ABSTRACT
Introduction: The presence of severe coronary artery calcification is associated with higher rates of angiographic complications during percutaneous coronary intervention (PCI), as well as higher major adverse cardiac events compared with non-calcified lesions. Incorporating orbital atherectomy (OAS) for effective preparation of severely calcified lesions can help maximize the benefits of PCI by attaining maximal luminal gain (or stent expansion) and improve long-term outcomes (by reducing need for revascularization).
Areas covered: In this manuscript, the prevalence, risk factors, and impact of coronary artery calcification on PCI are reviewed. Based on current data and experience, the authors review orbital atherectomy technique and best practices to optimize lesion preparation.
Expert Commentary: The coronary OAS is the only device approved for use in the U.S. as a treatment for de novo, severely calcified coronary lesions to facilitate stent delivery. Advantages of the device include its ease of use and a mechanism of action that treats bi-directionally, allowing for continuous blood flow during treatment, minimizing heat damage, slow flow, and subsequent need for revascularization. The OAS technique tips reviewed in this article will help inform interventional cardiologists treating patients with severely calcified lesions.
1. Introduction
An indicator of advanced coronary artery disease (CAD) is the presence of coronary artery calcification (CAC), and the extent of calcification strongly correlates with the degree of atherosclerosis and, therefore, with the rate of future cardiac events [Citation1-Citation4]. Percutaneous coronary intervention (PCI) in severely calcified lesions is also known to result in lower success rates, higher complication rates, and worse long-term outcomes compared with non-calcified lesions [Citation5–Citation7]. The prevalence of risk factors for CAC is increasing, hence the adverse impact of coronary calcification on PCI procedures and outcomes is likely to increase. Vessel preparation prior to PCI with orbital atherectomy can mitigate these issues and allows for both optimal stent delivery and complete stent expansion. In the present manuscript we review the prevalence, risk factors, and impact of CAC on PCI, as well as coronary atherectomy devices, and technique/best practices for the Diamondback 360® coronary orbital atherectomy system (OAS), Classic Crown (Cardiovascular Systems, Inc., St. Paul, MN, USA).
1.1. CAC prevalence and risk factors
The prevalence of moderate/severe CAC in the PCI patient population is 32%, of which 6–20% is considered severe [Citation5,Citation8]. Since intravascular imaging techniques, such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT), are underutilized as a diagnostic modality, coronary calcification is often underestimated or considered mild or moderate via angiography, when it may actually be severe [Citation9]. Several health behaviors and risk factors, as well as chronic inflammatory conditions, lead to calcium deposition in the coronary arteries () [Citation1,Citation7,Citation10–Citation12]. These conditions cause endothelial injury and subsequent cell dysfunction, escalating an inflammatory response from leukocytes and vascular smooth muscle cells which leads to calcium deposition in the intimal and medial layers of the arterial wall [Citation7,Citation12,Citation28]. Many of these risk factors are on the rise in the United States and worldwide [Citation10].
Table 1. CAC risk factors [Citation1,Citation7,Citation10–Citation12] and PCI adverse events due to CAC [Citation5,Citation13–Citation27].
1.2. Challenges related to the standard of care for severe CAC
The current standard of care for treating patients with severe and symptomatic CAD is lesion preparation with balloon angioplasty (high-pressure plain old balloon angioplasty [POBA] and/or cutting-scoring balloon [CB]) followed by implantation of drug-eluting stents (DES). Using DES in severely calcified coronary lesions, however, has been a challenge for interventional cardiologists and often results in worse outcomes for the patient [Citation5,Citation29,Citation30]. Calcified lesions have been shown to poorly yield to dilation with balloon angioplasty, resulting in poor or asymmetric stent expansion, adverse events, and increased major adverse cardiac events (MACE) () [Citation5,Citation13–Citation27]. In addition, a human autopsy study of DES indicated that stent deformation was associated with the presence of severe calcification [Citation20]. Extensive calcium may also damage the DES polymer coating [Citation31,Citation32] and potentially decrease the effectiveness of DES [Citation33]. The increased incidence of restenosis and target lesion revascularization (TLR) in the presence of CAC are likely due to stent underexpansion in addition to the reduction in drug concentration and uptake into the vessel wall from DES [Citation20,Citation21].
2. Atherectomy treatment options for calcified coronary arteries
Atherectomy devices have historically been used during PCI for undilatable or un-crossable lesions. Proactive vessel preparation of calcified coronary lesions can facilitate successful stent delivery and implantation as well as optimal stent expansion in the presence of CAC. Unfortunately for patients with CAC, atherectomy devices are likely underutilized. Recent evidence indicates that coronary atherectomy is utilized in less than 5% of PCI patients even though it has been shown that the prevalence of moderate/severe CAC in the PCI patient population is 32% [Citation5,Citation8,Citation34]. Thus, the majority of patients with CAC are still treated with POBA and DES. The commercially available coronary atherectomy devices currently include: orbital atherectomy (Diamondback 360) (Cardiovascular Systems, Inc., St. Paul, MN, USA); rotational atherectomy (RA), (Rotablator™) (Boston Scientific, Marlborough, MA, USA); and laser atherectomy (ELCA™), (Spectranetics, Colorado Springs, CO, USA). While RA and laser atherectomy have been used for decades, orbital atherectomy is the only atherectomy device specifically indicated by the US FDA as a treatment for de novo, severely calcified coronary lesions to facilitate stent delivery. A comparison of these atherectomy devices with regard to mechanism of action, clinical indication, and technical features is shown in . In-depth review articles comparing these devices and corresponding clinical data were recently published [Citation1,Citation6,Citation35–Citation37]. We present here a detailed review of orbital atherectomy trials/studies, technique, and best practices.
Table 2. Coronary atherectomy device comparison.
2.1. Orbital atherectomy and mechanism of action
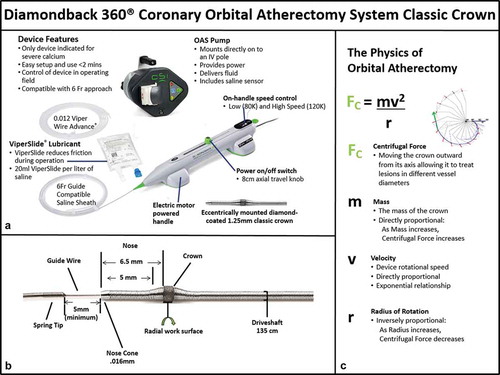
The OAS () provides a safe and effective option for physicians to improve treatment outcomes in patients via a proactive treatment approach to change the compliance of severely calcified coronary lesions. summarizes the mechanism of action and technique tips. Briefly, OAS uses a differential sanding mechanism of action to reduce plaque while potentially minimizing damage to the medial layer of the vessel [Citation6,Citation38]. A drive shaft with an eccentrically mounted diamond-coated crown provides proximal and distal sanding to modify the plaque and increase luminal size and compliance (). The crown’s orbital diameter expands radially via centrifugal force (). Softer tissue flexes away from the crown while fibrotic tissue or arterial calcium is engaged and treated. In addition, there may be pulsatile forces that indirectly impact deeper calcification in the coronary vessel wall [Citation39–Citation42], similar to what has been shown with the peripheral orbital atherectomy device [Citation43,Citation44]. OAS creates cracks in hard calcified lesions, changing the plaque morphology and lesion compliance allowing adequate stent expansion [Citation39]. Orbital atherectomy in the coronary arteries typically only requires a single crown to treat calcified plaques. Increasing the time in contact with the lesion, number of passes, or rotational speed allows for an increase in luminal gain as the crown is moved back and forth through the lesion. The elliptical orbit allows blood and micro-debris to flow past the crown, thus continually dispersing the particulate, cooling the crown, and reducing the risk of thermal injury to the target vessel which can be a potential cause of restenosis [Citation6]. The continuous perfusion both during rest periods and atherectomy treatment is unique to OAS as compared to RA with its centrally mounted and often larger burr. The flow rates of flush are different between OAS and RA. OAS has a pump-regulated flow rate as well as the ability to engage a prime button to allow for increased flush or flow rate – instead of depending on a standard IV flush rate. Saline and ViperSlide lubricant should always be continually infused during OAS operation to help minimize thermal injury. When orbital atherectomy is in the body, the device delivers approximately 18 ml/min of fluid (saline and ViperSlide minimum) to assist in cooling the device and treatment area(s). Continual flow allows plaque debris to be continuously washed away in the blood stream and may decrease ischemia and distal embolization. In carbon block testing, the average particle size created by OAS is 2.04 μm; 98.3% of particles are smaller than the diameter of a red blood cell; and 99.2% of particles are smaller than the diameter of a capillary [Citation37]. The combination of these features unique to OAS may lead to improved perfusion of the distal circulation, particularly during lesion treatment when the risk of embolization is highest. Compared to RA, OAS treatment may minimize the compromise in microcirculatory flow, as demonstrated in a recent study that reported that the index of microcirculatory resistance was significantly lower in patients treated with OAS versus RA (21 ± 6 vs. 31 ± 7 U, p = 0.04) [Citation45]. These potential putative protective effects of OAS on the microcirculation are currently being tested in the ORACLE trial (NCT03021577).
Table 3. Coronary orbital atherectomy system (OAS) mechanism of action and technique tips.
2.2. Orbital atherectomy clinical studies
Two major clinical trials (ORBIT I and II) and a multicenter real-world retrospective registry study have been completed on the coronary OAS Classic Crown () [Citation38,Citation46–Citation52]. In addition, numerous subanalyses have been published on orbital atherectomy, including such topics as gender, diabetes, left main, low ventricular ejection fraction, renal insufficiency, elderly, radial access, and intravascular imaging [Citation39,Citation53–Citation67]. Lastly, there is one major ongoing randomized trial of orbital atherectomy – The Evaluation of Treatment Strategies for Severe CaLcifIc Coronary Arteries: Orbital Atherectomy vs. Conventional Angioplasty Prior to Implantation of Drug Eluting StEnts (ECLIPSE).
Table 4. Coronary OAS: ORBIT I and II results [Citation38,Citation45–Citation51].
2.2.1. ORBIT I trial (first-in-human)
The ORBIT I trial (First-in-Human) was a prospective, non-randomized clinical trial that was conducted in two (2) centers in India to evaluate the safety of using the OAS in de novo calcified coronary lesions in 50 subjects [Citation38]. Device success (≤50% residual stenosis post-OAS treatment) occurred in 98% of patients. Angiographic complications included six dissections (types A–C) and one perforation. MACE rates of 6% at 30-days and 8% at 6 months were reported. Long-term follow-up was collected at a single center (N = 33). The MACE rate was 18.2% at 3 years and 21.2% at 5 years () [Citation46,Citation47].
2.2.2. ORBIT II trial (pivotal)
The ORBIT II trial (Pivotal) evaluated the use of coronary OAS Classic Crown to prepare de novo, severely calcified coronary lesions for stent placement (). It was a prospective, single-arm, multicenter clinical trial which enrolled 443 consecutive patients at 49 US sites from 25 May 2010 to 26 November 2012. Although there were other FDA-approved coronary atherectomy devices on the market, none were specifically indicated for the treatment of severely calcified coronary lesions and, therefore, could not be used as the comparator arm. Instead, the results of the ORBIT II primary endpoints were compared to outcomes reported for historical control subjects documented in the literature [Citation48]. Stents were successfully delivered in 97.7% of the time [Citation48]. The primary safety endpoint was freedom from MACE at 30 days which occurred in 89.6% of patients compared to the performance goal of 83% (95% confidence interval: 86.7–92.5%) with a total perforation rate of 1.8% (0.9% post-OAS device, 0.9% post balloon/stent) [Citation48]. The trial exceeded both primary endpoints by a significant margin; although the single arm design was a limitation, the ORBIT II trial demonstrated that OAS is safe and effective for the treatment of severely calcified coronary lesions. In the ORBIT II trial the 1-year MACE rate was 16.9%, including cardiac death (3.2%), myocardial infarction (MI) (10.6%), and TVR (5.8%). The 1-year TLR rate was 4.7%, and stent thrombosis occurred in one (0.2%) patient [Citation49]. Also, a sub-analysis of the ORBIT II 1-year data according to stent type revealed that the DES subset (n = 389 out of 440 treated subjects) had a TLR of 3.4% at 1 year [Citation49]. The low rate of TLR/TVR in the ORBIT II trial continued out to 3 years () [Citation51].
Numerous ORBIT II sub-analyses have been published [Citation53–Citation56,Citation66,Citation67]. For example, a gender sub-analysis indicated no difference in primary safety and efficacy endpoints; however, females had an increased risk for severe dissection [Citation53]. This finding was not consistent with a recent large coronary OAS registry which reported that the rate of dissection did not differ by gender [Citation53,Citation62].
Economic analyses have been completed and indicate that the coronary OAS device is projected to be highly cost-effective for patients who undergo PCI for severely calcified lesions owing to lower utilization of resources with improved outcomes. After adjusting for differences in age, gender, and comorbidities, the ORBIT II mean index procedure costs in elderly patients were 17% lower (p < 0.001; approximately US$ 2700), in comparison to historical data with a cost per life-year gained of US$ 11,895 offsetting the cost of the device in the first-year post-intervention [Citation68].
2.2.3. OAS real-world multicenter retrospective study
Lee and colleagues evaluated the safety and efficacy of OAS treatment prior to stent placement in real-world patients with severe CAC () [Citation52]. This single-arm, multicenter, retrospective study included 458 consecutive patients with heavily calcified coronary lesions treated with orbital atherectomy. The primary endpoint of 30-day major adverse cardiac and cerebrovascular events was 1.7%. Angiographic complication rates were low: perforation was 0.7%, dissection 0.9%, and no-reflow 0.7%; emergency coronary artery bypass graft surgery was performed in 0.2% of patients. This study demonstrated low rates of 30-day all-cause mortality (1.3%), stroke (0.2%), MI (1.1%), stent thrombosis (0.9%), and target vessel revascularization (0%). In this large real-world study of patients who underwent orbital atherectomy, including high-risk patients who would have been excluded from the ORBIT II trial, OAS was found to be safe and effective. The authors concluded that a randomized clinical trial is needed to identify the ideal treatment strategy for patients with severe CAC.
2.2.4. ECLIPSE trial
Minimal evidence is currently available to discern the ideal treatment strategy for severe CAC. Supplementary Table 1 summarizes the 2-year outcome rates among contemporary published data according to lesion preparation strategy and stent type [Citation49,Citation50,Citation69–Citation72]. Given the lack of appropriately powered comparative randomized controlled trials of lesion preparation strategy in severely calcified lesions (POBA and/or CB vs. atherectomy), as well as the improved outcomes seen with second-generation DES (Supplementary Table 1), a contemporary randomized controlled trial is warranted. The ECLIPSE trial (Clinicaltrials.gov identifier NCT03108456) is the largest randomized trial designed to study coronary atherectomy for severely calcified de novo lesions. Enrollment is underway in this multicenter, randomized trial that will enroll approximately 2000 patients with severe CAC assessing OAS vessel preparation compared to conventional angioplasty (POBA and/or CB). The co-primary trial endpoints – acute minimum stent area (assessed by OCT in a subset of 400 subjects) and 1-year target vessel failure (defined as the composite of cardiac death, target vessel-related MI, and clinically driven target vessel revascularization) – are powered to demonstrate superiority of OAS vessel preparation versus conventional angioplasty.
3. Orbital atherectomy treatment algorithm: technique and best practices
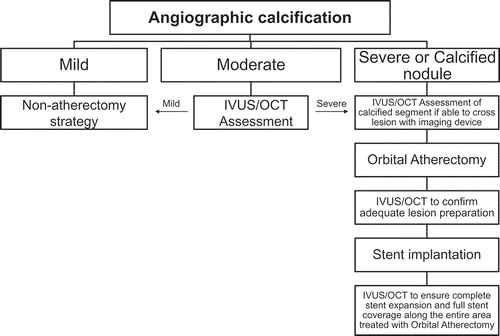
Historically, atherectomy has been a favored bailout tool for un-dilatable and/or un-crossable lesions which resulted in failure to place a stent. A better approach however may be a planned revascularization strategy that includes lesion preparation with atherectomy as the initial PCI approach for severely calcified lesions (). In a large comparison of bailout and planned atherectomy, shortened procedural time, lower amount of contrast dye, and lower rates of coronary dissections requiring additional stenting were observed with the strategy of planned atherectomy [Citation73]. Rather than seeking to debulk a calcified lesion, the goal of OAS treatment is to change the compliance of the lesion by altering its morphology, allowing for more effective lesion preparation with optimal stent expansion. In addition, complete lesion preparation may help to optimize PCI results, both peri-procedural and during the long term. OAS has a fully operator-controlled electric console that facilitates rapid setup. Briefly, the treatment with OAS can be performed by using a nine-step approach, , and is the description of the best practices and optimal technique tips for the OAS.
Table 5. Coronary OAS treatment algorithm – best practices and optimal technique.
3.1. Intravascular imaging
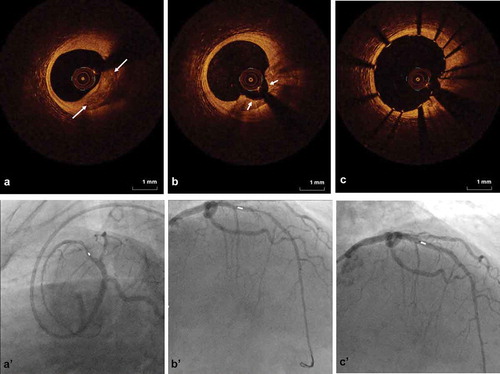
Proper lesion preparation as an initial strategy requires the ability to accurately detect and completely characterize CAC. Unfortunately, CAC is significantly under-appreciated by angiography alone, at least 50% of the time, as compared to IVUS [Citation9]. Utilization of intravascular imaging allows for accurate lesion assessment and measurement, particularly in patients where calcification is not readily apparent on fluoroscopy. Therefore, intravascular imaging, with either IVUS or OCT, should be performed when CAC is suspected based on patient demographics (e.g. elderly, chronic kidney disease, diabetic). IVUS utilizes ultrasound technology and thus does not have the ability to penetrate calcium to determine the depth of calcium. Therefore, OCT imaging may provide additional diagnostic information related to calcium depth that may aid in decision-making for optimizing PCI (). Imaging post-OAS intervention can also help assess the modification of calcification (), as well as post-stenting to determine strut apposition and minimum stent area ().
Figure 3. OCT imaging with angiographic co-registration of coronary OAS treatment. Top row is OCT imaging; bottom row is angiographic co-registration. a and a’. Baseline image of calcified coronary lesion (white arrows) pre-OAS treatment. b and b’. Post-OAS image indicating calcified plaque modification (short white arrows). c and c’. Final image showing stent expansion and apposition.
Intravascular imaging is an invaluable tool in optimizing outcomes when treating CAC. For example, intravascular imaging has provided insight into the prominent role of inadequate vessel preparation in the etiology of in-stent restenosis [Citation74]. In addition, IVUS-guided PCI has been shown to decrease rates of MACE, particularly in more complex lesion subsets [Citation75]. If the lesion is too stenotic to perform an initial intravascular imaging assessment, it is still useful to complete intravascular imaging post-OAS prior to stent deployment to confirm adequate vessel preparation, assess accurate sizing of the vessel, and to ensure the areas treated with OAS will be covered by the stent length chosen. Recently, a sub-analysis of the ORBIT II trial data found that there were significantly fewer stents placed and increased post-OAS minimum lumen diameter in patients with IVUS assessment of the degree of lesion calcification prior to OAS as compared to patients with angiographic assessment of the degree of lesion calcification [Citation66]. Thus, intravascular imaging post-OAS can ensure the calcium has been adequately treated and allow for more sensitive detection of dissections that may require treatment. Post-stenting intravascular imaging is also important to ensure complete stent expansion and treatment from normal to normal tissue and detect when post-dilation may be necessary.
3.2. ViperSlide
A number of combination of vasodilator and anticoagulation infusions have been used with atherectomy devices to lubricate and reduce the occurrence of adverse events. While the OAS IFU recommends following standard institution protocols with OAS, ViperSlide on its own has been shown to be adequate and does not routinely require the infusion of additional agents. Otherwise, if drug infusions are necessary, consider not including vasodilators with the ViperSlide, but instead use intracoronary nitroglycerin in between runs.
3.3. ViperWire
The 6 Fr compatible ViperWire guide wire is a smooth, moderately stiff, stainless steel wire, with a silicone coating, epoxy proximal tip bond, and a radiopaque distal spring tip. Although a stiff wire, the ViperWire is torquable, and, at times, can be used to initiate and complete the intervention. If there is any question about the ability to pass the wire, it is recommended that the lesion be crossed first with a standard 0.014” coronary wire and then utilize an exchange catheter to place the ViperWire. It is important to start with the wire as distal as possible but still keeping the tip in the main branch. Avoid locking the tip into a small side branch or the distal vessel if the wire is no longer free to move distally. If the lesion is a subtotal occlusion, a tapered crossing catheter may be useful to create a pilot channel and to facilitate the wire exchange. When using the device it is important to keep the tip of the device at least 5 mm for the spring tip of the wire. Running the device against the spring tip may result in damage to the wire. In addition, it is important to keep the guide coaxial aligned to avoid forcing the crown into the wall of the artery. Non-coaxial alignment increases the risk of angiographic complications. Extra-support guides are preferred, but the device is flexible and is able to navigate moderate tortuosity without much difficulty.
3.4. OAS initial pass and traverse speed
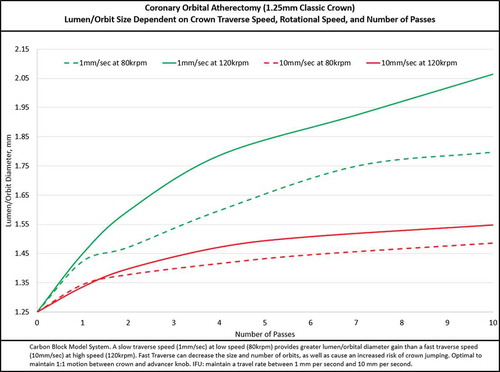
Once the wire is in the proper position and the advancer knob is locked at the 1-cm mark, advance the crown to approximately 5 mm proximal to the lesion. If the lesion is very severe, it may be useful to place the nose cone of the device partially into the lesion, but not fully occlusive, to facilitate crossing. Next, unlock the advancer knob and slide it to the 0-cm mark on the handle – this will relieve any built-up tension/torque in the drive shaft. This process is particularly important for tortuous vessels, as a greater amount tension/torque can build up in the drive shaft while the device is tracked to the lesion. If the tension/torque is not relieved prior to treating the lesion, there is a greater risk of rapid advancement (jumping) of the crown into or past the lesion during the initial activation. Therefore, always start on low speed and traverse slowly (targeting 1–3 mm/s) to engage the lesion. It is optimal to maintain 1:1 motion between the crown (as viewed via live angiography) and advancer knob. Slow and steady advancement at 1 mm/s traverse rate results in significantly larger luminal gain as compared to 10 mm/s traverse rate () in the same number of passes. Advancing the travel knob slowly can increase the radius of orbit and allow time to potentially create fractures within the calcium [Citation37]. Rapid advancement (>10 mm/s) may increase the risk for dissections and perforations with reduced treatment effect. The device works bi-directionally, so it is important to use the same slow movement when advancing the crown forward or backward while it is orbiting. On the first pass, with the device orbiting on low speed, engage the lesion with a target traverse speed of 1–3 mm/s while watching crown movement via angiography to ensure 1:1 movement. If 1:1 movement is lost, disengage the crown from the lesion by moving the advancer knob 1–2 mm proximal to remove tension from the drive shaft. Re-engage the lesion targeting the same 1–3 mm/s traverse speed while monitoring 1:1 movement. Repeat the engage and release technique whenever 1:1 movement between the crown and advancer knob is lost. The orbital nature of the device allows the device to contact the vessel wall, resulting in continued plaque modification even after the lesion has been treated for the first time. When performing subsequent runs, move the crown slowly forward and backward though the lesion, targeting 1–3 mm/s traverse speed, until there is no resistance and there is no change in pitch/sound from contacting calcium in the wall of the artery.
3.5. OAS run time
Orbital atherectomy is a time-dependent therapy. The recommended max run time should not exceed 30 s – the device will give a reminder beep at 25 s, allowing time to decide where to rest the device between runs. A single run should not exceed 30 s and the total treatment time should not exceed 5 min. If the device remains occlusive in a lesion when 30 s is approaching, continue treatment to allow tracking of device back to a non-occlusive resting area. Also, it is important not to pull the device backward rapidly to get to the original starting point. Instead move slowly out of lesion to find a good place for the device to rest between runs. Additionally, the orbiting crown should not remain in one location as it may lead to vessel damage due to uneven treatment or compression/tension build up in the driveshaft. Continuous crown movement should be maintained at all times during treatment. It is recommended that the rest time between runs is equal to or greater than the run time. For example, if there is a 30 s run there should be at least 30 s of rest between runs. Always wait until the hemodynamics have returned to baseline before starting then next pass. When treating a long lesion, do multiple short passes rather than longer runs over 30 s, maintaining slow and controlled movement. It is sometimes helpful to divide the long lesions into two shorter lesions. There is often a landing zone between lesions. If this is the case, treat the first lesion then track (release break and move entire device forward down the wire, and reposition crown 5mm from the lesion) the device forward to treat the second lesion rather than advancing the device though both at one time. Limiting the treatment interval reduces the potential for spasm and allows for particulate to be flushed away.
3.6. OAS speed settings
The orbital atherectomy device (OAD) has two unique speed settings. Low speed should always be used for the initial pass. Not all lesions treated with OAS require high speed. In fact, avoiding the high-speed setting in tortuous lesions, severe angulations, and vessels smaller than 3.0 mm in diameter are recommended. Ablation should always be initially performed at low rotational and traverse speeds and then only escalated to high-speed rotation as needed for more treatment potential – this will provide greater lumen gain and reduce the risk of complications (see and section above for more details). High speed should only be used when there is insufficient ablation or compliance change after two or more runs on low speed. Auditory, visual, and tactile feedback can be used to indicate the need for more passes or escalation to higher rotational speed. For example, inadequate pitch changes of sound from the OAS device handle (i.e. continued high pitched whining sound) or visually perceived crown resistance as seen on the live fluoroscopy, while ablating the lesion with tactile resistance of the travel knob (on the OAS device handle) may indicate the need for more passes at low speed. If treatment potential on low speed has been reached, indicated by lack of travel resistance as well as lack of audible change during treatment in a 3 mm or larger vessel, high speed may be used to increase device treatment potential.
3.7. Assessing the completeness of plaque modification and treatment endpoint
After the plaque has been modified by orbital atherectomy, remove the device and test the lesion compliance with a balloon sized 1:1 to the vessel. The balloon should be uniformly and completely expanded within the treated lesion. If the lesion does not yield at or below nominal pressure, consider additional runs of OAS or advancing to a high speed. If there is a visible dissection during treatment, remove the orbital atherectomy device and use other techniques to treat the lesion. If there is no angiographically visible dissection after balloon dilation, it is safe to proceed with further runs with the OAS. Calcified lesions should be treated completely to ensure optimal results. Lesions should be treated from normal tissue to normal tissue whenever possible, and the crown should never be stopped within an obstructive untreated lesion. Instead move the crown slowly to a distal or proximal position from the untreated lesion to minimize risk of limiting the crown’s ability to ramp to speed and occlusion of the vessel with the crown. A predefined number of runs should not be used, as it is difficult to predict the duration of treatment required by each individual lesion. Incorporating a combination of a tactile and audible endpoint can ensure the lesion has been adequately ablated. The operator must ‘feel and listen’ for the point where changes stop occurring and note a change in pitch, indicating the appropriate endpoint of treatment. Post-OAS intravascular imaging can also confirm that an adequate treatment endpoint has been reached with OAS. Premature termination of OAS treatment diminishes the benefits of vessel preparation.
3.8. Aorto-ostial lesions
Ostial lesions were excluded from the ORBIT II trial and are not described within the product labeling. However, since the commercial release of the product in October of 2013, many aorto-ostial lesions have been successfully treated [Citation76]. The ideal technique (if the device can cross the lesion) is to advance the crown through the ostial lesion to a relatively normal section of the artery, activate the device starting on low speed, and treat the lesion in a retrograde fashion. If the ostial lesion is too severe for the non-spinning crown to cross, make sure the guide catheter is coaxial and place the nose cone of the device into the ostium keeping the crown 5 mm from the lesion. This will constrain the orbit and allow the lesion to be treated in a more controlled manner. Utilizing low speed, slowly advance the crown though the lesion. After the initial pass consider treating in a retrograde fashion until the plaque is fully modified. If the nose cone cannot be engaged in the lesion, consider other treatment options. As standard OAS treatment for all lesions, it is important engage the lesion prior to escalating orbital speed – this is particularly important for aorto-ostial lesions, because a large unconstrained orbit in the aorta while advancing into the ostium may result in unconstrained device orbit.
3.9. Bifurcation lesions
Coronary artery bifurcation lesions are a common lesion subset in PCI accounting for 15–20% of the total number of interventions [Citation77]. Treatment of coronary artery bifurcation lesions represents a challenging area in interventional cardiology. When compared with non-bifurcation interventions, bifurcation interventions have a lower rate of procedural success, higher procedural costs, longer hospitalization, and a higher clinical and angiographic restenosis [Citation78,Citation79]. Plaque shift or carina shift are the likely mechanism for side-branch compromise/closure after main vessel intervention [Citation80,Citation81]. Bifurcation lesions are listed as a warning on the Instructions For Use (IFU) when exhibiting excessive tortuosity and were excluded from the ORBIT II trial; however, since the commercial release of the product in October of 2013, many bifurcation lesions have been successfully treated [Citation82]. When treating bifurcating lesions that do not create a tortuous path for the device, the guide may require upsizing to a 7-Fr system. After OAS treatment has been performed in the parent vessel, do not remove the wire from the parent vessel to wire the side branch until the parent vessel can be wired with certainty. Due to the orbital mechanism of the device and the risk of wire damage, a second wire cannot be placed in a side branch adjacent to the treatment area. The experience has been that when treating a calcified parent vessel, the plaque is removed from the ostium of the side branch and does not require protection with a wire in the side branch.
3.10. Temporary transvenous pacing
Temporary transvenous pacing (TVP) is often used during PCI in patients undergoing RA and is recommended for prophylactic use in all cases involving the right coronary artery (RCA) or left circumflex artery. The placement of TVP has cost implications, as well as associated procedural risk, including cardiac tamponade. Clinically significant bradycardia, however, rarely occurs with OAS. In one multicenter comparison, pacing activation occurred in just 0.1% of cases using OAS [Citation83]. Additionally, in a single center assessment, significant bradycardia was found to be uncommon with orbital atherectomy treatment [Citation60]. This is likely due to the mechanism of OAS that allows continuous blood flow and saline during ablation, decreasing thermal injury [Citation84]. In addition, a multicenter retrospective analysis revealed that temporary pacemakers were placed significantly less for OAS cases as compared with RA cases (p = 0.003) [Citation85]. Pacemaker activation showed a strong trend of lower activation in the OAS arm (p = 0.057) [Citation85]. Although the need for placing a temporary pacemaker is low, consideration may be warranted in patients with right coronary arteries or dominant circumflex lesions that are very long that may require extensive plaque modification.
Lastly, pharmacotherapy may serve as an alternative to TVP insertion. Aminophylline (250 mg infused over 10 min) can be administered to reduce bradycardia during ablation of the RCA. Additionally, atropine should be readily available during treatment. To help minimize the occurrence of transient heart block, adequate rest periods should occur between treatment runs, lasting at minimum an equal time to the prior run. If bradycardia is noted during ablation, shorter runs (15–20 s) with increased rest periods should be used.
3.11. Mechanical support
Given the low rates of slow flow and peri-procedural hypotension during OAS treatment, mechanical circulatory support (MCS) is not routinely used. The continuous blood flow that occurs during OAS treatment due to eccentricity of the crown attachment and orbital motion may reduce negative hemodynamic effects and necessity of MCS. Anecdotal use of OAS with MCS, however, suggests its usage may be both safe and feasible. As the number of complex higher risk-indicated patients (CHIP) treated with PCI increases, studies are needed to assess the optimal MCS strategy with atherectomy devices. Determining need for MCS should be done after considering lesion complexity, hemodynamic status, left ventricular systolic function, and comorbidities.
4. Conclusion
The interventional cardiology community is becoming more aware that patients with CAC are difficult to treat and have worse short- and long-term outcomes. OAS safely and effectively treats severely calcified lesions, allowing for successful stent implantation and expansion – key for optimal outcomes. Currently, OAS has been used in more than 33,860 cases and through that experience, important best practices and optimal technique have been determined. A key component for optimal coronary OAS utilization is a slow crown traverse speed of 1–3mm/sec – this will ensure enough time for the device to completely treat the lesion.
5. Expert commentary
The current standard of care for treating patients with severe and symptomatic CAD is balloon angioplasty (high-pressure POBA and/or CB) followed by DES implantation. Using DES in severely calcified coronary lesions, however, has been a challenge for interventional cardiologists and often results in worse outcomes for the patient [Citation5,Citation29,Citation30]. Calcified lesions have been shown to respond poorly to balloon angioplasty, resulting in poor or asymmetric stent expansion, adverse events, and increased MACE [Citation5,Citation13–Citation27]. Unfortunately for patients with CAC, atherectomy devices are underutilized. Recent evidence indicates that coronary atherectomy is only utilized in less than 5% of PCI patients even though it has been shown that the prevalence of moderate/severe CAC in the PCI patient population is 32% [Citation5,Citation8,Citation34]. Thus, the majority of patients with CAC are still only treated with POBA and DES. The Diamondback 360 Coronary OAS is the first and only device approved for use in the United States as a treatment for de novo, severely calcified coronary lesions to facilitate stent delivery. The advantage of OAS is the ease of use – all controls for activating the device and controlling the speed settings are on the electric handle (in the sterile field), allowing for the severely calcified lesions to be treated quickly and effectively. In addition, unlike other atherectomy systems, orbital atherectomy treats bi-directionally with an orbital mechanism that allows for continuous blood flow during treatment, minimizing heat damage, slow flow, and subsequent need for revascularization. Orbital atherectomy has not yet been studied in a randomized trial, but comparison to the literature has demonstrated a distinct advantage – namely, low rates of target vessel revascularization that have been shown to be durable out to 3 years. Given the lack of appropriately powered comparative randomized controlled trials of lesion preparation strategy in severely calcified lesions (POBA and/or CB vs. atherectomy), a contemporary randomized controlled trial is warranted. The Evaluation of Treatment Strategies for Severe CaLcifIc Coronary Arteries: Orbital Atherectomy vs. Conventional Angioplasty Prior to Implantation of Drug Eluting StEnts (Clinicaltrials.gov identifier NCT03108456) is the largest randomized trial designed to study coronary atherectomy for severely calcified de novo lesions. Enrollment is underway in this multicenter, randomized trial that will enroll approximately 2000 patients with severe CAC assessing OAS vessel preparation compared to conventional angioplasty (POBA and/or CB). The results of this unprecedented study and the OAS technique tips reviewed in this article will help inform interventional cardiologists treating patients with severely calcified lesions and will likely change the standard of care and guidelines.
6. Five-year view
Patients with severe CAD and a clinical indication for revascularization, but who are at high procedural risk because of patient comorbidities, complexity of coronary anatomy, severe calcification, and/or poor hemodynamics, represent an understudied and potentially underserved patient population – termed ‘complex higher-risk (and indicated) patient’ (CHIP) population [Citation86]. The impact of complex lesions is now of great interest and many feel that over the next several years dedicated coronary interventionalists, which develop the cognitive and technical skills to manage these patients, will shift the treatment paradigm for the benefit of these CHIP patients. By 2030, the AHA estimates that 40.5% of the US population will have some form of cardiovascular disease, costing an estimated USD $818 billion [Citation87]. Effective treatment strategies are needed if the growing burden of cardiovascular disease is to be mitigated. The risk factors for developing coronary calcification such as advanced age- and diabetes-induced kidney disease are increasing. Treating these patients will become an even bigger problem over the next 5 years. Orbital atherectomy will play a key role in the treatment of these complex patients.
Key issues
An indicator of advanced coronary artery disease is the presence of coronary artery calcification.
Advanced age, diabetes mellitus, and chronic kidney disease are prime risks factors for calcified coronary arteries.
Calcium remains underappreciated by conventional angiography.
Calcified coronary lesions are challenging to treat and are associated with increased risk for acute complications and worse long-term outcomes.
Proper technique and best practices of orbital atherectomy allows for modification of de novo, severely calcified plaque to facilitate optimal stent placement.
Declaration of interest
E Shlofmitz has a consulting agreement with Cardiovascular Systems, Inc. BJ Martinsen is an employee of Cardiovascular Systems, Inc. M Lee has a consulting agreement with Cardiovascular Systems, Inc. SV Rao has a consulting agreement with Cardiovascular Systems, Inc. P Généreux has a consulting agreement with Cardiovascular Systems, Inc. J Higgins is an employee of Cardiovascular Systems, Inc. JW Chambers has a consulting agreement with Cardiovascular Systems, Inc. AJ Kirtane reports institutional grants to Columbia University and/or Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, CathWorks, Siemens, Philips, ReCor Medical, Spectranetics. ES Brilakis has a consulting agreement with Cardiovascular Systems, Inc. consulting/speaker honoraria from Abbott Vascular, Amgen, Asahi, Elsevier, GE Healthcare, and Medicure; research support from Boston Scientific and Osprey; spouse is employee of Medtronic. DE Kandzari reports personal consulting honoraria: Biotronik, Boston Scientific, Medtronic, Micell Technologies; Institutional research/educational grant support: Abbott/St. Jude, Cardiovascular Systems, Biotronik, Boston Scientific, Orbus Neich, Medtronic, Micell Technologies. SK Sharma has a consulting agreement with Cardiovascular Systems, Inc. R Shlofmitz has a consulting agreement with Cardiovascular Systems, Inc. Writing assistance was utilized in the preparation of this manuscript, it was funded by Cardiovascular Systems, Inc and carried out by Nick Hargus, Ph.D.; Cardiovascular Systems, Inc and Ann Behrens, B.S.; Cardiovascular Systems, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Coronary_OAS_Best_Practices_SUPPL_TABLE_1_2017_09_08.docx
Download MS Word (16.4 KB)Supplementary-material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Lee MS, Shah N. The impact and pathophysiologic consequences of coronary artery calcium deposition in percutaneous coronary interventions. J Invasive Cardiol. 2016;28:160–167.
- Mintz GS. Intravascular imaging of coronary calcification and its clinical implications. JACC Cardiovasc Imaging. 2015;8:461–471.
- Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616.
- Yano Y, O’Donnell CJ, Kuller L, et al. Association of coronary artery calcium score vs age with cardiovascular risk in older adults: an analysis of pooled population-based studies. JAMA Cardiol. 2017 Jul 26:9. DOI: 10.1001/jamacardio.2017.2498
- Genereux P, Madhavan MV, Mintz GS, et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes: pooled analysis from the HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) and ACUITY (acute catheterization and urgent intervention triage strategy) trials. J Am Coll Cardiol. 2014;63:1845–1854.
- Chambers JW, Diage T. Evaluation of the Diamondback 360 coronary orbital atherectomy system for treating de novo, severely calcified lesions. Expert Rev Med Devices. 2014;11:457–466.
- Leopold JA. Vascular calcification: mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc Med. 2015;25:267–274.
- Bourantas CV, Zhang Y-J, Garg S, et al. Prognostic implications of coronary calcification in patients with obstructive coronary artery disease treated by percutaneous coronary intervention: a patient-level pooled analysis of 7 contemporary stent trials. Heart. 2014;100:1158–1164.
- Mintz GS, Popma JJ, Pichard AD, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91:1959–1965.
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322.
- Madhavan MV, Tarigopula M, Mintz GS, et al. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. 2014;63:1703–1714.
- Otsuka F, Sakakura K, Yahagi K, et al. Has our understanding of calcification in human coronary atherosclerosis progressed? Arter Thromb Vasc Biol. 2014;34:724–736.
- Mosseri M, Satler LF, Pichard AD, et al. Impact of vessel calcification on outcomes after coronary stenting. Cardiovasc Revasc Med. 2005;6:147–153.
- Colombo A, Stankovic G. Coronary perforations: old screenplay, new actors! J Invasive Cardiol. 2004;16:302–303.
- Onuma Y, Tanimoto S, Ruygrok P, et al. Efficacy of everolimus eluting stent implantation in patients with calcified coronary culprit lesions: two-year angiographic and three-year clinical results from the SPIRIT II study. Catheter Cardiovasc Interv. 2010;76:634–642.
- Schlüter M, Cosgrave J, Tübler T, et al. Rotational atherectomy to enable sorolimus-eluting stent implantation in calcified, nondilatable de novo coronary artery lesions. Vasc Manag. 2007;4:63–69.
- Cavusoglu E, Kini AS, Marmur JD, et al. Current status of rotational atherectomy. Catheter Cardiovasc Interv. 2004;62:485–498.
- Moussa I, Di Mario C, Moses J, et al. Coronary stenting after rotational atherectomy in calcified and complex lesions. Angiographic and clinical follow-up results. Circulation. 1997;96:128–136.
- Benezet J, Diaz de la Llera LS, Cubero JM, et al. Drug-eluting stents following rotational atherectomy for heavily calcified coronary lesions: long-term clinical outcomes. J Invasive Cardiol. 2011;23:28–32.
- Nakano M, Otsuka F, Yahagi K, et al. Human autopsy study of drug-eluting stents restenosis: histomorphological predictors and neointimal characteristics. Eur Heart J. 2013;34:3304–3313.
- Nishida K, Kimura T, Kawai K, et al. Comparison of outcomes using the sirolimus-eluting stent in calcified versus non-calcified native coronary lesions in patients on- versus not on-chronic hemodialysis (from the j-Cypher registry). Am J Cardiol. 2013;112:647–655.
- Moussa I, Ellis SG, Jones M, et al. Impact of coronary culprit lesion calcium in patients undergoing paclitaxel-eluting stent implantation (a TAXUS-IV sub study). Am J Cardiol. 2005;96:1242–1247.
- Clavijo LC, Steinberg DH, Torguson R, et al. Sirolimus-eluting stents and calcified coronary lesions: clinical outcomes of patients treated with and without rotational atherectomy. Catheter Cardiovasc Interv. 2006;68:873–878.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–e122.
- Fitzgerald PJ, Ports TA, Yock PG. Contribution of localized calcium deposits to dissection after angioplasty. An observational study using intravascular ultrasound. Circulation. 1992;86:64–70.
- Gilutz H, Weinstein JM, Ilia R. Repeated balloon rupture during coronary stenting due to a calcified lesion: an intravascular ultrasound study. Catheter Cardiovasc Interv. 2000;50:212–214.
- Kahn JK, Hartzler GO. Balloon rupture due to lesion morphology during coronary angioplasty. Cathet Cardiovasc Diagn. 1990;21:89–91.
- Bentzon JF, Otsuka F, Virmani R, et al. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866.
- Serruys PW, van Hout B, Bonnier H, et al. Randomised comparison of implantation of heparin-coated stents with balloon angioplasty in selected patients with coronary artery disease (Benestent II). Lancet. 1998;352:673–681.
- Pinto DS, Stone GW, Ellis SG, et al. Impact of routine angiographic follow-up on the clinical benefits of paclitaxel-eluting stents: results from the TAXUS-IV trial. J Am Coll Cardiol. 2006;48:32–36.
- Brown DL, George CJ, Steenkiste AR, et al. High-speed rotational atherectomy of human coronary stenoses: acute and one-year outcomes from the new approaches to coronary intervention (NACI) registry. Am J Cardiol. 1997;80:60K–67K.
- Garcia de Lara J, Pinar E, Ramon Gimeno J, et al. Percutaneous coronary intervention in heavily calcified lesions using rotational atherectomy and paclitaxel-eluting stents: outcomes at one year. Rev Esp Cardiol. 2010;63:107–110.
- Khattab AA, Otto A, Hochadel M, et al. Drug-eluting stents versus bare metal stents following rotational atherectomy for heavily calcified coronary lesions: late angiographic and clinical follow-up results. J Interv Cardiol. 2007;20:100–106.
- Arora S, Panaich SS, Patel N, et al. Coronary atherectomy in the United States (from a nationwide inpatient sample). Am J Cardiol. 2016;117:555–562.
- Chambers JW, Behrens AN, Martinsen BJ. Atherectomy devices for the treatment of calcified coronary lesions. Interv Cardiol Clin. 2016;5:143–151.
- Chambers J, Martinsen B Orbital atherectomy in the coronary arteries. Textb. Atherectomy. 1st ed. HMP Communications, LLC; 2016. p. 217–240.
- Sotomi Y, Shlofmitz R, Colombo A, et al. Patient selection and procedural considerations for coronary orbital atherectomy system. Interv Cardiol Rev. 2016;11:33–38.
- Parikh K, Chandra P, Choksi N, et al. Safety and feasibility of orbital atherectomy for the treatment of calcified coronary lesions: the ORBIT I trial. Catheter Cardiovasc Interv. 2013;81:1134–1139.
- Kini AS, Vengrenyuk Y, Pena J, et al. Optical coherence tomography assessment of the mechanistic effects of rotational and orbital atherectomy in severely calcified coronary lesions. Catheter Cardiovasc Interv. 2015;86:1024–1032.
- Ueda H, Kini A. Optical coherence tomography-guided bioresorbable vascular scaffold implantation with orbital atherectomy for calcified chronic total occlusion. Cath Lab Dig. 2016;24. Available from: http://www.cathlabdigest.com/article/Optical-Coherence-Tomography-Guided-Bioresorbable-Vascular-Scaffold-Implantation-Orbital
- Sotomi Y, Cavalcante R, Shlofmitz RA, et al. Quantification by optical coherence tomography imaging of the ablation volume obtained with the orbital atherectomy system in calcified coronary lesions. EuroIntervention. 2016;12:1126–1134.
- Galougahi KK, Shlofmitz RA, Ben-Yehuda O, et al. Guiding light: insights into atherectomy by optical coherence tomography. JACC Cardiovasc Interv. 2016;9:2362–2363.
- Zheng Y, Belmont B, Shih AJ. Experimental investigation of the abrasive crown dynamics in orbital atherectomy. Med Eng Phys. 2016;38:639–647.
- Sotomi Y, Shammas NW, Suwannasom P, et al. Impact of the orbital atherectomy system on a peripheral calcified lesion: quantitative analysis by intravascular echogenicity. JACC Cardiovasc Interv. 2015;8:e205–e206.
- Galougahi KK, Bhatti N, Shlofmitz R, et al. TCT-236 effects of orbital versus rotational atherectomy facilitated PCI on the coronary microcirculation. J Am Coll Cardiol. 2016;68:B96.
- Bhatt P, Parikh P, Patel A, et al. Orbital atherectomy system in treating calcified coronary lesions: 3-Year follow-up in first human use study (ORBIT I trial). Cardiovasc Revasc Med. 2014;15:204–208.
- Bhatt P, Parikh P, Patel A, et al. Long-term safety and performance of the orbital atherectomy system for treating calcified coronary artery lesions: 5-year follow-up in the ORBIT I trial. Cardiovasc Revasc Med. 2015;16:213–216.
- Chambers JW, Feldman RL, Himmelstein SI, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. 2014;7:510–518.
- Généreux P, Lee AC, Kim CY, et al. Orbital atherectomy for treating de novo severely calcified coronary narrowing (1-year results from the pivotal ORBIT II trial). Am J Cardiol. 2015;115:1685–1690.
- Généreux P, Bettinger N, Redfors B, et al. Two-year outcomes after treatment of severely calcified coronary lesions with the orbital atherectomy system and the impact of stent types: insight from the ORBIT II trial. Catheter Cardiovasc Interv. 2016;88:369–377.
- Lee M, Généreux P, Shlofmitz R, et al. Orbital atherectomy for treating de novo, severely calcified coronary lesions: 3-year results of the pivotal ORBIT II trial. Cardiovasc Revasc Med. 2017;18:261–264.
- Lee MS, Shlofmitz E, Kaplan B, et al. Real-world multicenter registry of patients with severe coronary artery calcification undergoing orbital atherectomy. J Interv Cardiol. 2016;29:357–362.
- Kim CY, Lee AC, Wiedenbeck TL, et al. Gender differences in acute and 30-day outcomes after orbital atherectomy treatment of de novo, severely calcified coronary lesions. Catheter Cardiovasc Interv. 2016;87:671–677.
- Lee MS, Shlofmitz E, Shlofmitz R, et al. Outcomes after orbital atherectomy of severely calcified left main lesions: analysis of the ORBIT II study. J Invasive Cardiol. 2016;28:364–369.
- Lee MS, Martinsen BJ, Shlofmitz R, et al. Orbital atherectomy treatment of severely calcified coronary lesions in patients with impaired left ventricular ejection fraction: one-year outcomes from the ORBIT II study. EuroIntervention. 2017;13:329–337.
- Lee MS, Lee AC, Shlofmitz RA, et al. ORBIT II sub-analysis: impact of impaired renal function following treatment of severely calcified coronary lesions with the orbital atherectomy system. Catheter Cardiovasc Interv. 2017;89:841–848.
- Lee MS, Shlofmitz E, Lluri G, et al. Outcomes in elderly patients with severely calcified coronary lesions undergoing orbital atherectomy. J Interv Cardiol. 2017;30:134–138.
- Shlofmitz E, Meraj P, Jauhar R, et al. Safety of orbital atherectomy in patients with left ventricular systolic dysfunction. J Interv Cardiol. 2017 Jul 19:6. DOI: 10.1111/joic.12405
- Lee MS, Shlofmitz E, Lluri G, et al. Outcomes of patients with myocardial infarction who underwent orbital atherectomy for severely calcified lesions. Cardiovasc Revasc Med. 2017 May 7. DOI:10.1016/j.carrev.2017.05.005.
- Lee MS, Nguyen H, Shlofmitz R. Incidence of bradycardia and outcomes of patients who underwent orbital atherectomy without a temporary pacemaker. J Invasive Cardiol. 2017;29:59–62.
- Lee MS, Shlofmitz E, Lluri G, et al. Impact of impaired renal function in patients with severely calcified coronary lesions treated with orbital atherectomy. J Invasive Cardiol. 2017;29:203–206.
- Lee MS, Shlofmitz E, Mansourian P, et al. Gender-based differences in outcomes after orbital atherectomy for the treatment of de novo severely calcified coronary lesions. J Invasive Cardiol. 2016;28:440–443.
- Lee MS, Shlofmitz E, Nguyen H, et al. Outcomes in diabetic patients undergoing orbital atherectomy system. J Interv Cardiol. 2016;29:491–495.
- Lee MS, Shlofmitz E, Kaplan B, et al. Percutaneous coronary intervention in severely calcified unprotected left main coronary artery disease: initial experience with orbital atherectomy. J Invasive Cardiol. 2016;28:147–150.
- Ruisi M, Zachariah J, Ratcliffe J, et al. Safety and feasibility of the coronary orbital atherectomy system via the transradial approach. J Invasive Cardiol. 2015;27:E252–E255.
- Shlofmitz E, Martinsen B, Lee M, et al. Utilizing intravascular ultrasound imaging prior to treatment of severely calcified coronary lesions with orbital atherectomy: an ORBIT II sub-analysis. J Interv Cardiol. 2017 Aug 7:7. DOI: 10.1111/joic.12423
- Lee MS, Martinsen BJ, Lee AC, et al. Impact of diabetes mellitus on procedural and one year clinical outcomes following treatment of severely calcified coronary lesions with the orbital atherectomy system: a subanalysis of the ORBIT II study. Catheter Cardiovasc Interv. 2017 Jul 22:8. DOI: 10.1002/ccd.27208
- Chambers J, Généreux P, Lee A, et al. The potential cost-effectiveness of the Diamondback 360® coronary orbital atherectomy system for treating de novo, severely calcified coronary lesions: an economic modeling approach. Ther Adv Cardiovasc Dis. 2016;10:74–85.
- Abdel-Wahab M, Richardt G, Joachim Büttner H, et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (rotational atherectomy prior to taxus stent treatment for complex native coronary artery disease) trial. JACC Cardiovasc Interv. 2013;6:10–19.
- de Waha S, Allali A, Büttner H-J, et al. Rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: two-year clinical outcome of the randomized ROTAXUS trial. Catheter Cardiovasc Interv. 2016;87:691–700.
- Généreux P, Redfors B, Witzenbichler B, et al. Two-year outcomes after percutaneous coronary intervention of calcified lesions with drug-eluting stents. Int J Cardiol. 2017;231:61–67.
- Redfors B, Maehara A, Witzenbichler B, et al. Outcomes after successful percutaneous coronary intervention of calcified lesions using rotational atherectomy, cutting balloon angioplasty, or balloon-only angioplasty before drug-eluting stent implantation. J Invasive Cardiol. 2017 Jun 15: 11. pii: JIC2017615-2
- Allali A, Abdel-Wahab M, Sulimov DS, et al. Comparison of bailout and planned rotational atherectomy for heavily calcified coronary lesions: a single-center experience. J Interv Cardiol. 2017;30:124–133.
- Buccheri D, Piraino D, Andolina G, et al. Understanding and managing in-stent restenosis: a review of clinical data, from pathogenesis to treatment. J Thorac Dis. 2016;8:E1150–E1162.
- Witzenbichler B, Maehara A, Weisz G, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. 2014;129:463–470.
- Lee MS, Shlofmitz E, Kaplan B, et al. Outcomes of patients with severely calcified aorto-ostial coronary lesions who underwent orbital atherectomy. J Interv Cardiol. 2017 Sep 4:6. DOI: 10.1111/joic.12432
- Al Suwaidi J, Berger PB, Rihal CS, et al. Immediate and long-term outcome of intracoronary stent implantation for true bifurcation lesions. J Am Coll Cardiol. 2000;35:929–936.
- Iakovou I, Ge L, Colombo A. Contemporary stent treatment of coronary bifurcations. J Am Coll Cardiol. 2005;46:1446–1455.
- Sharma SK, Sweeny J, Kini AS. Coronary bifurcation lesions: a current update. Cardiol Clin. 2010;28:55–70.
- Suárez de Lezo J, Medina A, Martín P, et al. Predictors of ostial side branch damage during provisional stenting of coronary bifurcation lesions not involving the side branch origin: an ultrasonographic study. EuroIntervention. 2012;7:1147–1154.
- Karanasos A, Tu S, van der Heide E, et al. Carina shift as a mechanism for side-branch compromise following main vessel intervention: insights from three-dimensional optical coherence tomography. Cardiovasc Diagn Ther. 2012;2:173–177.
- Chambers JW, Warner C, Behrens A, et al. Outcomes after atherectomy treatment of severely calcified coronary bifurcation lesions: a single center experience (SCAI-A046). Catheter Cardiovasc Interv. 2017;89:S1–S226.
- Shlofmitz E, Chambers J, Lee M, et al. Incidence of temporary transvenous pacemaker placement with coronary orbital atherectomy compared to rotational atherectomy. J Am Coll Cardiol. 2016;67:218.
- Adams GL, Khanna PK, Staniloae CS, et al. Optimal techniques with the Diamondback 360 degrees system achieve effective results for the treatment of peripheral arterial disease. J Cardiovasc Transl Res. 2011;4:220–229.
- Shlofmitz E, Chambers J, Moses JW, et al. TCT-389 temporary pacemaker placement incidence with the diamondback 360® coronary orbital atherectomy system compared to rotational atherectomy. J Am Coll Cardiol. 2015;66:B157.
- Kirtane AJ, Doshi D, Leon MB, et al. Treatment of higher-risk patients with an indication for revascularization: evolution within the field of contemporary percutaneous coronary intervention. Circulation. 2016;134:422–431.
- Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944.