1. Introduction
Paliperidone palmitate once monthly (PP1M) is a long-acting injectable (LAI) antipsychotic, which appeared in 2013 as the first monthly second-generation antipsychotic. The dosing of PP1M is described differently in Europe than in the United States (US). In Europe the equivalent dose of paliperidone (without the palmitate contribution) is used, while the US uses the full dose of paliperidone palmitate. The comparison of Europe vs. US doses are: 1) 25 mg: 39 mg, 2) 50 mg: 78 mg 3) 75 mg: 117 mg, 4) 100 mg: 156 mg, and 4) 150 mg: 234 mg [Citation1,Citation2].
The marketer recommends a first dose of 150 mg followed by the administration of 100 mg at day 8, both administered into the deltoid muscle to achieve therapeutic concentrations quickly. Monthly administration starts at day 36 after the first injection. The recommended administration in maintenance treatment is 75 mg/4 weeks, there being a therapeutic window of ±1 week for administration [Citation1,Citation2]. In real-world conditions, higher injection doses and different dosing intervals are common [Citation3,Citation4]. Besides, there is off-label clinical experience with changing the dosing interval versus the injection dosing of PP1M [Citation3].
The pharmacokinetics of PP1M has been studied at various doses, and at the beginning of treatment and steady state. Its half-life ranges between 29 and 45 days [Citation2] and steady state is probably reached after the 9th injection [Citation5]. Interindividual variations are well-known with pharmacokinetically relevant variables including sex, age, injection volume and injection site [Citation5,Citation6].
For schizophrenia, the plasma concentration of paliperidone required to achieve approximately 60% dopamine D2 receptor occupancy is considered to be 7.5 ng/mL [Citation2]. At least 73% of the patients achieve concentrations above 7.5 ng/mL within one week of the first administration [Citation2]. Peak plasma concentrations are reached around day 22 from first administration [Citation7]. In the last week of treatment, a decrease in plasma levels is observed, finding subtherapeutic concentrations, and it is therefore recommended that a second dose be administered at day 8 to bridge this drop in levels [Citation2].
The variations between deltoid and gluteal administration are especially relevant at the beginning of treatment, with higher and faster plasma concentrations observed for deltoid injections [Citation2,Citation8]. However, these differences are not considered clinically significant at steady state [Citation2,Citation8]. Plasma concentrations at steady state are almost linear in respect to doses administered, with no significant differences between administration sites [Citation2]. There are no data on intraindividual variations for injection site at the start of treatment [Citation1].
Various time intervals for reaching a steady state have been reported. A review of the limited available literature proposed that steady state would be reached no earlier than the 8th month of treatment [Citation4]. A recent case with paliperidone detectable concentrations 2.5 years after the last PP1M injection indicates that in the clinical environment the half-life of PPM1 may be much longer than what was originally proposed [Citation9].
Regarding variations of paliperidone levels within the dosing interval, slight differences between maximum (Cmax) and minimum (Cmin) plasma concentrations with a ratio of 1.56 at steady state have been reported [Citation10].
We suggest that, in certain scenarios, the differences between peak and trough levels may pose a potential risk to patient stability, especially in the period between the last few days of monthly administration and the first few days after monthly administration, as the lowest plasma concentrations would be faced during the steady-state period. This risk would therefore lead to the onset of psychotic outbreak symptoms at some point during maintenance treatment, although there is little literature assessing the risk of relapse with respect to the injection timepoint.
2. Total annual dosing is influenced by the dose and the interval of the injection
Since there are five different dosages ranging from 25 mg to 150 mg and given its linearity, the initial option is to increase or reduce the injection dose if psychotic symptoms or adverse drug reactions (ADRs) appear, respectively. An alternative strategy may include adjustment of the dose administration intervals, moving them from 4 weeks to 3 or 5 weeks.
To calculate the average dose administered at steady state, a calculation of the dose per patient per year was performed. We estimated the mean annual dose that would be administered to a patient depending on the dose and week of administration of the treatment at steady state ().
Table 1. Annual doses per patient/year and injection intervals for paliperidone palmitate one-month.
For example, assuming that a patient is being treated with the monthly maintenance dose of 75 mg/4 weeks, the annual dose received would be 975 mg/year (). Should psychotic symptoms appear in a stationary situation, the dose increase to 100 mg/4 weeks would lead to 1300 mg/year, while the dose advance of 75 mg/3 weeks would mean an annual administration of 1297.5 mg/year (). Although the annual doses do not essentially differ (although we did not perform statistical comparisons as no data is available), there are essential differences between these strategies with regard to pharmacokinetics, which refer to the peak and the overall plasma concentrations of paliperidone.
3. Expert opinion
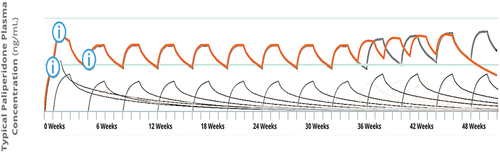
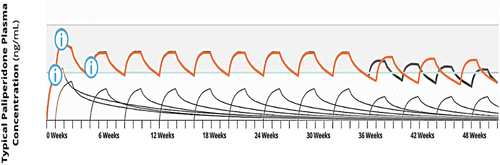
We used a publicly available pharmacokinetic simulation model, offered by the marketer for patients with schizophrenia [Citation11], to assess the differences between increasing/decreasing the dose versus advancing/delaying administration once steady state has been reached.
When the injection dose is increased, increases in global and peak plasma concentrations will occur (), but reducing the dosing interval to three weeks at same dose leads to a more moderate overall increase in plasma concentration and also affects trough concentration, which increases (). This increase in trough plasma concentrations may prevent the onset of psychotic symptoms related to low antipsychotic exposure, a risk potentially expected in the last week. Moreover, as the peak values are less impacted (compared to increases in the injection dose) this strategy might be related to lower ADR risk associated with high peak values [Citation12].
When comparing the injection dose reduction vs. dosing interval extension, reducing the injection dose leads to reduction for overall and peak plasma concentrations. If clinicians choose to delay administration (i.e. prolong the time interval till the next dose), a more moderate reduction in the overall plasma concentration is observed, without greatly impacting the peak plasma value.
We propose a new treatment approach using PP1M once steady state has been reached that can be modified according to the clinical needs of each individual patient. The findings of a previous population pharmacokinetic study [Citation13] support the figures generated by the online dose simulator.
3.1. How to proceed in case of lack of clinical response/relapse
If the onset of psychotic symptoms occurs in the first 3 weeks following the PP1M injection, the proposed strategy is to increase the injection dose, aiming for an increase in peak and trough plasma concentrations. If relapse symptoms occur toward the end of the treatment period, the proposed strategy is to advance the administration of the drug, preventing a further decrease of plasma concentrations (as trough levels are expected to reach their lowest at 4 weeks) which could be related to further limited effectiveness.
3.2. How to proceed in case of ADRs
In case of ADRs in a clinically stable patient, prolonging the dose injection may lead to a lower overall plasma concentration without impacting the peak concentrations compared to an injection dose reduction.
3.3. Further improvements
This treatment algorithm based on the adjustment of the injection interval could gain additional precision by adjusting for days (instead of weeks) of administration (e.g. 75 mg/25 days). Moreover, this algorithm is based on a simulation model that does not allow for modification according to renal or hepatic function or age, although we expect regular assessments of levels (known as therapeutic drug monitoring [TDM]) to account for such aspects. In fact, TDM can be a valuable tool for LAI dosing adjustments; a schematic overview of integrating TDM in LAI dosing is provided in a prior article [Citation13]. Further, as simulated plasma levels are based on aggregated pharmacokinetic information, they may not be fully representative of individual patient variations that can only be studied by TDM in that specific patient. Consequently, and as there may be a lag between PP1M dose changes and clinical outcomes, patients should be assessed periodically for clinical response and TDM. Additionally, similar algorithms should be developed for formulations with longer administration intervals such as paliperidone palmitate 3-month formulation (PP3M) and 6-month formulation (PP6M). Lastly, information regarding validation of the simulation model is not available online.
4. Conclusion
Combined with regular TDM, this algorithm may also be useful in the search for the minimum effective dose, or in the progressive withdrawal of treatment with the combination of dose reduction vs. the prolongation of the injection interval to five weeks.
In addition, this strategy may help ADR management strategies by introducing a novel approach keeping effective but low long-term dose [Citation14].
Real-world evidence is needed to test the effectiveness of this strategy in daily clinical practice, although some reports of off-label injection intervals are available [Citation3]. Ultimately, patients’ preferences regarding dosing intervals need to be considered.
Declaration of interests
G Schoretsanitis has served as a consultant for Dexcel Pharma, HLS Therapeutics, Saladax and Thermo Fisher and has received speaker’s fees from HLS Therapeutics and Saladax. E Baca-Garcia has been a consultant to or has received honoraria or grants from Janssen Cilag, Lundbeck, Otsuka, Pziffer, Servier, Deprexis and Sanoffi. E Baca-Garcia is founder of eB2. E Baca-Garcia has designed MEmind. J de Leon personally develops his presentations for lecturing, has never lectured using any pharmaceutical or pharmacogenetic company presentations, and has never been a consultant for pharmacogenetic or pharmaceutical companies. In the past, J de Leon received researcher-initiated grants from Eli Lilly (one ended in 2003 and the other, as co-investigator, ended in 2007); from Roche Molecular Systems, Inc. (ended in 2007); and, in a collaboration with Genomas, Inc., from the NIH Small Business Innovation Research program (ended in 2010). J de Leon has been on the advisory boards of Bristol-Myers Squibb (2003/04) and AstraZeneca (2003). Roche Molecular Systems supported one of his educational presentations, which was published in a peer-reviewed journal (2005). His lectures were supported once by Sandoz (1997), twice by Lundbeck (1999 and 1999), twice by Pfizer (2001 and 2001), three times by Eli Lilly (2003, 2006, and 2006), twice by Janssen (2000 and 2006), once by Bristol-Myers Squibb (2006), and seven times by Roche Molecular Systems, Inc. (once in 2005 and six times in 2006). S Ovejero has served as consultant and provided medical training for Otsuka, Rovi and Johnson & Johnson. S Sánchez-Alonso has been a consultant, provided medical training and received honoraria or grants from Otsuka, Rovi, Angelini, Casen-Recordati and Johnson & Johnson. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
One reviewer has received manuscript or speaker’s fees from Astellas, Eisai, Eli Lilly, Elsevier Japan, Janssen Pharmaceuticals, Kyowa Yakuhin, Lundbeck Japan, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, MSD, Nihon Medi-Physics, Novartis, Otsuka Pharmaceutical, Shionogi, Shire, Sumitomo Pharma, Takeda Pharmaceutical, Tsumura, Viatris, Wiley Japan, and Yoshitomi Yakuhin, and research grants from Eisai, Mochida Pharmaceutical, Meiji Seika Pharma, Shionogi and Sumitomo Pharma. The remaining reviewers have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- xeplion-epar-public-assessment-report_en.pdf [Internet]. [cited 2023 Sep 15]. Available from: https://www.ema.europa.eu/en/documents/assessment-report/xeplion-epar-public-assessment-report_en.pdf
- Samtani MN, Gopal S, Gassmann-Mayer C, et al. Dosing and switching strategies for paliperidone palmitate: based on population pharmacokinetic modelling and clinical trial data. CNS Drugs. 2011;25:829–845. doi: 10.2165/11591690-000000000-00000
- Cohen JY, Dumoulin-Charette A, Meraabi N, et al. Serum concentration of paliperidone palmitate administered every 3 weeks. Psychopharmacol Bull. 2019;49:57–62.
- Cicala G, de Filippis R, Barbieri MA, et al. Tolerability profile of paliperidone palmitate formulations: a pharmacovigilance analysis of the EUDRAVigilance database. Front Psychiatry. 2023;14:1130636. doi: 10.3389/fpsyt.2023.1130636
- Schoretsanitis G, Spina E, Hiemke C, et al. A systematic review and combined analysis of therapeutic drug monitoring studies for long-acting paliperidone. Expert Rev Clin Pharmacol. 2018;11(12):1237–1253. doi: 10.1080/17512433.2018.1549489
- Samtani MN, Vermeulen A, Stuyckens K. Population pharmacokinetics of intramuscular paliperidone palmitate in patients with schizophrenia: a novel once-monthly, long-acting formulation of an atypical antipsychotic. Clin Pharmacokinet. 2009;48:585–600. doi: 10.2165/11316870-000000000-00000
- Schoretsanitis G, Meyer JM, Conca A, et al. Personalized switching from oral to long-acting injectable second-generation antipsychotics in schizophrenia treatment using pharmacokinetic considerations. Expert Opin Drug Metab Toxicol. 2023;19:189–202. doi: 10.1080/17425255.2023.2220962
- Hough D, Lindenmayer J-P, Gopal S, et al. Safety and tolerability of deltoid and gluteal injections of paliperidone palmitate in schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33(6):1022–1031. doi: 10.1016/j.pnpbp.2009.05.014
- Nersesjan M, Wagner M, Dalhoff KP, et al. Paliperidone poisoning and measurable plasma concentrations 2.5 years after last administered dose: a case report. Br J Clin Pharmacol. 2024. doi: 10.1111/bcp.16036
- Sheehan JJ, Reilly KR, D-J F, et al. Comparison of the peak-to-trough fluctuation in plasma concentration of long-acting injectable antipsychotics and their oral equivalents. Innov Clin Neurosci. 2012;9(7–8):17–23.
- Maintenance Dosing | Educational Dose Illustrator [Internet]. [cited 2023 Nov 5]. Available from: https://www.educationaldoseillustrator.com/pp1m/schizophrenia/scenario/maintenance-dosing
- Schoretsanitis G, Haen E, Piacentino D, et al. Pharmacokinetic correlates of once-monthly paliperidone palmitate-related adverse drug reactions. Clin Pharmacokinet. 2021;60(12):1583–1589. doi: 10.1007/s40262-021-01044-8
- Magnusson MO, Samtani MN, Plan EL, et al. Dosing and switching strategies for paliperidone palmitate 3-month formulation in patients with schizophrenia based on population pharmacokinetic modeling and simulation, and clinical trial data. CNS Drugs. 2017;31(4):273–288. doi: 10.1007/s40263-017-0416-1
- Schoretsanitis G, Baumann P, Conca A, et al. Therapeutic drug monitoring of long-acting injectable antipsychotic drugs. Ther Drug Monit. 2021;43:79–102. doi: 10.1097/FTD.0000000000000830