1. Introduction
Antiretroviral treatments have greatly improved over the years from complex regimens with a high pill burden, multiple daily dosing, and considerable toxicities to highly effective daily single-pill regimens with good tolerability. Another outstanding milestone has been reached with the approval of the first long-acting (LA) intramuscular treatment combining cabotegravir and rilpivirine. After an optional oral lead-in phase, followed by an intramuscular loading dose (cabotegravir/rilpivirine 600/900 mg), cabotegravir/rilpivirine is administered intramuscularly at a maintenance dose of 400/600 mg every 4 weeks or 600/900 mg every 8 weeks [Citation1–3]. Thus, by eliminating the need for daily administration, LA injectable antiretrovirals may improve adherence. Other advantages include the possibility of treating people with swallowing difficulties and the prevention of drug–drug interactions (DDIs) occurring at the gastrointestinal level. The slow release of cabotegravir/rilpivirine from the muscle combined with the avoidance of the first-pass metabolism will have an impact on the magnitude of DDIs. This editorial provides insight into the intramuscular administration of cabotegravir/rilpivirine, presents available DDI data, and discusses how to interpret and manage DDIs after intramuscular administration.
2. Intramuscular administration of cabotegravir and rilpivirine
Cabotegravir and rilpivirine are injected as solid nanoparticles suspended in a liquid vehicle [Citation1,Citation1,Citation3] thus, upon administration, the drug formulation creates a depot in the muscle from which the antiretroviral drugs are slowly released thereby resulting in sustained plasma concentrations. The muscle is characterized by a rich vascular supply, which favors the drug release from the depot, and therefore, the injection technique is key to ensure that the antiretroviral drugs reach the muscle. Of interest, the inadvertent injection of cabotegravir in the subcutaneous adipose tissue, where blood flow and drug release from the depot are reduced, was shown to result in lower initial concentrations compared to intramuscular injection [Citation4]. Thus, a longer needle is advised when administering cabotegravir/rilpivirine to individuals with a BMI of >30 kg/m2 in order to reach the muscle through the thicker adipose layer. Another factor that can impact the release of the drug from the depot and contribute to pharmacokinetic variations includes high physical activity, which can increase the blood flow in the muscle and consequently enhance the drug release from the depot [Citation5]. Of interest, a real-world study from the Swiss HIV Cohort study reported an individual with repetitive low cabotegravir and rilpivirine concentrations after intramuscular administration. This individual was athletic and was injecting anabolic steroids [Citation6].
For evaluating the risk of a DDI with LA drugs, it is necessary to consider that the absorption (i.e. drug release from the depot) occurs in the muscle and not in the gastrointestinal tract. This allows to prevent DDIs occurring in the gastrointestinal tract due to changes in gastric pH (i.e. rilpivirine requires a low pH for optimal absorption), chelation (i.e. cabotegravir forms a complex with divalent cations, thereby impairing its absorption), or inhibition/induction of intestinal drug metabolizing enzymes (i.e. rilpivirine is metabolized by CYP3A4 and cabotegravir mainly by UGT1A1 and therefore are subject to DDIs at the intestinal level) [Citation5]. However, escaping the first-pass metabolism does not necessarily mitigate the DDI magnitude if the drug is minimally metabolized in the gut. This is notably the case for cabotegravir whose exposure (i.e. area under the curve (AUC)) in the presence of the strong inducer rifampicin was predicted to be reduced by 61% and 64% after intramuscular and oral administration, respectively [Citation7]. Conversely, rilpivirine has a high first-pass metabolism so that the DDI with rifampicin is mitigated with the intramuscular compared to oral administration (rilpivirine AUC reduced by 38% and 74%) [Citation7].
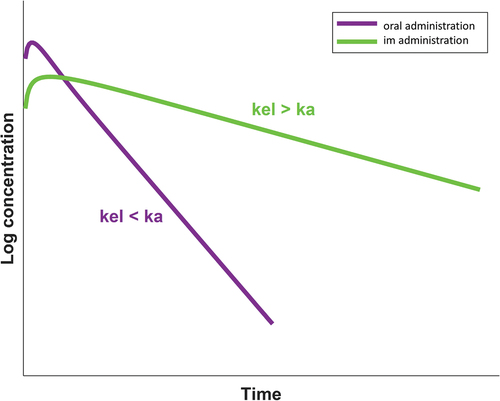
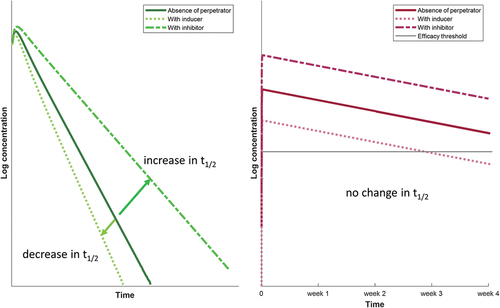
Another consideration to take into account when interpreting the clinical relevance of DDIs with the depot injection is the flip-flop pharmacokinetics whereby the rate of absorption (ka) is slower than the rate of elimination (kel). This implies that the elimination half-life (t1/2) of the drug is driven by ka in the case of intramuscular administration, whereas it is driven by kel in the case of oral administration. This difference explains the longer elimination t1/2 of the intramuscular compared to the oral formulation (i.e. cabotegravir t1/2: 5.6–11.5 weeks for intramuscular versus 41 hours for oral; rilpivirine t1/2: 13–28 weeks versus 45 hours) () [Citation5]. Importantly, since ka is not impacted by inhibitors or inducers of drug metabolism and since ka governs t1/2 in the case of intramuscular administration, the net effect of a DDI will be a parallel shift up (inhibitor) or down (inducer) of intramuscular cabotegravir or rilpivirine exposure, whereas the elimination rate remains unchanged. However, inhibitors and inducers will increase and decrease t1/2 of oral cabotegravir or rilpivirine since kel governs t1/2 in the case of oral administration (). Thus, since the pharmacokinetic profile after intramuscular administration is ‘flatter’ (as opposed to oral administration), even a modest decrease in the AUC can be clinically relevant as drug concentrations can reach the efficacy threshold days or weeks before the next dosing as illustrated in . For cabotegravir, the current efficacy target threshold corresponds to the fourfold protein-adjusted concentration required for 90% viral inhibition (4× PA-IC90, i.e. 664 ng/mL). This threshold has been associated with high treatment efficacy in phase 3 trials and with high protective efficacy in vaginal and rectal simian HIV challenge models [Citation8]. For rilpivirine, the minimal concentration associated with therapeutic response is 50 ng/mL [Citation9]. Not achieving the necessary therapeutic levels can put people at risk of treatment failure or acquisition of HIV infection when LA cabotegravir is used for PrEP.
3. DDIs and strategies to overcome DDIs after intramuscular administration
As mentioned earlier, cabotegravir and rilpivirine undergo UGT1A1- and CYP3A4-mediated metabolism and therefore can be impacted by drugs inhibiting or inducing (perpetrator) these enzymes. On the other hand, cabotegravir and rilpivirine have a low potential to cause DDIs as these agents do not inhibit or induce drug metabolizing enzymes or drug transporters to a clinically significant extent (except OAT1 and OAT3 for cabotegravir so caution is needed when coadministering with sensitive substrates such as methotrexate) [Citation5].
It should be noted that clinical DDI data are only available for oral cabotegravir and rilpivirine as, given the long dosing interval, the conduct of DDI studies with LA formulation is challenging. However, this limitation can be overcome by using physiologically based pharmacokinetic (PBPK) modeling, an approach recognized by the regulatory authorities, that allows to simulate unstudied DDI scenarios by combining in vitro and clinical observed data [Citation10]. PBPK modeling also presents the advantage of capturing the population variability and thereby ensures that the simulated DDI magnitude and the related dosing adjustment can apply to most individuals. Using PBPK modeling, strong inhibitors of UGT1A1 or UGT1A9 were predicted to cause a minimal increase (approximately 10%) in LA cabotegravir AUC, an effect that is not clinically relevant [Citation11]. No studies have evaluated the effect of strong CYP3A4 inhibitors on LA rilpivirine. However, considering the high first-pass metabolism of rilpivirine, the DDI magnitude with a strong CYP3A4 inhibitor is expected to be lower with the intramuscular formulation. Thus, strong inhibitors can be used with intramuscular rilpivirine without dose adjustment given that oral rilpivirine at the standard dose was shown to be well tolerated in the presence of strong inhibitors [Citation12].
Conversely, strong inducers cause clinically relevant DDIs as rifampicin reduced oral cabotegravir and rilpivirine AUC by 59% [Citation13] and 80% [Citation14], respectively. The moderate inducer rifabutin decreased oral cabotegravir AUC by 23%, an effect that is not considered to be clinically relevant [Citation15]. However, the exposure of oral rilpivirine was reduced by 42%, and therefore, the coadministration with a moderate inducer requires to double the oral rilpivirine dose (i.e. 50 mg once daily) [Citation16]. Using PBPK modeling, rifampicin was predicted to reduce LA cabotegravir and rilpivirine AUC (at steady-state) by 61% and 38%, respectively [Citation7]. An increase in the cabotegravir/rilpivirine dosing frequency (i.e. every 4 or 3 weeks instead of the recommended 8 weeks in the absence of inducer) did not overcome the DDI with rifampicin as a large proportion of individuals were predicted to have concentrations below the efficacy threshold during the dosing interval. Therefore, the coadministration of rifampicin (or other strong inducers) should be avoided with LA cabotegravir and rilpivirine. The moderate inducer rifabutin was predicted to decrease LA cabotegravir and rilpivirine AUC (at steady-state) by 16% and 18%, respectively [Citation7]. Although the decrease is modest, the DDI with rifabutin is clinically relevant as a large proportion of individuals are predicted to have concentrations below the efficacy threshold during the dosing interval after the first loading dose (cabotegravir, rilpivirine) and after the maintenance dose (rilpivirine) (). However, the DDI with rifabutin (or other moderate inducers) can be overcome by administering LA cabotegravir/rilpivirine monthly together with a daily oral rilpivirine dose of 25 mg (). While a dose adjustment is needed during the coadministration with a moderate inducer, switching from an efavirenz (moderate inducer) containing regimen to LA cabotegravir/rilpivirine is possible without dose adjustment as the residual induction is predicted to minimally impact cabotegravir/rilpivirine AUC [Citation17].
Table 1. Proportion of individuals with cabotegravir and rilpivirine concentrations above the efficacy threshold during the intramuscular administration dosing interval for various simulated DDI scenarios. Reprinted from reference [Citation7], with permission from Oxford University Press License.
4. Expert opinion
The intramuscular administration of LA cabotegravir/rilpivirine allows to avoid DDIs with rilpivirine due to gastric pH changes and with cabotegravir due to chelation with divalent cations. However, bypassing the gastrointestinal tract does not eliminate DDIs with strong and moderate inducers, even though the magnitude of the DDI is mitigated for rilpivirine. When interpreting the clinical significance of DDIs with LA cabotegravir/rilpivirine, it is important to consider not only the decrease in drug exposure but also the proportion of individuals likely to have concentrations above the efficacy threshold during the dosing interval. Monthly dosing together with a 25 mg daily oral dose of rilpivirine was predicted to overcome the interaction with moderate but not with strong inducers. Of interest, splitting cabotegravir 400 mg intramuscular injection into 2 × 200 mg intramuscular injections was shown to increase AUC0-week4 by twofold, likely explained by the increased absorption due to the greater surface area [Citation18]. Thus, considering that LA cabotegravir alone is used for prevention, it could be of interest to evaluate whether splitting cabotegravir dose in two injections together with a monthly dosing could overcome the DDI with strong inducers. Another potential question of interest relates to the impact of the injection site on the magnitude of DDIs as alternative sites of administration are being considered. Obesity was found to be associated with lower cabotegravir concentrations, thus the magnitude of DDIs with moderate inducers will need to be evaluated in individuals with a high BMI. Lastly, more data are needed during pregnancy as the related physiological changes can impact both the pharmacokinetics and pharmacodynamics of LA cabotegravir and rilpivirine. Relevant physiological changes include the expansion of the plasma volume, which leads to a larger volume of distribution. The drug distribution is also impacted by the progressive decrease in albumin level and the gradual increase in fat during pregnancy. Importantly, pregnancy is characterized by an increase in drug metabolism due to the induction of drug metabolizing enzymes by progesterone and estrogen. Finally, the glomerular filtration rate is increased during pregnancy [Citation19]. Data on the pharmacokinetics of LA cabotegravir and rilpivirine during pregnancy are available only for a small number of women with HIV who became pregnant while participating in phase 2b/3/3b clinical trials and who were required to discontinue LA cabotegravir/rilpivirine and use an alternative antiretroviral regimen [Citation20]. Quarterly pharmacokinetic sampling, performed after the last injected dose, showed that cabotegravir and rilpivirine washout concentrations during pregnancy were within the range of those in non-pregnant women [Citation20]. Nevertheless, prospective studies are warranted to evaluate the pharmacokinetics of LA cabotegravir and rilpivirine in women who continue or initiate this treatment during pregnancy. Furthermore, data are needed to characterize the placental transfer as well as the secretion of LA cabotegravir and rilpivirine into the breastmilk.
Declaration of interest
C. Marzolini has received speaker honoraria from ViiV, Merck Sharp & Dohme, and Pfizer unrelated to this work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers of this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- US. Food and drug administration. Cabenuva product label. 2023 Mar. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/212888s005s006lbl.pdf
- European Medicines Agency. Rekambys summary of product characteristics. 2023 Mar. Available from: https://www.ema.europa.eu/en/documents/product-information/rekambys-epar-product-information_en.pdf
- European Medicines Agency. Vocabria summary of product characteristics. 2023 Mar. Available from: https://www.ema.europa.eu/en/documents/product-information/vocabria-epar-product-information_en.pdf
- Jucker BM, Fuchs EJ, Lee S, et al. Multiparametric magnetic resonance imaging to characterize cabotegravir long-acting formulation depot kinetics in healthy adult volunteers. Br J Clin Pharmacol. 2021;88(4):1655–1666. doi: 10.1111/bcp.14977
- Hodge D, Back DJ, Gibbons S, et al. Pharmacokinetics and drug-drug interactions of long-acting intramuscular cabotegravir and rilpivirine. Clin Pharmacokinet. 2021;60(7):835–853. doi: 10.1007/s40262-021-01005-1
- Thoueille P, Alves Saldanha S, Schaller F, et al. Real-life therapeutic concentration monitoring of long-acting cabotegravir and rilpivirine: preliminary results of an ongoing prospective observational study in Switzerland. Pharmaceutics. 2022;14(8):1588. doi: 10.3390/pharmaceutics14081588
- Bettonte S, Berton M, Stader F, et al. Management of drug-drug interactions between long-acting cabotegravir and rilpivirine and comedications with inducing properties: a modelling study. Clin Infect Dis. 2022;76(7):1225–1236. [epub ahead print]. doi: 10.1093/cid/ciac901
- Landovitz RJ, Li S, Eron JJ, et al. Tail-phase safety, tolerability, and pharmacokinetics of long-acting injectable in HIV-uninfected adults: a secondary analysis of the HPTN 077 trial. Lancet HIV. 2020;7(7):e472–481. doi: 10.1016/S2352-3018(20)30106-5
- Aouri M, Barcelo C, Guidi M, et al. Population pharmacokinetics and pharmacogenetics analysis of rilpivirine in HIV-1-infected individuals. Antimicrob Agents Chemother. 2017;61(1):e00899–16. doi: 10.1128/AAC.00899-16
- Wagner C, Pan Y, Hsu V, et al. Predicting the effect of CYP3A inducers on the pharmacokinetics of substrate drugs using physiologically based pharmacokinetic (PBPK) modeling: an analysis of PBPK submissions to the US FDA. Clin Pharmacokinet. 2016;55(4):475–483. doi: 10.1007/s40262-015-0330-y
- Taskar K, Patel P, Cozens S, et al. Utilization of physiologically based pharmacokinetic modelling (PBPK) to predict the effect of UGT enzyme inhibition and induction on the systemic exposure of cabotegravir, International Workshop on Clinical Pharmacology of HIV, hepatitis, and other antiviral drugs. Noordwijk 14-16 May 2019, abstract 18.
- Liverpool HIV Drug Interactions website. 2023 Mar. Available from: https://www.hiv-druginteractions.org
- Ford SL, Sutton K, Lou Y, et al. Effect of rifampin on the single-dose pharmacokinetics of oral cabotegravir in healthy subjects. Antimicrob Agents Chemother. 2017;61(10):e00487–e517. doi: 10.1128/AAC.00487-17
- Van Heeswijk R, Hoetelmans R, Kestens D, et al. The effects of CYP3A4 modulation on the pharmacokinetics of TMC278, an investigational non-nucleoside reverse transcriptase inhibitor. 7th International Workshop on Clinical Pharmacology of HIV Therapy, Lisbon 20-22 Apr, 2006, abstract 74.
- Ford SL, Lou Y, Lewis N, et al. Effect of rifabutin on the pharmacokinetics of oral cabotegravir in healthy subjects. Antivir Ther. 2019;24(4):301–308. doi: 10.3851/IMP3306
- Crauwels H, van Heeswijk R, Kestens D, et al. The pharmacokinetic interaction between rifabutin and TMC278, a next generation non-nucleoside reverse transcriptase inhibitor. 17th International AIDS conference, Mexico 3-8 Aug 2008, abstract TUPE0080.
- Bettonte S, Berton M, Stader F, et al. Intramuscular cabotegravir/rilpivirine concentrations after switching from efavirenz. 30th Conference on Retroviruses and Opportunistic Infections, Seattle 19-22 Feb 2023, abstract 508.
- Spreen W, Ford SL, Chen S, et al. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr. 2014;67(5):481–486. doi: 10.1097/QAI.0000000000000301
- Bettonte S, Berton M, Marzolini C. Magnitude of drug-drug interactions in special populations. Pharmaceutics. 2022;14(4):789. doi: 10.3390/pharmaceutics14040789
- Patel P, Ford SL, Baker M, et al. Pregnancy outcomes and pharmacokinetics in pregnant women living with HIV exposed to long-acting cabotegravir and rilpivirine in clinical trials. HIV Med. 2023;24(5):568–579. doi: 10.1111/hiv.13439