67(11):5262–5271. doi: 10.1007/s10620-022-07403-0
- Louissaint J, Kassab I, Yeboah-Korang A, et al. Combining K-72 Hepatic Failure with 15 Individual T-Codes to Identify Patients with Idiosyncratic Drug-Induced Liver Injury in the Electronic Medical Record. Dig Dis Sci. 2022;67(8):4243–4249. doi: 10.1007/s10620-021-07223-8
- Oliveira JdFMd, Wagner GA, Romano-Lieber NS, et al. Medicine poisoning mortality trend by gender and age group, São Paulo State, Brazil, 1996-2012. Cien Saude Colet. 2017;22(10):3381–3391. doi: 10.1590/1413-812320172210.12782017
- Xiao L, Ye Y, Wang Y, et al. A 9-year retrospective study of poisoning-related deaths in Southwest China (Sichuan). Forensic Sci Int. 2021;318:110558. doi: 10.1016/j.forsciint.2020.110558
- Koskela L, Raatiniemi L, Bakke HK, et al. Do pre-hospital poisoning deaths differ from in-hospital deaths? A retrospective analysis. Scand J Trauma Resusc Emerg Med. 2017;25(1):48. doi: 10.1186/s13049-017-0391-z
- Gunnarsdottir OS, Rafnsson V. Accidental poisoning, intentional self-harm and event of undetermined intent mortality over 20 years in Iceland: a population-based cohort study. BMJ Open. 2020;10(5):e034590. doi: 10.1136/bmjopen-2019-034590
- Lynn E, Cousins G, Lyons S, et al. Comparing characteristics of suicide to non-suicide drug poisoning deaths, by sex, in Ireland. J Affect Disord. 2022;306:80–89. doi: 10.1016/j.jad.2022.03.030
- Magalhães AFA, Caldas ED. Underreporting of fatal poisonings in Brazil - a descriptive study using data from four information systems. Forensic Sci Int. 2018;287:136–141. doi: 10.1016/j.forsciint.2018.03.040
- Koskela L, Raatiniemi L, Bakke HK, et al. Fatal poisonings in Northern Finland: causes, incidence, and rural-urban differences. Scand J Trauma Resusc Emerg Med. 2017;25(1):90. doi: 10.1186/s13049-017-0431-8
- González-Santiago O, Morales-San Claudio PC, Cantú-Cárdenas LG, et al. Unintentional and self-poisoning mortalities in Mexico, 2000–2012. PLoS ONE. 2017;12(7):e0181708. doi: 10.1371/journal.pone.0181708
- Gharbaoui M, Ben Khelil M, Harzallah H, et al. Pattern of suicide by self-poisoning in Northern Tunisia: an eleven-year study (2005-2015). J Forensic Leg Med. 2019;61:1–4. doi: 10.1016/j.jflm.2018.10.004
- Schmidt LE, Rasmussen DN, Petersen TS, et al. Fewer adverse effects associated with a modified two-bag intravenous acetylcysteine protocol compared to traditional three-bag regimen in paracetamol overdose. Clin Toxicol (Phila). 2018;56(11):1128–1134. doi: 10.1080/15563650.2018.1475672
- Wong A, Isbister G, McNulty R, et al. Efficacy of a two bag acetylcysteine regimen to treat paracetamol overdose (2NAC study). EClinicalMedicine. 2020;20:100288. doi: 10.1016/j.eclinm.2020.100288
- Shoib S, Patel V, Khan S, et al. Over-the-counter drug use in suicidal/self-harm behavior: scoping review. Health Sci Rep. 2022;5(3):e662. doi: 10.1002/hsr2.662
- Wazaify M, Kennedy S, Hughes CM, et al. Prevalence of over-the-counter drug-related overdoses at Accident and Emergency departments in Northern Ireland – a retrospective evaluation. J Clin Pharm Ther. 2005;30(1):39–44. doi: 10.1111/j.1365-2710.2004.00607.x
- Morthorst BR, Erlangsen A, Nordentoft M, et al. Availability of Paracetamol Sold Over the Counter in Europe: a Descriptive Cross‐Sectional International Survey of Pack Size Restriction. Basic Clin Pharmacol Toxicol. 2018;122(6):643–649. doi: 10.1111/bcpt.12959
- Gedeborg R, Svennblad B, Holm L, et al. Increased availability of paracetamol in Sweden and incidence of paracetamol poisoning: using laboratory data to increase validity of a population-based registry study. Pharmacoepidemiol Drug Saf. 2017;26(5):518–527. doi: 10.1002/pds.4166
- Hopkins AG, Spiller HA, Kistamgari S, et al. Suicide-related over-the-counter analgesic exposures reported to United States poison control centers, 2000-2018. Pharmacoepidemiol Drug Saf. 2020;29(9):1011–1021. doi: 10.1002/pds.4997
- Medicines Classification Database [Internet]. MEDSAFE: New Zealand medicines and medical devices safety authority; [Updated 2023 Jan 27; cited 2022 Dec 28]. Available from: https://www.medsafe.govt.nz/profs/class/classintro.asp.
- Poisons Standard October 2022. Canberra. Standard No. 37. Australian Capital Territory (Australia): Department of Health and Aged Care; 2022.
- Modified-release paracetamol-containing products to be suspended from EU market [Internet]. London (UK): European Medicines Agency; 2018 [cited 2023 Jan 19]. Available from: https://www.ema.europa.eu/en/medicines/human/referrals/paracetamol-modified-release
- 1.4. Final decision in relation to paracetamol (modified release) [Internet]. Canberra (Australia): Department of Health and Aged Care (Therapeutic Goods Administration); 2019 [updated 2019 Aug 26; cited 2022 Aug 31]. Available from. https://www.tga.gov.au/resources/publication/scheduling-decisions-final/notice-final-decision-amend-or-not-amend-current-poisons-standard-august-2019/14-final-decision-relation-paracetamol-modified-release
- Morgan O, Majeed A. Restricting paracetamol in the United Kingdom to reduce poisoning: a systematic review. J Public Health. 2005;27(1):12–18. doi: 10.1093/pubmed/fdh200
- Turvill JL, Burroughs AK, Moore KP. Change in occurrence of paracetamol overdose in UK after introduction of blister packs. Lancet. 2000;355(9220):2048–2049. doi: 10.1016/S0140-6736(00)02355-2
- Hawton K, Simkin S, Deeks J, et al. UK legislation on analgesic packs: before and after study of long term effect on poisonings. BMJ. 2004;329(7474):1076. doi: 10.1136/bmj.38253.572581.7C
- Morgan O, Griffiths C, Majeed A. Impact of paracetamol pack size restrictions on poisoning from paracetamol in England and Wales: an observational study. J Public Health. 2005;27(1):19–24. doi: 10.1093/pubmed/fdh216
- Morgan OW, Griffiths C, Majeed A, et al. Interrupted time-series analysis of regulations to reduce paracetamol (acetaminophen) poisoning. PLOS Med. 2007;4(4):e105. doi: 10.1371/journal.pmed.0040105
- Bateman DN, Gorman DR, Bain M, et al. Legislation restricting paracetamol sales and patterns of self-harm and death from paracetamol-containing preparations in Scotland. Br J Clin Pharmacol. 2006;62(5):573–581. doi: 10.1111/j.1365-2125.2006.02668.x
- Hawton K, Bergen H, Simkin S, et al. Long term effect of reduced pack sizes of paracetamol on poisoning deaths and liver transplant activity in England and Wales: interrupted time series analyses. BMJ. 2013;346():f403. doi: 10.1136/bmj.f403.
- Greene SL, Dargan PI, Leman P, et al. Paracetamol availability and recent changes in paracetamol poisoning: is the 1998 legislation limiting availability of paracetamol being followed? Postgrad Med J. 2006;82(970):520–523. doi: 10.1136/pgmj.2005.042036
- Molloy P, Chambers R, Cork T. How well are national guidelines relating to the general sales of aspirin and paracetamol, adhered to by retail stores: a mystery shopper study. BMJ Open. 2016;6(1):e010081. doi: 10.1136/bmjopen-2015-010081
- Buckley NA, Gunnell D. Does restricting pack size of paracetamol (acetaminophen) reduce suicides? PLOS Med. 2007;4(4):e152. doi: 10.1371/journal.pmed.0040152
- Morthorst BR, Erlangsen A, Chaine M, et al. Restriction of non-opioid analgesics sold over-the-counter in Denmark: a national study of impact on poisonings. J Affect Disord. 2020;268:61–68. doi: 10.1016/j.jad.2020.02.043
- Steeg S, Carr MJ, Mok PLH, et al. Temporal trends in incidence of hospital-treated self-harm among adolescents in Denmark: national register-based study. Soc Psychiatry Psychiatr Epidemiol. 2020;55(4):415–421. doi: 10.1007/s00127-019-01794-8
- Goldberger D, Vearrier D. Evaluation of a food and drug administration mandate to limit acetaminophen in prescription combination products. J Med Toxicol. 2017;13(4):303–308. doi: 10.1007/s13181-017-0622-8
- Antoniou T, Guan Q, Martins D, et al. Impact of acetaminophen product labelling changes in Canada on hospital admissions for accidental acetaminophen overdose: a population-based study. CMAJ. 2022;194(15):E542–E548. doi: 10.1503/cmaj.210842
- Cairns R, Noghrehchi F, Buckley NA. The impact on poisonings of up-scheduling of modified release paracetamol to Schedule 3 (pharmacist only medicine).Med J Aust. 2023;218(9):412–414. doi: https://doi.org/10.5694/mja2.51888
- de Silva V, Ratnayake A. Increased use of medicinal drugs in self-harm in urban areas in Sri Lanka. Arch Suicide Res. 2008;12(4):366–369. doi: 10.1080/13811110802325265