ABSTRACT
Introduction
Every year thousands of children undergo surgery for congenital heart disease. Cardiac surgery requires the use of cardiopulmonary bypass, which can have unexpected consequences for pharmacokinetic parameters.
Areas covered
We describe the pathophysiological properties of cardiopulmonary bypass that may influence pharmacokinetic parameters, with a focus on literature published in the last 10 years. We performed a PubMed database search with the keywords ‘Cardiopulmonary bypass’ AND ‘Pediatric’ AND ‘Pharmacokinetics’. We searched related articles on PubMed and checked the references of articles for relevant studies.
Expert opinion
Interest in the influence of cardiopulmonary bypass on pharmacokinetics has increased over the last 10 years, especially due to the use of population pharmacokinetic modeling. Unfortunately, study design usually limits the amount of information that can be obtained with sufficient power and the best way to model cardiopulmonary bypass is yet unknown. More information is needed on the pathophysiology of pediatric heart disease and cardiopulmonary bypass. Once adequately validated, PK models should be integrated in the patient electronic database integrating covariates and biomarkers influencing PK, making it possible to predict real-time drug concentrations and guide further clinical management for the individual patient at the bedside.
1. Introduction
With an incidence of 17.9 per 1000 neonates worldwide, congenital heart disease is the most common birth defect [Citation1]. Due to improvements in diagnostic imaging, catheter-based treatments and cardiac surgery survival has increased significantly [Citation1]. Current mortality rates of surgery are 3%, including all ages and defects [Citation2]. Thousands of children undergo cardiac surgery annually, either for correction or palliation of their congenital heart defect.
A plethora of drugs are administered during and after cardiac surgery. For anesthesia, sedatives, opiates, and neuromuscular relaxants are used. Antibiotics are used for the prevention of surgical wound infections, anti-inflammatory drugs for the amelioration of inflammatory responses associated with surgery, antifibrinolytic drugs for prevention of excessive blood loss, as well as drugs to support cardiovascular function.
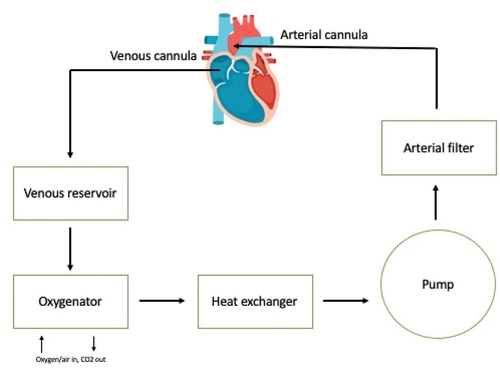
In most cardiac surgery procedures, cardiopulmonary bypass (CPB) is used to facilitate surgery (). To provide a motionless and blood-free operating field for the surgeon, the heart must be arrested during surgery, while at the same time perfusion and gas exchange for the rest of the body must be maintained. The patient’s blood is passively drained to the CPB system through a cannula placed in the right atrium or the vena cava of the patient. The system’s tubing transports the blood to the venous reservoir, from which it is subsequently pumped to the oxygenator by ways of a centrifugal or roller pump. The oxygenator removes carbon dioxide and adds oxygen to the blood. After this, an arterial filter filters the blood to eliminate any embolic materials. A heater cooler device is used to regulate blood temperature. Blood from the CPB system is returned to the patient via an arterial cannula placed in the aorta. The heart and lungs are thus bypassed. The CPB system is primed with a priming fluid consisting of various fluids, such as red blood cell concentrates, fresh frozen plasma, albumin or colloid and crystalloid fluids. The exact composition depends on patient age and preoperative hemoglobin values. This priming fluid dilutes the patient’s blood at initiation of CPB. The CPB system is composed of plastics such as polyvinyl chloride (PVC), polycarbonate, polymethylpentene (PMP), polyurethane, and silicone which are coated with biocompatible materials such as heparin, phosphorylcholine polymers, or poly(2-methoxyacrylate) to decrease activation of the inflammatory and coagulation system.
In 2013, our research group published an extensive review regarding the influence of CPB on the pharmacokinetics (PK) of drugs commonly used during cardiac surgery, with a focus on the pediatric population [Citation3]. Since then, a number of studies have been performed attempting to further delineate this influence (), which are reviewed here-in.
Table 1. Summary of current evidence for the mechanisms behind the influence of cardiopulmonary bypass on pharmacokinetic parameters.
2. Pathophysiology of CPB and the influence of CPB covariates on PK parameters
2.1. Adsorption of drugs to plastic components of the CPB system
It has long been known that there is an interaction between drugs and biological molecules with container materials [Citation4,Citation5]. This interaction also exists with the plastic components of the CPB system. Physicochemical characteristics of the drug and characteristics of the plastic are the primary determinants of the amount of absorption of drug to plastic components [Citation4,Citation5]. Currently, there is still inadequate knowledge regarding the characteristics of drugs and plastic which are important in determining the interaction between drugs and CPB system components. In vitro drug adsorption in extracorporeal membrane oxygenation (ECMO) systems has been shown to correlate with logP, a measure for lipophilicity of a drug [Citation6,Citation7]. In in vitro studies performed in CPB systems used in our own center, we were not able to show this correlation [Citation8]. Our study did suggest adsorption to be positively correlated with plasma protein binding, which is in line with previous findings in ECMO systems [Citation9,Citation10], and with pKa [Citation8]. This latter factor has not been described to influence adsorption of drugs in in vitro CPB systems before. The negative charging of surface-coated CPB systems makes electrostatic attraction of positively charged molecules a possible mechanism for adsorption of drugs to CPB system components. Because drugs with a high pKa are unlikely to be dissociated at normal pH, electrostatic attraction is not expected to be a factor of specific interest.
It must be kept in mind that, due to the costs involved, in vitro studies are generally performed with a limited number of drugs, making correlations between adsorption and drug characteristics preliminary at best. A large, carefully designed study of numerous drugs with a sufficient range in values of physicochemical characteristics would be necessary, but is unlikely to be performed.
Apart from drug characteristics, adsorption of drugs to CPB system components may also be dependent on the drug concentration in the CPB system. It has been shown that the percentage adsorption of, for instance, dexmedetomidine is lower at therapeutic concentrations compared to supra-therapeutic concentrations [Citation11], while for propofol, relative adsorption was lower at higher concentrations, without indication of saturation of the system even at very high concentrations [Citation12]. It is currently not known what causes the differences between these two drugs with similar logP and no further studies have been performed for other drugs.
Additionally, the presence of concomitant medication may further impact drug adsorption to the CPB system, possibly resulting from a complex interplay between competition for plasma protein binding and for binding to the CPB system components. In an in vitro study of CPB systems, there was 0% adsorption of midazolam in systems with and systems without an oxygenator, but adsorption of midazolam was higher in the presence of fentanyl if an oxygenator was present in the system [Citation13]. In the presence of morphine, midazolam adsorption was also much higher, with no difference between systems with and without an oxygenator. On the contrary, adsorption of fentanyl decreased when it was combined with midazolam, but only when an oxygenator was present in the system [Citation13]. Since a large number of drugs are used in combination during pediatric cardiac surgery, it is imperative that interactions between drugs are further studied.
The impact of various CPB system components in relation to in vitro adsorption of drugs has also been investigated. With regard to the tubing used in CPB systems, an increased adsorption of drugs to silicone tubing compared to PVC tubing has been reported [Citation6,Citation14]. Tubing is often coated to improve biocompatibility with blood and different types of coating may impact drug adsorption differently [Citation12,Citation15–17].
In earlier days, the largest amount of adsorption was attributed to the oxygenator, due to the presence of polyurethane defoaming sponges in bubble oxygenators [Citation18] and later due to silicone membranes in membrane oxygenators [Citation18,Citation19]. Newer oxygenators with hollow fiber PMP and polypropylene microporous membranes appear to be less important in drug adsorption, as shown in more recent in vitro studies. In current ECMO systems, no significant difference has been shown for morphine adsorption between uncoated PVC tubing systems with and without an oxygenator [Citation20]. Likewise, only 5% of fentanyl is lost to modern oxygenators, compared to 80% adsorption to uncoated PVC tubing [Citation20]. A recent in vitro study of midazolam in CPB systems showed 0% adsorption in systems with uncoated tubing, whether a hollow fiber polypropylene membrane oxygenator was present or not [Citation13]. A study investigating which key components affect dexmedetomidine adsorption in in vitro ECMO systems confirmed that PMP hollow fibers or polypropylene microporous fibers for gas exchange were not involved in drug loss in the oxygenator [Citation17]. The loss was attributed to polyurethane fibers or stainless steel used for heat exchange [Citation17]. The presence of an integrated arterial filter in the oxygenator may be a factor of importance. Forty-five percent adsorption of fentanyl was shown in uncoated tubing systems without an oxygenator present, vs 74% adsorption in the presence of an oxygenator [Citation13]. A previous study, in contrast, showed that just 5% of adsorption of fentanyl was to be attributed to the oxygenator [Citation20]. The major difference between the oxygenators used in both studies [Citation13,Citation20], was the presence of an integrated arterial filter (pore size 32 μm) in the first study [Citation13]. This suggests the arterial filter may have been the cause for increased adsorption of fentanyl, rather than the oxygenator itself. Further methodological differences between the studies preclude a definitive conclusion, however.
Larger studies of drugs with an array of physicochemical properties, CPB system components with varying properties and interactions between drugs may be able to further elucidate the exact interaction between drugs and CPB system components. This may enable us to make better predictions regarding future drugs and components and making future studies of individual components and drugs unnecessary. Also, it is yet unknown whether binding of drugs to components of the CPB system is reversible, and if so, whether this is a clinically relevant factor to take into account. Another factor to take into account is the difference between blood binding and plasma binding of drugs [Citation6]. The initial concentration of drugs in the CPB system is often calculated based on the amount of drug added to the system divided by the priming volume of the system. Since the priming fluid in pediatric systems often contains red blood cells, this initial calculated concentration is by definition a blood concentration. Subsequent concentrations of drugs are usually measured in plasma. The blood plasma ratio of drug concentrations thus has to be taken into account when comparing the calculated initial concentration of a drug with subsequent measured concentrations in plasma. Another method to calculate adsorption of drugs to CPB system components is comparison of subsequent drug concentrations with an initial concentration measured after the first circulation of the priming fluid through the CPB system. Since both initial and subsequent concentrations are measured in plasma, blood plasma ratio does not have to be taken into account. With this methodology, however, one can speculate how much time is necessary for adequate mixing of drug and priming fluid, and thus when the initial concentrations should be measured relatively to the timing of addition of drugs to the CPB system. Also, very fast binding of drug during first pass of the priming fluid through the CPB system may be missed with this methodology. As we have shown in our in vitro study, the difference between the maximum calculated initial concentration and the plasma concentration measured in plasma after 1 minute of circulation of the priming fluid is often large [Citation8].
2.2. Retention of drug in cell saver blood
The large volume of priming fluid in the CPB system relative to a child’s circulating blood volume may lead to unacceptable hemodilution of hemoglobin. Most cardiac surgeries in children thus require the use of allogeneic donor blood. An increased incidence of pulmonary complications, prolonged duration of mechanical ventilation, increased incidence of hospital acquired infections, and a prolonged length of hospital stay have been associated with blood product transfusion in pediatric cardiac surgery [Citation21]. To reduce the amount of red blood cell concentrates used during pediatric cardiac surgery, cell savers have been used [Citation22]. Blood is suctioned from the operating field during surgery, and residual blood from the CPB system is collected. After washing, an end product with a hematocrit of 60% is obtained. This is subsequently returned to the patient, usually in the early postoperative phase. In our institution, we investigated the concentrations of drugs present in cell saver blood after processing with an Electa cell saver system [Citation23]. We measured plasma concentrations of midazolam, propofol, sufentanil, and cefazolin in the reservoir of the cell saver system after initial collection. These concentrations were comparable to in vivo concentrations in our patients. After processing of the blood present in the reservoir by the cell saver, there were significantly decreased concentrations for all drugs. Propofol had disappeared completely after washing, even before passage through a lipophilic filter. After passage through the lipophilic filter, concentrations of midazolam had decreased to a median of 6% (IQR 4–10%) and concentrations of cefazolin to a median of 5% (IQR 2–6) compared to concentrations in the reservoir. For sufentanil, a median of 34% (IQR 27–50%) of drug was retained in the end product ready for transfusion to the patient. We were surprised to find both lipid soluble and protein bound drugs in the end product after washing and passage through a lipophilic filter, since proteins and lipids should be completely removed from the end product so that it only contains red blood cells and saline, according to the manufacturer. The differences in retention of sufentanil vs propofol and midazolam, both with comparable lipophilicity and protein binding, remain unexplained. We unfortunately could not confirm the importance of lipophilicity and protein binding in retention of drug in the end product during the cell saving process. Cefazolin, a hydrophilic drug, was almost completely washed from the reservoir blood during the cell saving process. The clinical consequences of the retention of sufentanil in the cell saver blood end product are unknown. We expect them to be larger in small children when a relatively large amount of cell saver blood is available and is administered as a bolus.
No further studies have been reported, to our knowledge. Given the possible clinical consequences of drugs retained in cell saver blood, comprehensive research of more drugs used in clinical practice is needed. The influence of drug properties and cell saver component properties on retention of drugs should be investigated, so in the future it may be possible to predict these effects without the need for studies of individual drugs.
2.3. Hemodilution
The CPB system is filled with prime fluid prior to attachment to the patient. The prime fluid is devoid of medication, and when CPB is initiated, mixing of the prime fluid with the patient’s blood leads to hemodilution. The amount of hemodilution depends on the amount of prime fluid relative to the patient’s circulating volume and is especially large in small children, amounting up to 100% of their circulating volume. Due to the differences in pediatric CPB setups and the large differences in body size in this population, variation in the percentage of volume being added to the circulation is also large within the pediatric population.
Hemodilution would lead to an apparent increase in volume of distribution (V) on initiation of CPB. It has been suggested that the degree of increase of V is larger for drugs with a small V, and that for drugs with a larger V the change in V may be mitigated by redistribution of drug from the peripheral to the central compartment. The influence of hemodilution may be confounded by adsorption of drugs to the plastic components of the CPB system and inflammation causing redistribution of fluids to the tissues. No studies have been performed to establish a correlation between the decrease in blood drug concentration on initiation of CPB and known information about drug adsorption to plastics.
Hemodilution has been shown to occur in multiple studies in pediatric patients. A decrease in the unbound concentration of cefazolin on initiation of CPB was found in a PK study of 56 children aged 1 day to 15 years, leading to an increased calculated Vd on initiation of CPB [Citation24]. To account for this, a separate CPB compartment was modeled in this population PK study, with V for this compartment estimated to be equal to prime volume, suggesting no adsorption of drug to the plastic components of the CPB system [Citation24]. In contrast, a second study of cefazolin PK measuring total cefazolin concentration found no increase in central V on initiation of CPB in children aged 0.75–8 months weighing 3–7.5 kg [Citation25]. The authors attribute this difference with the first study to the single prime volume used for all children.
In a study of tranexamic acid PK including 55 children aged 2 days to 4 years and 10 months old CPB prime volume did not enhance a population PK model [Citation26]. V at steady state was, however, significantly increased during CPB compared to before CPB, with increases in especially the central but also the peripheral V [Citation26]. In a second study of tranexamic acid PK including 43 children aged 6–348 days the lack of a direct enhancement of a population PK model by CPB prime volume was confirmed [Citation27]. An effect of CPB as an on/off variable on central V could also not be demonstrated, but peripheral V was decreased. For other water-soluble drugs such as vancomycin [Citation25], cefoxitin [Citation28], and cefuroxime [Citation29], no effect of CPB prime volumes on distribution volumes has been reported.
In a study of methylprednisolone total concentrations in pediatric cardiac surgery our study group has found a significant 26.8% (median, range 13.9–48.1%) decrease in concentration on initiation of the first run of CPB in children aged 0.2–117 months [Citation30]. The median time difference between these concentration measurements was 13 minutes, thus excluding metabolism as a cause. We found no correlation of the percentage decrease in total methylprednisolone concentrations on initiation of CPB with the absolute amount of prime fluid, prime fluid per kg bodyweight or prime fluid as a percentage of patient circulating volume [Citation30]. This might suggest drug adhesion to the plastics of the CPB components, rather than hemodilution. A PK study of methylprednisolone in 64 children aged 3–30 days showed a five-fold greater V at steady state during CPB compared to previously published values in children [Citation31]. V at steady state may not be a very good parameter to model the effects of hemodilution, since for lipophilic drugs it is expected to consist especially of peripheral distribution. Also, the children in the comparison study were children with inflammatory bowel disease with a mean age of 11.3 years, likely to have a lower Vd due to differences in body composition [Citation31].
The evidence regarding the influence of prime volume is supported by multiple drug simulations and clinical studies for cefazolin [Citation24,Citation32,Citation33], epsilon aminocaproic acid [Citation34] and TXA [Citation27], all drugs that are not expected to significantly adhere to the plastic components of the CPB system. Addition of drug to the prime fluid or administration of an extra drug dose before initiation of CPB attenuates the influence of hemodilution in these studies. The attainment of simulated and obtained drug concentrations in the therapeutic range was facilitated. Unfortunately, the clinical studies were of an unrandomized, observational nature and none of the drug simulations have been validated in clinical practice. To reach the same drug concentration in the prime fluid across the population, the prime dose should be based on prime volume rather than patient weight [Citation33].
It thus appears difficult to model hemodilution as a covariate. Adequately modeling this effect would require sampling before and after the initiation of CPB, spaced in such a way that metabolism can be taken out of the equation. Even then, the decrease in concentration on initiation of CPB may be caused by adhesion of drug to the plastics of the CPB system components, rather than hemodilution. A specific study investigating the differences between compounds that have been shown to have high rates of adsorption to plastics vs. compounds with low rates of adsorption to plastics may shed further light on this subject.
Interpretation of the differences between studies is complicated by the inadequate description of study procedures such as the duration of time between administration of a drug and the start of CPB and volume and composition of priming fluids.
2.4. Protein binding
Apart from the volume of the CPB prime fluid, the composition of said fluid is also an important consideration. In adult patients, the CPB prime fluid consists mostly of protein-free fluids, leading to decreases in concentrations of total protein, albumin, and alpha-1-acid-glycoprotein [Citation35–37]. This leads to decreased protein binding of drugs, with an increase in free fraction. This phenomenon has been shown for multiple drugs in adult patients, such as alfentanil [Citation35], sufentanil [Citation38], midazolam [Citation39] and propofol [Citation36,Citation37,Citation39]. Unbound concentrations are thus less markedly decreased or even increased compared to total concentrations on initiation of CPB [Citation35,Citation38,Citation39]. Obviously, the influence of decreased protein binding is higher for highly protein bound drugs.
Heparin, used for anticoagulation during CPB, may also decrease protein binding. This has been shown for alpha-1-acid-glycoprotein bound alfentanil, where unbound fraction increased significantly from 0.08 to 0.16 after a loading dose of 200 U/kg of heparin was administered [Citation35]. A similar, albeit not statistically significant effect has been shown for albumin bound propofol [Citation37]. This phenomenon has been attributed to activation of lipases by heparin, causing an increase in circulating concentrations of free fatty acids and displacement of drugs from protein [Citation35,Citation37]. Subsequent neutralization of heparin with protamine after CPB may reverse this process [Citation37].
At discontinuation of CPB total protein concentrations, albumin concentrations and concentrations of alpha-1-acid glycoprotein generally increase but remain lower than preoperative values in adult patients, likely due to the redistribution of part of the CPB prime fluid to the tissues [Citation35,Citation37]. The influence on protein binding of drugs is thus partially reversed during the post-CPB period.
Unfortunately, extensive studies have not been performed in children. The influence of the CPB prime on protein binding in children may be very different from adults, since the prime fluid has a different composition in children. In small children, fresh frozen plasma and albumin are often added to the prime fluid. The older the child, the more the composition of the prime fluid mimics that of adults. A significant decrease in protein binding of cefazolin by 12% has been reported on initiation of CPB in a PK study of children during cardiac surgery, accompanied by a 25% decrease in albumin concentrations [Citation24]. Unfortunately, the composition of the prime fluid was not reported and likely varied significantly, since children aged 0.013–15 years old were included in the study.
In a study regarding the tissue distribution of cefazolin during CPB with and without deep hypothermic circulatory arrest (DHCA) in children, a significant decrease in protein binding was demonstrated in blood samples obtained during DHCA vs. samples obtained at other timepoints, suggesting an influence of temperature management on protein binding during CPB [Citation40].
Theoretically, protein binding may have effects on PK parameters due to the increase in unbound concentrations. We have not been able to find studies directly investigating this influence in children.
2.5. Hypothermia
All active processes in the body are mediated by proteins (enzymes, transporter proteins). These proteins display optimal function at normothermia, and their efficiency decreases with decreasing temperatures. The most comprehensive studies on the influence of hypothermia on PK parameters in children have been performed in children undergoing therapeutic hypothermia after perinatal asphyxia and/or hypoxic-ischemic encephalopathy showing a positive effect of whole body cooling on neurodevelopmental outcome [Citation41,Citation42]. These patients are generally cooled to temperatures of 33.5°C for 12–72 hours within 6 hours after birth. In a pooled population PK analysis of seven drugs (morphine, midazolam, lidocaine, phenobarbital, amoxicillin, gentamicin, and benzylpenicillin) and five metabolites (morphine-3-glucuronide, morphine-6-glucuronide, 1-hydroxymidazolam, hydroxy-midazolam glucuronide, and mono-ethylglycylxylidide), the PharmaCool study group was able to distinguish the influence of hypothermia from the influence of ischemic organ dysfunction caused by the original insult, an important confounding factor for clearance (Cl) in this patient group [Citation43]. Their study group consisted of 192 neonates undergoing therapeutic hypothermia after hypoxic-ischemic encephalopathy on 12 NICUs in the Netherlands and Belgium, with samples obtained on days 2–5 after birth. After capturing the effects of bodyweight, maturation of organ function and recovery of organ function represented by postnatal age on Cl, temperature (modeled as a continuous variable set at 33.5°C during the hypothermic phase, 36.5°C during the normothermic phase and rewarming at a rate of 0.4°C/hour) was only a significant covariate for intermediate Cl compounds, decreasing Cl by 6.83%/°C (95% confidence interval 5.16–8.34), likely because the influence of hypothermia on Cl in high Cl compounds was confounded by the large influence of organ function recovery on Cl.
Changes in physiology such as cardiac output, heart rate, and blood flow during hypothermia, which are especially important for renally cleared drugs and drugs with high hepatic Cl, may also be an important confounder for decreases in Cl during hypothermia, but to our knowledge no studies have been performed to explore the relationship between the two [Citation41,Citation44]. The influence of hypothermia on metabolism of drugs may extend into the posttreatment period after rewarming, especially for drugs with a long half-life [Citation41].
Transport of drugs across natural barriers often needs to take place for drugs to exert their effect and may be influenced by hypothermia. In vitro, the influence of hypothermia on the transporter protein P-glycoprotein has been established, in contrast to passive processes [Citation41]. To our knowledge, no further in vivo research has been performed.
Hypothermia is commonly used for organ protection during CPB in pediatric cardiac surgery. Depending on the surgical procedure, temperature management can range from normothermia to deep hypothermic circulatory arrest (DHCA) at 18°C. Unfortunately, studies as comprehensive as the PharmaCool study have not been performed for hypothermia during cardiac surgery procedures in pediatric patients. Studies regarding the influence of hypothermia on PK in these patients are likely to be more complicated, since hypothermia is applied for a much shorter duration during CPB, a maximum of several hours, and over a larger range of temperatures. It is as of yet unknown whether this shorter duration of hypothermia also leads to clinically relevant changes in PK parameters. There are many PK studies for many different drugs showing that temperature is not an influencing factor for the PK of both renally and hepatically cleared drugs during CPB [Citation24,Citation26,Citation28,Citation29,Citation34,Citation45,Citation46]. In these studies, time on CPB ranged from 22 to 400 minutes and minimum temperature from 17.4 to 34.2°C, often with large ranges for both values in the same study. Temperatures as low as 17°C could not be confirmed as a factor influencing Cl of TXA [Citation26] and epsilon aminocaproic acid [Citation34] in PK modeling efforts. One PK study of dexmedetomidine has shown an influence of temperature on central V during CPB, with an increase in V for temperatures lower than 37°C [Citation46]. To our knowledge, no head-to-head comparison of the influence of normothermic versus hypothermic CPB on the PK of drugs has been performed in children undergoing cardiac surgery with CPB. Rewarming from hypothermia during CPB is a much faster process, performed at a maximum rate of 1°C per 10 minutes in neonates undergoing CPB as compared to 0.3–0.5°C per hour for neonates rewarming after therapeutic hypothermia for hypoxic-ischemic encephalopathy. It is difficult to separate the effects of hypothermia during CPB from changes in blood flow, blood pressure, and ischemic changes. CPB pump flow is generally decreased during hypothermic periods, causing difficulty in ruling out the influence of decreased hepatic and renal blood flow.
2.6. Blood flow and organ perfusion
The influence of hemodynamic parameters on PK parameters has been extensively described in a review dating back to 1975 [Citation47]. Given this influence, it is not surprising that changes in systemic and regional blood flow associated with CPB have been implicated as factors influencing PK in children with congenital heart disease. During CPB, blood flow is controlled by CPB pump flow settings, which may vary during cardiac surgery due to surgical needs and decreases in blood flow are often employed during hypothermia. Also, during CPB, blood flow is usually continuous, compared to the pulsatile blood flow of normal cardiac output, the relevance of which has been debated for years [Citation48].
CPB has been known to cause redistribution of blood flow. Organ blood flow during CPB may depend on several factors including temperature, perfusion pressure, blood flow rate, pulsatile vs non-pulsatile blood flow, hematocrit, and pH-management.
Liver blood flow during CPB has been measured with indocyanine green clearance and auricular densitometry in a study of 36 children with congenital heart disease with a mean age of 3.4 years [Citation49]. CPB was performed with a blood flow of 2.4 l/min/m2 at 37°C. Blood flow was reduced to 1.2–1.6 l/min/m2 at temperatures of 25–30°C. Hematocrit was maintained at 25–30%. The disappearance rate of indocyanine green was approximately 67% lower during CPB, with no correlation with either temperature or CPB flow rate. To our knowledge, no further studies in children have been published, which is likely caused by the difficulty measuring hepatic blood flow during cardiac surgery in a minimally invasive and practical way. Portal vein flow can be measured with transesophageal echocardiography [Citation50], but arterial supply to the liver is highly variable and venous blood is returned to the caval vein by multiple venous branches.
Compared to liver blood supply, renal blood supply is generally simpler, with a single artery and vein for each kidney. As with liver blood flow, animal studies and studies in adult patients have been performed. There is a paucity of information regarding renal blood flow in children with congenital heart disease and children undergoing CPB. A method for interrogating left renal blood flow with TEE in children with a success rate of 96% and excellent inter- and intra-observer reproducibility has been described 10 years ago [Citation51]. In this proof-of-concept study of 24 children volumetric renal blood flow was directly correlated with CPB pump flow rate and linearly correlated with mean arterial pressure [Citation51].
It is unknown whether changes in organ blood flow during CPB can cause clinically relevant changes in PK parameters. In a PK study of hepatically cleared dexmedetomidine of 18 patients with a median age of 3.3 months (range 0.1–34 months) for a variety of congenital cardiac surgeries a decrease in Cl was found during CPB [Citation45]. The addition of flow rate as a covariate did not decrease interindividual variability in Cl values. Flow rates of <0.135 L/min/kg were compared with flow rate >0.135 L/min/kg with nine patients in each group, with two patients undergoing regional low-flow CPB and one patient undergoing DHCA. For renally cleared drugs, CPB flow rate was not a significant covariate in PK modeling efforts for drugs such as tranexamic acid [Citation26,Citation27,Citation52], epsilon aminocaproic acid [Citation34], cefazolin [Citation24,Citation25], vancomycin [Citation25], cefoxitin [Citation28], and cefuroxime [Citation29].
Changes in central and peripheral blood flow may also cause changes in distribution parameters. In a study of cefazolin PK in 14 children with single ventricle physiology with a median age of 141 days undergoing PCPC with CPB cefazolin concentrations were measured in blood and tissue of the deltoid muscle [Citation40]. Seven children also underwent DHCA. There were significant delays in peak tissue concentration of cefazolin in children undergoing DHCA vs. children undergoing CPB without DHCA, even though cefazolin exposure was higher in the DHCA group due to decreased Cl calculated with non-compartmental PK analysis [Citation40]. This study was unable to determine whether this delay in peripheral distribution of cefazolin was caused by circulatory arrest (and thus differences in CPB pump flow rate) or hypothermia.
To our knowledge, no further studies have been reported, especially no head-to-head comparisons of high and low CPB flow rate. Also, the isolated effect of flow rate may be difficult to investigate, given the fact that organ blood flow is determined by many other physiological changes that take place during CPB and are often difficult to control for, such as temperature and blood pressure.
2.7. Inflammation and capillary leak
CPB and cardiac surgery are associated with an intense inflammatory reaction. Younger and smaller patients are more susceptible to the effects of CPB on the inflammatory reaction. The CPB system is large compared to the patient’s circulating volume and the higher metabolic demand of small children requires a higher pump flow rate. Both these factors lead to increased contact of blood with the components of the CPB system and increased shear forces exerted on blood components [Citation53,Citation54]. Young children’s immune systems are immature, relying more on the innate immune system with a still developing adaptive immune system [Citation54]. Older children show a more prolonged but less severe inflammatory response than neonates in response to tissue injury [Citation55,Citation56].
Capillary leak associated with the inflammatory reaction leads to fluid shifts with loss of fluid and protein from the central circulation to the tissues. This is accompanied by a need for fluid resuscitation, diluting any drugs present in the circulation. Capillary leak is expected to cause diffusion of water and water-soluble drugs from the central circulation to peripheral tissue. Inflammation also causes changes in organ blood flow due to vasodilation and hypovolemia and has also been shown to decrease hepatic expression and/or activity of CYP450 mediated drug PK and the function of drug transporting receptor complexes through inflammatory cytokines [Citation57]. In a study of critically ill children admitted to the ICU, C-reactive protein as a measure of the severity of inflammation at values >300 mg/L was associated with a 65% decrease in Cl of midazolam [Citation58]. To our knowledge, no studies have confirmed the effect of the inflammatory response on PK parameters during CPB. Also, the individual influence of inflammation and capillary leak will be difficult to ascertain, due to inherent entanglement with other CPB associated covariates influencing PK.
2.8. Conventional and modified ultrafiltration
Conventional, zero-balance and modified ultrafiltration are often performed during pediatric CPB to regulate fluid balance and to influence the inflammatory response. Several PK studies have not shown an influence of the use of conventional [Citation29,Citation34,Citation46] or modified [Citation26,Citation27,Citation46] ultrafiltration on PK parameters, whether modeled as a continuous or dichotomous variable, for water-soluble or fat-soluble drugs. This may be because drug concentrations in ultrafiltrate are similar to plasma concentrations, as has been shown for epsilon-aminocaproic acid [Citation34].
2.9. Duration of CPB
One would expect that the longer the duration of CPB, the easier the influence of CPB can be shown in PK studies with sparse blood sampling. Also, since longer CPB duration is often associated with lower temperatures, decreased flow rates, increased inflammation, and use of ultrafiltration, the influence of these parameters would be expected to be increased with longer time on CPB. However, conflicting results have been reported in the pediatric population [Citation28,Citation31,Citation45].
3. Discussion
There has been an increased interest in the influence of CPB on PK in pediatric cardiac surgery in the past years, likely due to the increased use of population PK analysis. Population PK analyses are based on simultaneous analysis of all data from the entire population, increasing statistical power, while still taking into account that observations are obtained in individual patients, which are thus correlated [Citation59]. Population PK analysis is especially useful in the pediatric population, since it allows for the analysis of limited numbers of observations per individual and for the analysis of data that are unequally distributed across the concentration–time profile. This is often the case in pediatric pharmacological research, since restrictions on the number of blood samples that can be drawn for research purposes are in place in this population. Population PK is also able to disentangle inter-individual variability and residual, unexplained variability. This allows for the quantification of differences between population acquired typical PK parameter values and individual PK parameter values. This facilitates a covariate analysis, aiming to identify correlations between individual PK parameter values and patient- or treatment characteristics [Citation59].
Unfortunately, the population PK approach has not yet led to an increased knowledge of the causes behind changes in PK parameters during CPB. An important limiting factor decreasing the amount of information that can be obtained from concentration time profiles is study design. It is important to adjust sampling schedules to be able to determine the influence of CPB on PK parameters. For example, the decrease in drug concentration on initiation of CPB may be missed by not sampling shortly before and after the start of CPB. Collinearity between covariates may also be problematic, especially in studies with insufficient power [Citation28]. An adequate number of patients in each covariate category must be ensured, as well as a sufficient range of covariate values. Time periods, such as the time from initiation of surgery to initiation of CPB, time on CPB, and time from weaning of CPB to transfer to the ICU, are also generally short, which may complicate calculation of PK parameters.
Since study methodology has to be adequate for the research question, there is not one best way to model the effects of CPB covariates on PK parameters. It is as of yet unknown whether there is an influence of the way CPB is incorporated in pharmacological models. Currently, CPB is generally included as an on/off covariate, but alternatively it may be modeled by covariates individually describing processes such as temperature, prime volume, or flow rate. Models that have already been published are not uniformly generalizable to different institutions and patient populations outside of the population used to construct the model, due to differences in parameterization and covariate relationships [Citation60]. Usually, models are based on single-center studies with internal validation at the most. There are only sparse studies describing external validation, especially in populations outside of the research center where the model was developed.
Complicating matters further, there is a lack of fundamental knowledge on the many components which affect PK parameters in pediatric cardiac surgery. The influence of congenital cardiac disease on the physiology of other organs than the heart and the differences between disease with decreased systemic circulation or decreased pulmonary circulation is emerging [Citation61–63]. Also, evidence regarding the in vitro interaction between drugs with the CPB system materials has not been fully determined. There is also a lack of information regarding the influence of CPB on patient physiology in pediatric cardiac surgery, likely due to limitations in minimally and noninvasive measurement methods easily accessible at the bedside.
4. Expert opinion
The ultimate goal in all our efforts is to individualize drug administration, taking into account covariates that influence PK in the individual patient, together with maximum therapeutic effect with minimum toxicity. This may be of particular advantage to patients who are likely to deviate far from the population PK model or for patients with varying PK parameters over time, such as patients undergoing cardiac surgery, frail elderly patients and children [Citation64,Citation65]. In pediatric cardiac surgery, this patient group consists mostly of neonates with complex cardiac disease such as hypoplastic left heart syndrome, transposition of the great arteries with or without pulmonary hypertension or complex tetralogy of Fallot. These children are often admitted to the ICU prior to surgery and are thus everything but drug naïve, having received benzodiazepines and opiates for sedation in the ICU to facilitate mechanical ventilation. Longer periods of sedation, pain treatment, antibiotic use, and use of a myriad of other drugs can be anticipated in these children postoperatively in the ICU. Combined with the greater adverse effects of cardiac disease and surgery on metabolizing and eliminating organ function in this population, a much greater impact on drug metabolism may be expected in this vulnerable population. This may, for instance, cause an increased risk of drug toxicity and prolonged recovery from sedative drugs.
CPB has been shown to have many potential covariates that may influence PK parameters during cardiac surgery. In the past 10 years, there has been an increase in the number of studies regarding the influence of CPB on PK. Unfortunately, study design usually limits the amount of information that can be obtained with sufficient power and the best way to model cardiopulmonary bypass is yet unknown, causing conflicting results between studies. PK models are generally not uniformly generalizable, and there is a lack of studies conducted for external validation. Cooperation on a national and international level is expected to increase the speed with which information can be obtained. Cooperation with clinical pharmacology departments and experts in population PK modeling may lead to improvements in study design. Cooperation will also be needed with a myriad of other departments, to close important knowledge gaps regarding the physiology of congenital cardiac disease and CPB and its influence on PK parameters. Also, including a sufficient number of patients in individual studies will likely require national or even international cooperation between centers. As has been shown in a PBPK study of milrinone, data sharing between industry and academia is feasible and beneficial [Citation66]. Combining datasets already obtained in similar patient populations may lead to increased knowledge and the prevention of superfluent research with limited yield, maximizing the gain from data already available and decreasing patient risk [Citation66]. Existing datasets may also be used for much needed external validation of PK models.
Another area for improvement is the actual lack of knowledge regarding the influence of congenital heart disease and CPB on patient physiology, further limiting the conclusions that can be drawn regarding the pathophysiology of changes in PK parameters. Newly developed methods for minimally or noninvasive measurements of organ perfusion may improve knowledge critical for model development. This also presents a limitation when these data are not readily available.
For the future, we expect a large role of PBPK modeling in the understanding of CPB induced changes in PK parameters. Systems biology approaches such as physiology-based PK modeling may serve as a unifying theory of pharmacology and physiology. The premise is that a system of connected and interacting biological components as a whole is greater than the sum of its parts and has properties that cannot be discovered by investigation of the separate components in isolation. The goal is to assemble all the known components of the biologic system and to quantify the dynamic interactions among individual elements within a system. In pharmacology, these systems describe all components from distribution to the effect site, to formation of drug-receptor complexes, to post-receptor processes involved in the achievement of a PD parameter [Citation67]. This methodology represents an extension of standard PK/PD models, which study a single transduction pathway as the basis of drug action [Citation67]. The feasibility of systems biology to adequately describe PK and PD has been shown for milrinone in the prevention and treatment of low cardiac output syndrome (LCOS) in pediatric cardiac surgery [Citation66]. With the systems approach for milrinone, drug models in healthy volunteers were merged with a disease model reflecting physiological changes in adult patients during cardiac surgery with CPB [Citation66]. This model was then be adjusted for pediatric patients by incorporating growth- and maturation-dependent changes in physiology, pathophysiology, and PK [Citation66]. It was shown that current prescription is suboptimal with regard to achieving plasma concentrations within the therapeutic target range and optimized dosing strategies were developed for both pediatric and adult patients for the prevention and the treatment of LCOS after cardiac surgery [Citation66]. All in all, the PBPK model for milrinone complemented existing knowledge established by population PK models and improved this knowledge with regard to the influence of organ growth and maturation and pathophysiological factors on PK parameters [Citation66].
A large advantage of this methodology is that it allows the inclusion of pathophysiological parameter changes. The pathophysiological interactions between growth and maturation, pediatric cardiac disease, surgery, and CPB are likely too complex to be adequately described by population PK methods alone. Also, PBPK modeling is more flexible than conventional population PK modeling, allowing integration of new data when they emerge and use for multiple compounds. It must be stated that PBPK models are still population based, precluding their use for dose individualization. However, it is possible to use Bayesian forecasting and individual patient data to replace population-based parameter estimations [Citation66]. Once adequately validated, models should be integrated in the patient electronic database integrating covariates and biomarkers influencing PK, making it possible to predict real-time drug concentrations and guide further clinical management for the individual patient at the bedside [Citation65,Citation68]. This may lead to more effective treatment, fewer side effects, and more economic use of drugs and ICU resources.
The development of a PBPK model in this field will be no easy task, however. A recent publication has shown the complexities of developing a PBPK framework for the influence of hypothermia on PK parameters [Citation42]. Initiating a PBPK modeling effort may lead to great gains, as this approach allows for easier identification of knowledge gaps, and may thus streamline research efforts for the years to come.
Article highlights
Cardiopulmonary bypass can have unexpected consequences for pharmacokinetic parameters due to absorption of drugs to plastic system components, hemodilution, changes in protein binding, hypothermia, changes in organ and peripheral blood flow, inflammation and capillary leak and the use of ultrafiltration
Over the past 10 years, population pharmacologic modeling has been used to determine the influence of cardiopulmonary bypass on pharmacokinetic parameters with varying degrees of success due to limitations in study design and a lack of knowledge on the influence of the underlying congenital heart disease and CPB on physiology.
Pharmacokinetic models are generally not uniformly generalizable, and there is a lack of studies conducted for external validation.
Physiology-based pharmacokinetic modeling may enable us in the prediction of real-time drug concentrations and guide further clinical management for the individual patient at the bedside.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or mending, or royalties.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they are an employee and stockholder of Certara. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Additional information
Funding
References
- Scott M, Neal AE. Congenital heart disease. Prim Care. 2021 Sep;48(3):351–366. doi: 10.1016/j.pop.2021.04.005
- Pettitt TW. Quality improvement in congenital heart surgery. NeoReviews. 2020 Mar;21(3):e179–e192. doi: 10.1542/neo.21-3-e179
- van Saet A, de Wildt SN, Knibbe CA, et al. The effect of adult and pediatric cardiopulmonary bypass on pharmacokinetic and pharmacodynamic parameters. Curr Clin Pharmacol. 2013 Nov;8(4):297–318.
- Jenke D, Couch T, Gillum A, et al. Modeling of the solution interaction properties of plastic materials used in pharmaceutical product container systems. PDA J Pharm Sci Technol. 2009 Jul;63(4):294–306.
- Wang M, Li Y, Srinivasan P, et al. Interactions between biological products and product packaging and potential approaches to overcome them. AAPS Pharm Sci Tech. 2018 Nov;19(8):3681–3686.
- Wildschut ED, Ahsman MJ, Allegaert K, et al. Determinants of drug absorption in different ECMO circuits. Intensive care Med. 2010 Dec;36(12):2109–2116.
- Raffaeli G, Allegaert K, Koch B, et al. In vitro adsorption of analgosedative drugs in new extracorporeal membrane oxygenation circuits. Pediatr Crit Care Med. 2018 May;19(5):e251–e258.
- van Saet A, Zeilmaker-Roest GA, van Hoeven MPJ, et al. In vitro recovery of sufentanil, midazolam, propofol, and methylprednisolone in pediatric cardiopulmonary bypass systems. J Cardiothorac Vasc Anesth. 2020 Apr;34(4):972–980.
- Shekar K, Roberts JA, Barnett AG, et al. Can physicochemical properties of antimicrobials be used to predict their pharmacokinetics during extracorporeal membrane oxygenation? Illustrative data from ovine models. Crit Care. 2015 Dec 15;19(1):437. doi:10.1186/s13054-015-1151-y
- Shekar K, Roberts JA, McDonald CI, et al. Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: results from an ex vivo study. Crit Care. 2015 Apr 14;19(1):164. doi:10.1186/s13054-015-0891-z
- Wilder NS, Andropoulos DB, Paugh T, et al. Sequestration of dexmedetomidine in ex vivo cardiopulmonary bypass circuits. Asaio J. 2021 Aug 3;68:592–598. doi: 10.1097/MAT.0000000000001536
- Hammaren E, Rosenberg PH, Hynynen M. Coating of extracorporeal circuit with heparin does not prevent sequestration of propofol in vitro. Br J Anaesth. 1999 Jan;82(1):38–40. doi: 10.1093/bja/82.1.38
- Kuntz MT, Pereira LM, Matte GS, et al. Sequestration of midazolam, fentanyl, and morphine by an ex vivo cardiopulmonary bypass circuit. Asaio J. 2021 Jun 24;67(12):1342–1348. doi:10.1097/MAT.0000000000001506
- Koren G, Crean P, Klein J, et al. Sequestration of fentanyl by the cardiopulmonary bypass (CPBP). Eur J Clin Pharmacol. 1984;27(1):51–56. DOI:10.1007/BF02395206
- Preston TJ, Ratliff TM, Gomez D, et al. Modified surface coatings and their effect on drug adsorption within the extracorporeal life support circuit. J Extra Corpor Technol. 2010 Sep;42(3):199–202.
- Myers GJ, Voorhees C, Eke B, et al. The effect of Diprivan (propofol) on phosphorylcholine surfaces during cardiopulmonary bypass–an in vitro investigation. Perfusion. 2009 Sep;24(5):349–355.
- Park J, Shin DA, Lee S, et al. Investigation of key circuit constituents affecting drug sequestration during extracorporeal membrane oxygenation treatment. Asaio J. 2017 May;63(3):293–298.
- Booth BP, Henderson M, Milne B, et al. Sequestration of glyceryl trinitrate (nitroglycerin) by cardiopulmonary bypass oxygenators. Anesth Analg. 1991 Apr;72(4):493–497.
- Rosen D, Rosen K, Davidson B, et al. Fentanyl uptake by the Scimed membrane oxygenator. J Cardiothorac Anesth. 1988 Oct;2(5):619–626.
- Preston TJ, Hodge AB, Riley JB, et al. In vitro drug adsorption and plasma free hemoglobin levels associated with hollow fiber oxygenators in the extracorporeal life support (ECLS) circuit. J Extra Corpor Technol. 2007 Dec;39(4):234–237.
- Cholette JM, Faraoni D, Goobie SM, et al. Patient blood management in pediatric cardiac surgery: a review. Anesth Analg. 2018 Oct;127(4):1002–1016.
- Cholette JM, Powers KS, Alfieris GM, et al. Transfusion of cell saver salvaged blood in neonates and infants undergoing open heart surgery significantly reduces RBC and coagulant product transfusions and donor exposures: results of a prospective, randomized, clinical trial. Pediatr Crit Care Med. 2013 Feb;14(2):137–147.
- Zeilmaker-Roest GA, van Saet A, van Rosmalen J, et al. Potentially clinically relevant concentrations of cefazolin, midazolam, propofol, and sufentanil in auto-transfused blood in congenital cardiac surgery. J Cardiothorac Surg. 2018 Jun 8;13. doi:10.1186/s13019-018-0747-0
- De Cock PA, Mulla H, Desmet S, et al. Population pharmacokinetics of cefazolin before, during and after cardiopulmonary bypass to optimize dosing regimens for children undergoing cardiac surgery. J Antimicrob Chemother. 2017 Mar 1;72(3):791–800. doi:10.1093/jac/dkw496
- Ingrande J, Gutierrez K, Lemmens HJ, et al. Pharmacokinetics of cefazolin and vancomycin in infants undergoing open-heart surgery with cardiopulmonary bypass. Anesth Analg. 2019 May;128(5):935–943.
- Wesley MC, Pereira LM, Scharp LA, et al. Pharmacokinetics of tranexamic acid in neonates, infants, and children undergoing cardiac surgery with cardiopulmonary bypass. Anesthesiology. 2015 Apr;122(4):746–758.
- Gertler R, Gruber M, Grassin-Delyle S, et al. Pharmacokinetics of tranexamic acid in neonates and infants undergoing cardiac surgery. Br J Clin Pharmacol. 2017 Aug;83(8):1745–1757.
- Ricci Z, Benegni S, Cies JJ, et al. Population pharmacokinetics of cefoxitin administered for pediatric cardiac surgery prophylaxis. Pediatr Infect Dis J. 2020 Jul;39(7):609–614.
- Gertler R, Gruber M, Wiesner G, et al. Pharmacokinetics of cefuroxime in infants and neonates undergoing cardiac surgery. Br J Clin Pharmacol. 2018 Sep;84(9):2020–2028.
- van Saet A, Zeilmaker-Roest GA, Veen KM, et al. Methylprednisolone plasma concentrations during cardiac surgery with cardiopulmonary bypass in pediatric patients. Front Cardiovasc Med. 2021;8:640543. doi:10.3389/fcvm.2021.640543
- Hornik CP, Gonzalez D, Dumond J, et al. Population pharmacokinetic/pharmacodynamic modeling of methylprednisolone in neonates undergoing cardiopulmonary bypass. CPT Pharmacometrics Syst Pharmacol. 2019 Dec;8(12):913–922.
- Cies JJ, Moore WS, Parker J, et al. Pharmacokinetics of cefazolin delivery via the cardiopulmonary bypass circuit priming solution in infants and children. J Antimicrob Chemother. 2019 May 1;74(5):1342–1347. doi:10.1093/jac/dky574
- Jaworski R, Dzierzanowska-Fangrat K, Czajkowska A, et al. Cefazolin prophylaxis in children undergoing cardiac surgery with the use of cardiopulmonary bypass-is the dosing correct? Eur J Cardiothorac Surg. 2021 Jul 16;27:27–33. doi: 10.1093/ejcts/ezab251
- Eaton MP, Alfieris GM, Sweeney DM, et al. Pharmacokinetics of epsilon-aminocaproic acid in neonates undergoing cardiac surgery with cardiopulmonary bypass. Anesthesiology. 2015 May;122(5):1002–1009.
- Hynynen M, Hynninen M, Soini H, et al. Plasma concentration and protein binding of alfentanil during high-dose infusion for cardiac surgery. Br J Anaesth. 1994 May;72(5):571–576.
- Takizawa E, Hiraoka H, Takizawa D, et al. Changes in the effect of propofol in response to altered plasma protein binding during normothermic cardiopulmonary bypass. Br J Anaesth. 2006 Feb;96(2):179–185.
- Hammaren E, Yli-Hankala A, Rosenberg PH, et al. Cardiopulmonary bypass-induced changes in plasma concentrations of propofol and in auditory evoked potentials. Br J Anaesth. 1996 Sep;77(3):360–364.
- Jeleazcov C, Saari TI, Ihmsen H, et al. Changes in total and unbound concentrations of sufentanil during target controlled infusion for cardiac surgery with cardiopulmonary bypass. Br J Anaesth. 2012 Nov;109(5):698–706.
- Dawson PJ, Bjorksten AR, Blake DW, et al. The effects of cardiopulmonary bypass on total and unbound plasma concentrations of propofol and midazolam. J Cardiothorac Vasc Anesth. 1997 Aug;11(5):556–561.
- Himebauch AS, Nicolson SC, Sisko M, et al. Skeletal muscle and plasma concentrations of cefazolin during cardiac surgery in infants. J Thorac Cardiovasc Surg. 2014 Dec;148(6):2634–2641.
- Anderson KB, Poloyac SM, Kochanek PM, et al. Effect of hypothermia and targeted temperature management on drug disposition and response following cardiac arrest: a comprehensive review of preclinical and clinical investigations. Ther Hypothermia Temp Manag. 2016 Dec;6(4):169–179.
- Smits A, Annaert P, Van Cruchten S, et al. A physiology-based pharmacokinetic framework to support drug development and dose precision during therapeutic hypothermia in neonates. Front Pharmacol. 2020;11:587. doi:10.3389/fphar.2020.00587
- Favie LMA, de Haan TR, Bijleveld YA, et al. Prediction of drug exposure in critically Ill encephalopathic neonates treated with therapeutic hypothermia based on a pooled population pharmacokinetic analysis of seven drugs and five metabolites. Clin Pharmacol Ther. 2020 Nov;108(5):1098–1106.
- Lutz IC, Allegaert K, de Hoon JN, et al. Pharmacokinetics during therapeutic hypothermia for neonatal hypoxic ischaemic encephalopathy: a literature review. BMJ Paediatr Open. 2020;4(1):e000685. DOI:10.1136/bmjpo-2020-000685
- Zimmerman KO, Wu H, Laughon M, et al. Dexmedetomidine pharmacokinetics and a new dosing paradigm in infants supported with cardiopulmonary bypass. Anesth Analg. 2019 Dec;129(6):1519–1528.
- Zuppa AF, Nicolson SC, Wilder NS, et al. Results of a phase 1 multicentre investigation of dexmedetomidine bolus and infusion in corrective infant cardiac surgery. Br J Anaesth. 2019 Dec;123(6):839–852.
- Wilkinson GR. Pharmacokinetics of drug disposition: hemodynamic considerations. Ann Rev Pharmacol. 1975;15(1):11–27. doi:10.1146/annurev.pa.15.040175.000303
- Miyamoto T, Karimov JH, Fukamachi K. Acute and chronic effects of continuous-flow support and pulsatile-flow support. Artif Organs. 2019 Jul;43(7):618–623. doi: 10.1111/aor.13446
- Mitchell IM, Pollock JC, Jamieson MP. The effects of congenital heart disease and cardiac surgery on liver blood flow in children. Perfusion. 1995 Jul;10(4):210–218. doi: 10.1177/026765919501000403
- Denault AY, Azzam MA, Beaubien-Souligny W. Imaging portal venous flow to aid assessment of right ventricular dysfunction. Can J Anaesth. 2018 Nov;65(11):1260–1261. doi: 10.1007/s12630-018-1125-z
- Zhu D, Yu H, Zhou Y, et al. Feasibility of measuring renal blood flow using transesophageal echocardiography in pediatric patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2012 Feb;26(1):39–45.
- Grassin-Delyle S, Couturier R, Abe E, et al. A practical tranexamic acid dosing scheme based on population pharmacokinetics in children undergoing cardiac surgery. Anesthesiology. 2013 Apr;118(4):853–862.
- Kapitein B, van Saet AW, Golab HD, et al. Does pharmacotherapy influence the inflammatory responses during cardiopulmonary bypass in children? J Cardiovasc Pharmacol. 2014 Aug;64(2):191–197.
- Kozik DJ, Tweddell JS. Characterizing the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg. 2006 Jun;81(6):S2347–54. doi: 10.1016/j.athoracsur.2006.02.073
- Alcaraz AJ, Sancho L, Manzano L, et al. Newborn patients exhibit an unusual pattern of interleukin 10 and interferon gamma serum levels in response to cardiac surgery. J Thorac Cardiovasc Surg. 2002 Mar;123(3):451–458.
- Alcaraz AJ, Manzano L, Sancho L, et al. Different proinflammatory cytokine serum pattern in neonate patients undergoing open heart surgery. Relevance of IL-8. J Clin Immunol. 2005 May;25(3):238–245.
- Adiraju SKS, Shekar K, Fraser JF, et al. Effect of cardiopulmonary bypass on cytochrome P450 enzyme activity: implications for pharmacotherapy. Drug Metab Rev. 2018 May;50(2):109–124.
- Vet NJ, de Hoog M, Tibboel D, et al. The effect of inflammation on drug metabolism: a focus on pediatrics. Drug Discov Today. 2011 May;16(9–10):435–442.
- De Cock RF, Piana C, Krekels EH, et al. The role of population PK-PD modelling in paediatric clinical research. Eur J Clin Pharmacol. 2011 May;67(Suppl 1):5–16.
- Mian P, Valkenburg AJ, Allegaert K, et al. Population pharmacokinetic modeling of acetaminophen and metabolites in children after cardiac surgery with cardiopulmonary bypass. J Clin Pharmacol. 2019 Jun;59(6):847–855.
- Hill KD, Sampson MR, Li JS, et al. Pharmacokinetics of intravenous sildenafil in children with palliated single ventricle heart defects: effect of elevated hepatic pressures. Cardiol Young. 2016 Feb;26(2):354–362.
- Su F, El-Komy MH, Hammer GB, et al. Population pharmacokinetics of etomidate in neonates and infants with congenital heart disease. Biopharm Drug Dispos. 2015 Mar;36(2):104–114.
- Hasija S, Chauhan S, Jain P, et al. Comparison of speed of inhalational induction in children with and without congenital heart disease. Ann Card Anaesth. 2016 Jul;19(3):468–474.
- Jankovic SM. A critique of pharmacokinetic calculators for drug dosing individualization. Eur J Drug Metab Pharmacokinet. 2020 Apr;45(2):157–162. doi: 10.1007/s13318-019-00589-1
- Gambus PL, Troconiz IF. Pharmacokinetic-pharmacodynamic modelling in anaesthesia. Br J Clin Pharmacol. 2015 Jan;79(1):72–84. doi: 10.1111/bcp.12286
- Vogt W. Evaluation and optimisation of current milrinone prescribing for the treatment and prevention of low cardiac output syndrome in paediatric patients after open heart surgery using a physiology-based pharmacokinetic drug-disease model. Clin Pharmacokinet. 2014 Jan;53(1):51–72. doi: 10.1007/s40262-013-0096-z
- Ayyar VS, Jusko WJ, Barker EL. Transitioning from basic toward systems pharmacodynamic models: lessons from corticosteroids. Pharmacol Rev. 2020 Apr;72(2):414–438. doi: 10.1124/pr.119.018101
- Van Driest SL, Marshall MD, Hachey B, et al. Pragmatic pharmacology: population pharmacokinetic analysis of fentanyl using remnant samples from children after cardiac surgery. Br J Clin Pharmacol. 2016 Jun;81(6):1165–1174.