ABSTRACT
Introduction: Intraoperative surgical sealants and hemostatic agents have been shown to reduce postoperative complications, transfusions, and hospital resource utilization. Despite availability of these agents, the incidence and burden of bleeding remains high and surgeons’ requirements for hemostatic control continue to evolve. A burgeoning class of hemostatic agents are hemostatic patches, which offer package-to-patient readiness and direct application. In addition, hemostatic patches may provide tissue sealing capabilities.
Areas covered: This review focuses on the clinical effectiveness, versatility, and surgical efficiency of HEMOPATCH as a surgical sealant and hemostatic agent in various surgical specialties including: cardiac, digestive (hepatic, gastrointestinal, pancreatic), urological, neurological, and endocrine.
Expert commentary: Among hemostatic patches, HEMOPATCH is a valuable tool to stop bleeding without adverse events across various surgical specialties. Clinical evidence demonstrates the safety, clinical effectiveness, and versatility of HEMOPATCH as a unique surgical adjunct in patients undergoing complex and routine surgical procedures. Larger randomized-controlled clinical studies, or clinical registries, will continue to be used to evaluate its performance and versatility, particularly for sealing tissues and closing the dura. In the current field of surgical sealing and hemostasis, however, HEMOPATCH represents the next step in improving patient outcomes.
1. Introduction
1.1. Background
Globally, over 300 million surgical procedures are conducted annually [Citation1]. Overall, 3%–17% of all surgeries result in major complications; approximately 50% of which are considered preventable [Citation2,Citation3]. Bleeding and leakage of body fluids were among the top five most common surgical adverse events at Dutch hospitals of which 26% and 46% were considered preventable, respectively [Citation3]. Eliminating these avoidable complications with the adjunctive use of hemostatic agents to reduce blood loss and surgical sealants to seal suture lines and prevent leaks [Citation4] can contribute to favorable patient outcomes and reduce the additional hospital resource utilization and costs associated with bleeding and leakage-related surgical complications [Citation5,Citation6].
Among the hemostatic agents and surgical sealants, hemostatic patches are being quickly adopted and researched. These agents offer unique versatility for their hemostatic and sealing effects and prevention of complications across various surgical settings [Citation4,Citation7]. Secondarily, these benefits can lead to reductions in the need for multiple products and possibly provide additional cost offsets through more favorable outcomes and reduced hospital resource utilization [Citation8,Citation9]. A recent study found that use of a hemostatic patch, HEMOPATCH, could offset costs or reduce total healthcare costs accounting for product costs, cost of cardiac surgery, incremental cost of revision, and incremental cost of blood transfusion, by €39,958 in Italy, €54,178 in Spain, €29,505 in France, and £24,291 in the UK for an average hospital with an annual caseload of 574 mixed cardiac surgeries relative to standard of care [Citation9].
1.2. Hemostatic pads and patches
The first hemostatic agents were gelatin and oxidized cellulose pads [Citation10,Citation11]. These agents continue to serve as the basis for several hemostatic agents. GELFOAM (Pfizer, Inc., New York, NY, USA) was the first gelatin pad introduced. It induces hemostasis by binding fibronectin to which platelets adhere, spread, and activate [Citation12–Citation14]. SURGICEL Original (Ethicon Inc., Somerville, NJ, USA) was the first oxidized cellulose introduced. It induces hemostasis by generating a primary local hemostyptic action and secondary platelet activation to form a temporary platelet plug [Citation15]. These agents offered a low-cost and high ease-of-use solution to treat mild bleeds.
After these pads were adopted in clinical use, the development of hemostatic agents shifted to fibrin sealants and flowable agents such as TISSEEL Fibrin Sealant (Baxter Healthcare Corporation, Deerfield, IL, USA) [Citation16], EVICEL Fibrin Sealant (Ethicon Inc., Somerville, NH, USA) [Citation16–Citation18], and FLOSEAL Hemostatic Matrix (Baxter Healthcare Corporation) [Citation19,Citation20].
Recently, development has shifted back to hemostatic pads and patches and, specifically, on advanced hemostatic patches that can provide hemostasis and sealant properties similar to the fibrin sealants. The first-generation advanced hemostatic patch, TACHOSIL (Takeda Pharmaceutical Company, Osakashi, Japan), has been utilized by surgeons for over a decade. However, the high burden of uncontrolled bleeding and the surgeon’s unmet need of more immediate hemostasis, safety, and efficacy have led to the development of newer pad technology [Citation21–Citation23]. Among these newer second-generation technologies is HEMOPATCH Sealing Hemostat (Baxter AG, Vienna, Austria), EVARREST Fibrin Sealant Patch (Ethicon Inc., Somerville, NJ, USA) [Citation21,Citation24], and VERISET Hemostatic Patch (Medtronic, Minneapolis, MN, USA) [Citation22,Citation25–Citation27].
While this device profile focuses on HEMOPATCH, it is noteworthy to briefly discuss the differences among these second-generation patches. All advanced hemostatic pads consist of a sheet-like backing and a single, self-binding surface. The backing materials include collagen (HEMOPATCH), neutralized oxidized cellulose (VERISET), or an oxidized cellulose–polyglactin 910 composite (EVARREST); binding agents include fibrinogen and thrombin (EVARREST) or a synthetic, protein-reactive monomer (HEMOPATCH, VERISET). As reported in the literature, collagen provides advantages to the acidic nature of oxidized cellulose and synthetic, protein-reactive monomers provide advantages to human-derived proteins [Citation28]. HEMOPATCH consists of a collagen backing and a protein-reactive polyethylene glycol monomer (PEG-NHS) that exerts its effects by rapidly adhering to and sealing the targeted tissue site [Citation29,Citation30].
In this context, HEMOPATCH is a next-generation topical hemostat (capable of inducing coagulation) and sealant (providing a liquid-tight barrier) that is approved for control of bleeding or leaking of other body fluids or air when conventional techniques are impractical or ineffective and for the closure of dural defects.
HEMOPATCH provides its hemostatic performance via a dual mechanism of action which involves the interaction of two components: rapid adherence to applied tissue due to the electrophilic cross-linking with a protein-reactive polyethylene glycol monomer (PEG-NHS) and collagen mediation of the intrinsic hemostatic action responsible for forming a fibrin clot. The combination of these two independent mechanisms can create rapid and lasting hemostasis by sealing off the bleeding surface and accelerating the body’s clotting mechanism [Citation29].
1.3. HEMOPATCH review objective
In preclinical studies, HEMOPATCH is demonstrated to provide superior hemostatic efficacy as compared to TACHOSIL and SURGICEL [Citation28,Citation31–Citation34]. In another animal study, HEMOPATCH has also been proven to be safe as a dural substitute [Citation34]. In this study, HEMOPATCH’s biocompatibility was similar to a dural regeneration matrix DuraGen XS (Integra LifeSciences Corporation, Plainsboro, NJ, USA), and HEMOPATCH’s dural sealing effectiveness was similar to a fibrin sealant patch, TACHOSIL.
Before now, HEMOPATCH clinical data were limited to case series and reports [Citation35–Citation40]. These data along with the preclinical studies noted above have previously been summarized in two comprehensive review articles [Citation29,Citation36]. Since those reviews, data from randomized-controlled trials (RCTs) and observational studies investigating the utilization and clinical effectiveness of HEMOPATCH across multiple surgical specialties have been published or presented. The purpose of this review is to summarize, synthesize, and perform an in-depth examination of HEMOPATCH’s hemostatic performance in these newly available clinical studies.
2. Literature search
2.1. Search strategy
In August 2017, using OVID and Google Scholar, a literature search was conducted to identify published results from relevant clinical studies evaluating the utilization and clinical effectiveness of HEMOPATCH in human studies. The OVID search used Biosis, Embase, MEDLINE, and International and Pharmaceutical Abstracts. Two search strategies were employed, first utilizing more restrictive keywordsFootnote1 and then using broader keywords.Footnote2 Google Scholar searches used ‘hemopatch’ or ‘haemopatch’ alone or in combination with additional keywords.Footnote3 Database searches were supplemented with references identified by key opinion leaders (KOLs). The Cochrane Database of Systematic Reviews was checked to confirm that no similar review has been undertaken. Similarly, ClinicalTrials.gov was searched; however, results are not included in the literature review as these studies are active and ongoing.
2.2. Inclusion/exclusion criteria
Studies were screened based on a predetermined set of inclusion/exclusion criteria. Studies (journal and research abstract publications) were included that collected data on clinical or resource utilization outcomes of HEMOPATCH use in human subjects and that were published no earlier than 2014. Reviews, editorials, case series, and reports were excluded from the synthesis but noted for discussion in the context of included studies. Publications were translated into English, as required.
2.3. Data extraction and synthesis
Data were extracted from publications into an evidence table. The extracted data included study identifying information as well as study characteristics such as country, study type, number of patients, surgical settings, interventions, and clinical results. Studies were grouped into subcategories by surgery setting (cardiac, digestive, urinary, neurology, and endocrine) and graded according to level of evidence using the U.S. Preventive Services Task Force (USPSTF) Levels of Clinical Evidence [Citation41].
2.4. Search results
Of 1317 citations and abstracts reviewed (literature search: 1313, added by KOLs: 4), only eight studies (literature search: 4, added by KOLs: 4) were included in the literature synthesis including five manuscripts, two research abstract posters, and one research abstract oral presentation ().
Figure 1. Flow diagram of literature search for clinical evidence of HEMOPATCH Sealing Hemostat in various surgical settings.
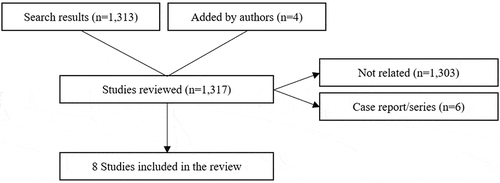
The review includes literature on the use of HEMOPATCH in cardiac (1), digestive (3) (including hepatic (1), gastrointestinal (1), and pancreatic (1)), urological (1), neurological (2), and endocrine (1) procedures () [Citation42–Citation49]. The results summarized in show that HEMOPATCH, when compared to standard of care or other comparator, can improve hemostatic efficacy, and it may also improve surgical outcomes including blood loss, transfusion need, complications, and surgical revision and reduce resource utilization outcomes including theater time and length of hospital stay. No cost outcomes were reported in the reviewed literature. The results summarized in also show that HEMOPATCH, when compared to a similar patch, provides equal sealing efficacy, not improved efficacy. What follows is a summary of this evidence by procedure with additional context provided.
Table 1. Summary of clinical evidence by outcome and surgery setting for HEMOPATCH Sealing Hemostat.
3. Cardiac
In a prospective randomized-controlled study, Weltert et al. [Citation42] investigated the hemostatic efficacy of HEMOPATCH in comparison to that of conventional methods during ascending aortic aneurysm surgery. The primary end point was the cessation of moderate, non-pulsating bleeds from the suture line of the aortic Dacron graft within 3 minutes of applying the hemostatic agent. Secondary endpoints included post-surgical blood loss, the number of units of autologous blood transfused, and surgical revision rates due to bleeding within 4 days of surgery.
HEMOPATCH had a statistically significant higher rate of hemostatic success than that of conventional treatments, achieving hemostasis within 3 minutes of application at the assessed suture line (p < 0.001). HEMOPATCH-treated patients also had statistically significant less postsurgical bleeding compared with the conventional treatment group at the 2-hr (p = 0.01) and 6-hr (p = 0.02) time points. Autologous transfusions (p = 0.24) and surgical revisions due to bleeding (p = 0.30) were also lower in the HEMOPATCH group (not statistically significant). The authors cite the low number of patients (n = 85 per group) as a possible reason for non-statistical significance [Citation42].
Between 2.7% and 29% [Citation50] of cardiac patients experience severe perioperative bleeding leading to transfusion of blood products [Citation5] and surgical revision [Citation51–Citation53], which negatively impacts surgical time, readmission, long-term patient outcomes, and healthcare costs [Citation54]. In cardiac applications, HEMOPATCH has prevented suture-line bleeding without the need for surgical revision and the benefit of the flexibility of a sizeable patch that is effective in otherwise difficult placements [Citation36]. Weltert et al. [Citation42] confirmed the improvement in time to hemostasis, transfusion rates, drain output or blood loss, and surgical revision when using HEMOPATCH [Citation5,Citation52,Citation55].
4. Digestive
4.1. Gastrointestinal
Torres et al. [Citation47] presented research abstract data derived from an observational study and noted that intestinal suture or bowel anastomosis reinforcement with HEMOPATCH might contribute to the reduction of surgical complications such as dehiscence, leaks, and fistula formation. The study included 12 peritoneal carcinomatosis patients who underwent complete cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy (CRS-HIPEC using coliseum technique). In all subjects, HEMOPATCH was applied to cover up to 1 cm beyond the 5 colo-colonic, 3 ileorectal, and 4 jejunojejunal mechanical suture lines. Compared to an anastomotic dehiscence rate of 7.5% in series of 305 CRS-HIPEC patients, clinical and radiological examinations revealed no anastomotic leakage or dehiscence in any of the 12 patients treated with HEMOPATCH.
Gastrointestinal surgery applications of HEMOPATCH have shown good adherence to irregular surfaces without anastomotic leakage or further bleeding complications in case studies [Citation36]. The observational study by Torres et al. [Citation47] reported no complications such as leakage, dehiscence of the anastomosis, and fistulas. Future publication of the research would provide more insight into the study methods and clinical outcomes.
4.2. Hepatic
In a peer-reviewed research abstract poster, Scheurer et al. [Citation43] presented limited findings from an RCT investigating the efficacy of HEMOPATCH in controlling intraoperative bleeding during hepatic resections compared to TACHOSIL. Patients were included and randomized if the surgeon asked for a hemostatic patch during surgery. Each group consisted of 50 patients, wherein 87 units of HEMOPATCH and 84 units of TACHOSIL were used in total. To determine the hemostatic success rate of the patches, surgeons evaluated each patch based on five qualitative properties (properties not specified in the abstract) and considered a patch application a success only if it met all five qualities. The authors reported no statistically significant differences in the hemostatic success rate of HEMOPATCH and TACHOSIL (p = 0.5).
Case studies of HEMOPATCH in liver surgery have shown good adherence to irregular and cauterized surfaces with good outcomes including stopping residual oozing and no need for blood transfusion [Citation36]. HEMOPATCH’s ability to tightly adhere independent of the coagulation cascade and unaffected by body fluids is beneficial in these applications. Although no statistical difference was found when comparing the success of HEMOPATCH vs. TACHOSIL for liver resection (as noted above) [Citation43], superior performance was noted for gastrointestinal and pancreatic applications relative to standard of care [Citation45,Citation47].
4.3. Pancreatic
Serradilla et al. [Citation45] presented the research abstract results of a prospective observational study that examined the ability of HEMOPATCH to prevent postsurgical pancreatic fistula formation following duct-to-mucosa pancreaticojejunostomies. HEMOPATCH was used in 13 of 26 consecutive pancreaticoduodenectomies performed between July 2015 and October 2016. The rate of postoperative complications in the HEMOPATCH group was compared to that of a control group (n = 13); both groups were considered statistically homogeneous.
The HEMOPATCH group demonstrated lower postsurgical complication rates compared to the control group in overall pancreatic and biliary fistulae formation, bleeding, hospital readmission, mean hospital stay, and mortality (see ). However, the authors noted a higher rate of other complications with the HEMOPATCH group (61.5% vs. 46.2%) without describing their nature [Citation45]. Publication of the observational study would provide more insight into the study methods including control group procedures and clinical outcomes associated with hemostatic agents.
5. Urology
Imkamp et al. [Citation46] presented, in a research abstract, the results of a prospective observational study evaluating the use of HEMOPATCH by a single, experienced surgeon during zero-ischemia laparoscopic partial nephrectomies (LPNs) (16 patients) and LPNs with renal artery clamping (3 patients). Suspected solid renal masses, the reason for the nephron-sparing surgeries, ranged from 8.79 to 74 mm (median: 37 mm) in size. Preoperative Aspects and Dimensions Used for an Anatomical (PADUA) and Radius, Exophytic/endophytic properties, Nearness of tumor deepest portion to the collecting system or sinus, Anterior/posterior descriptor, Location relative to polar line (RENAL) nephrometry scores ranged from 6 to 8 (median: 7) and 4 to 8 (median: 5), respectively. Only 17 of the 19 patients required parenchymal suturing at the resection site [Citation46].
HEMOPATCH successfully provided hemostasis in all cases. The median operation duration, blood loss, and hospitalization length were 139 min (range 103–194), 325 mL (range 50–700), and 6 days (range 4–8), respectively. The only postoperative complication reported was the development of pneumonia by a single patient which required conservative treatment [Citation46].
When aggressive surgery is potentially dangerous to surrounding organs or tissues, HEMOPATCH can successfully control bleeding as demonstrated in a case study of an extensive resection where energy-driven hemostasis would potentially damage the ureter [Citation36]. Imkamp et al. [Citation46] have confirmed the success of HEMOPATCH for LPN in draining output or bleed amount, surgery time, length of stay, and other complications. The authors noted that in comparison with previously used hemostatic agents, HEMOPATCH was advantageous in urologic applications [Citation35]. Additionally, the authors’ previous study noted the favorable porosity and material memory facilitating intracorporal handling as well as the ability to rapidly and tightly adhere to persistent oozing [Citation35].
6. Neurology
González et al. [Citation48], in a retrospective observational study, evaluated the safety and efficacy of HEMOPATCH in heterologous duraplasty. Dural reconstruction procedures included 64 craniotomies, 58 spinal detachments, 72 endoscopies, and 6 others. Patients were considered at high risk for developing postsurgical fistulae due to underlying factors such as young age, posterior fossa lesions, ventricular system opening lesions and endoscopies, and extensive dural reconstructions in complex dysraphia undergoing spinal detachment [Citation48].
No complications secondary to surgical or staining materials such as infection, cerebrospinal fluid (CSF) fistula, or pseudo-meningocele, etc., were identified up to 2 years after the dural reconstruction was performed. Notably, there was a reduction in the incidence of CSF fistula from 26% pre-surgery to 8% post-surgery (varied by indication). The authors demonstrated HEMOPATCH to be a safe and effective for dural sealing, providing an impervious reinforcement even in patients at high risk of developing CSF fistulae [Citation48].
In another neurosurgery prospective observational study, Tardáguila et al. [Citation49] examined the hemostatic and sealing efficacy of HEMOPATCH in dura closures. One hundred and thirty-six procedures were performed (90 craniotomies, 30 endonasal procedures, and 16 spinal procedures). HEMOPATCH was applied to non-sutured dural defects during craniotomies, sites with difficult to control venous bleeding such as sinus tears, suture lines during spinal procedures, and the reconstruction floor during endonasal procedures.
Computed tomography (CT) scans performed 24 hr after the craniotomies revealed five non-symptomatic hematomas (no intervention required) and a single CSF fistula (required surgical repair). Two cases of CSF fistulae formations developed following endonasal surgery (one required external lumbar drainage, one resolved with rest). No complications occurred after the spinal procedures, and no postsurgical infections occurred [Citation49].
CSF fistulae formation and its complications such as meningitis, pseudo-meningocele, pneumocephalus, and hydrocephalus are particularly of concern during duraplasty. Tardáguila et al. [Citation49] noted HEMOPATCH was easy to apply and had no associated complications.
7. Endocrine
Ruggiero et al. [Citation44] in a single-center prospective observational study investigated the hemostatic efficacy and safety of HEMOPATCH in patients undergoing total thyroidectomies performed using a harmonic scalpel. Thirty patients were enrolled to receive HEMOPATCH and 30 to receive standard hemostatic treatment (gauze, ligature, electrocauterization). Apart from the type of thyroid disease, which differed slightly, the groups had similar demographic and baseline characteristics [Citation44].
HEMOPATCH achieved a statistically significant better hemostatic performance compared to standard hemostatic treatment and was reported to be safe and effective as an adjunct hemostatic agent for total thyroidectomy. The mean drain output over 24 hr and mean surgery time in the HEMOPATCH group was statistically significantly lower than in the standard treatment group (p < 0.0001). No surgical complications were reported in the HEMOPATCH group compared to three reports in the standard group: one case of dysphonia with a saturated O2 of 98% and two cases of temporary laryngeal nerve paralysis that resolved within 3 months of surgery. Postsurgical seroma formation was statistically significantly less with HEMOPATCH than with standard treatment (p < 0.0001) [Citation44].
In a case study of thyroid surgery, neither hypocalcemia nor aerodigestive symptoms were observed, and HEMOPATCH avoided damaging the nerve with energy-driven hemostasis or suture [Citation36]. The prospective study of HEMOPATCH in thyroidectomy by Ruggiero et al. [Citation44] confirmed a statistically significant improvement compared to other standard treatments in draining output or bleed amount, surgery time, and dry surgical field but no clinically significant difference in hypocalcemia.
8. Conclusion
Based on the clinical studies published to date and summarized above, HEMOPATCH is more effective for hemostasis when compared to other standard therapies. The clinical effectiveness and versatility have been shown across multiple surgical specialties (cardiac, gastrointestinal, pancreatic, urological, neurological, and endocrine), key clinical outcomes (reduced blood loss, transfusion need, complications, and surgical revision when compared to other standard therapies), and resource utilization outcomes (reduced theater time and length of hospital stay when compared to other standard therapies). In contrast, HEMOPATCH is only equally effective for sealing when compared to other standard therapies. To this end, additional studies are needed to explore its sealing capabilities in relevant surgical specialties.
9. Expert commentary
The findings of this review confirm the clinical effectiveness and ease of use of HEMOPATCH, an advanced hemostatic patch, reported by European general, cardiac, pulmonary, and urological surgeons [Citation56] and previously reported in case studies. These experiences further demonstrate the clinical predictiveness of earlier preclinical models. Although only a small number of RCT and observational studies were available to review for HEMOPATCH, they provide the best evidence to date of comparative clinical effectiveness for multiple surgical procedures including cardiac, hepatic, gastrointestinal, pancreatic, urological, neurological, and endocrine.
Surgeons of many surgical specialties are further investigating the effectiveness and versatility of HEMOPATCH. Ten clinical trials of HEMOPATCH are underway for liver resection (3), lung surgery/resection (3), coronary artery bypass graft surgery (1), laparoscopic cholecystectomy (1), rectal anastomosis (1), and renal transplant (1) [Citation57–Citation66]. Investigators of these studies currently in recruitment should be careful to adequately power their studies to potentially show statistically significant differences between treatment and control groups, where applicable. These additional studies should also further investigate the sealing capabilities of HEMOPATCH, which, although equal, are not yet demonstrated to be improved relative to other existing therapies.
In addition to primary clinical outcomes, economic outcomes should be included as secondary endpoints in future studies to investigate the value of HEMOPATCH for healthcare providers. Reductions in complications that increase hospital costs [Citation67,Citation68] could produce cost offsets when using HEMOPATCH. Recent health economic and outcome research finds that hemostatic agents provide a statistically significant benefit to patients and healthcare providers by improving outcomes and reducing costs. A hospital cost analysis in soft tissue and hepatic surgical bleeding predicted a net hospital cost savings with fibrin sealant patches of $2,846 per patient (range = $1,483–$5,575) with the greatest net hospital cost savings of $9,287 per patient in the coagulopathic subgroup [Citation8]. In a retrospective analysis of patients treated with hemostats using the Premier Perspectives Database, uncontrolled bleeding despite hemostat use occurred in 57% of cases (range = 32%–68%) and was associated with higher hospital costs compared to cases with bleeding controlled by hemostat use (uncontrolled: range = $24,203–$61,323, controlled: range = $14,420–$45,593, p < 0.001) indicating a continued unmet need for newer hemostats [Citation23]. Among the classes of hemostatic agents, hemostatic patches also provide benefit to patients and healthcare providers.
A cost-effectiveness analysis of HEMOPATCH versus standard of care in cardiac surgery from a European hospital perspective shows the potential cost offsets from and cost-effectiveness of using HEMOPATCH [Citation9]. When conservatively considering only product acquisition cost, HEMOPATCH was shown to be cost-effective in Italy, Spain, France, and the UK with incremental cost-effectiveness ratios (ICERs) of €1,659, €1,519, €1,623, and £1,725, respectively, per hemostasis success (within 3 minutes) relative to standard of care [Citation9]. The authors refer the reader to the manuscript by Weltert et al. [Citation9] for the full calculation of the ICER. This analysis identified the benefits of using HEMOPATCH to be derived from the potential to improve patient outcomes and offset costs of transfusions, readmissions, surgical revisions, surgery time, as well as other surgical complications. The results are supported in additional surgical settings by cost research on other advanced hemostat products.
Cost studies of a novel fibrin sealant matrix, EVARREST, compared to standard of care have shown cost savings for hepatic surgery in a US hospital setting [Citation8] and for soft tissue surgery in Italian, German, and UK hospital settings [Citation69–Citation71]. Similarly, a retrospective cost analysis of brain/cerebral surgery, cardiovascular surgery, and carotid endarterectomy using a US hospital database found lower resource utilization and costs associated with the use of SURGICEL advanced products [Citation72]. While many of these agents provide benefit over standard of care, future research should focus on comparing the advanced topical hemostats. This type of research can help guide clinical use as it did for flowable hemostatic matrices [Citation73–Citation75].
10. Five-year view
Advancements in the next 5 years will focus on improving patient outcomes and reducing resource utilization through novel biomaterials that increase versatility and efficacy of hemostatic agents. There is early evidence for HEMOPATCH usability as a hemostat and sealant, and the authors anticipate further clinical evidence to be generated given the initial, positive efficacy. Hemostatic agents have evolved––and will continue to evolve––to achieve this goal.
HEMOPATCH represents the next step in the evolution of hemostatic patches. Its clinical evidence supports the importance of adopting blood conservation strategies, which include the use of hemostatic agents to stop intraoperative bleeding [Citation76–Citation78]. Doing so correlates to improved patient outcomes, surgical efficiency, and resource utilization (e.g. reduce blood loss, transfusions, complications, surgical revisions, theater time and length of hospital stay). To this end, the expanded use of hemostatic agents, such as HEMOPATCH, will also be tied to the evidence of cost-effectiveness compared to the standard of care, which requires that economic data be collected and analyzed in combination with clinical efficacy.
As hemostatic agents evolve further, the move to more synthetic agents and/or recombinant proteins will likely improve the clinical effectiveness and cost-effectiveness profiles as evidenced by clinical and cost analysis of hemostatic patches that utilize human-derived proteins. Furthermore, the increased versatility of these agents––such as being able to act as a surgical sealant, hemostatic agent and/or antimicrobial agent––will drive the value proposition for healthcare providers. Collectively, these advancements must be driven by improved patient outcomes to be successful.
In next 5 years, the authors foresee that research related to HEMOPATCH will focus in four areas. The first is that its hemostatic properties will continually be investigated across several surgical specialties. The second is that its sealing properties will become a greater research focus. The initial studies are promising in this regard [Citation28,Citation33,Citation34,Citation47,Citation48]; however, additional research is needed to investigate whether HEMOPATCH provides improved sealing relative to other treatments. Furthermore, research on the concurrent use of bicarbonate is of interest as it is known to accelerate and strengthen the adherence of HEMOPATCH to tissue. The third area, as noted above, is in terms of health economic and outcome data. The last area of focus is comparative studies among the various hemostatic patches. While there are multiple new hemostatic patches commercially available (i.e. EVARREST, VERISET), there is little research to the advantages and disadvantages of each. These agents will likely be compared in all three of the previously mentioned dimensions: hemostatic efficacy, sealing efficacy and health economic outcomes.
Key issues
Hemostatic agents continue to evolve with a focus on efficacy and ease of use. HEMOPATCH Sealing Hemostat represents an advanced hemostatic agent and provides clinical versatility in multiple surgical specialties with benefits for improved patient outcomes and resource utilization.
Cardiac: A recent RCT and cost model confirms that HEMOPATCH prevents suture-line bleeding in cardiac applications without the need for surgical revision and the benefit of the flexibility of a sizeable patch that is effective in otherwise difficult placements with cost offsets for hospitals.
Gastrointestinal: Gastrointestinal surgery applications of HEMOPATCH have shown good adherence to irregular surfaces without anastomotic leakage or further bleeding complications in case studies and no complications such as leakage, dehiscence of the anastomosis, and fistulas in an observational study.
Hepatic: Applications of HEMOPATCH in hepatic surgery have shown good adherence to irregular and electrocoagulated surfaces with good outcomes including stopping residual oozing and no need for blood transfusion.
Pancreatic: An observational study of HEMOPATCH in pancreatic surgery confirms reductions in length of stay, surgical complications and mortality also shown in case studies that reported HEMOPATCH prevented bleeding with no postoperative complications and no fistula formation.
Urology: A prospective observational study showed the success of HEMOPATCH for laparoscopic partial nephrectomy reporting draining output/bleed amount, surgery time, length of stay, and other complications.
Neurology: Cerebrospinal fluid fistulae formation and its complications are particularly of concern during duraplasty, but HEMOPATCH controlled difficult bleeds with no associated complications.
Endocrine: A prospective study of HEMOPATCH in thyroidectomy confirmed statistically significant improvement compared to other standard treatments in draining output/bleed amount, surgery time, and dry surgical field.
Although RCT and observational clinical studies in this review did not include economic outcomes, the reduction of preventable adverse events related to surgery when using HEMOPATCH compared to standard of care or other comparator has the potential to offset hospital costs and be a cost-effective alternative.
Declaration of interest
K Lewis, S Ikeme, and CE Kuntze are employees of Baxter International Inc. subsidiaries. T Olubunmi was retained by Baxter for this work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. One peer reviewer has disclosed that they have worked on various hemostat products in the past for different companies as a consultant and currently have staff working on another hemostat project. Peer reviewers have no other relevant financial relationships to disclose.
Acknowledgments
The authors thank Barbara Blaylock and Josh Epstein of Stratevi (Santa Monica, CA) for their concept development and writing assistance during the preparation of the manuscript, and Manuel Ramirez of Baxter Healthcare Corporation for his scientific contributions and health economic and outcome insights. A significant amount of appreciation goes to the numerous researchers, clinicians and scientific investigators that contributed to the body of evidence for the named hemostatic agents.
Additional information
Funding
Notes
1. Restrictive keywords: hemopatch OR hemopatch OR ‘hemo-patch’ OR ‘hemo-patch’ OR (‘polyethylene glycol’ adj5 collagen) OR ((‘polyethylene glycol’ OR ‘n-hydroxylsuccinimide’ OR ‘n hydroxylsuccinimide’ OR peg OR (hydroxylsuccinimide adj2 polyeth*) OR ((collagen OR hemostat* OR hemostat*) adj2 seal*)) adj5 (patch* OR pad*4)).
2. Broader keywords: ((‘polyethylene glycol’ OR ‘n-hydroxylsuccinimide’ OR ‘n hydroxylsuccinimide’ OR peg OR (hydroxylsuccinimide adj2 polyeth*) OR collagen OR hemostat* OR hemostat*) adj5 (seal*5 OR patch*4 OR pad*4)).
3. Collagen pad OR collagen patch OR NHS-PEG OR n-hydroxylsuccinimide polyethylene glycol OR n-hydroxylsuccinimide functionalized polyethylene glycol OR collagen sealant.
References
- Weiser TG, Haynes AB, Molina G, et al. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet. 2015;385(Suppl 2):S11.
- Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–499.
- Zegers M, de Bruijne MC, de Keizer B, et al. The incidence, root-causes, and outcomes of adverse events in surgical units: implication for potential prevention strategies. Patient Saf Surg. 2011;5:13.
- Bracey A, Shander A, Aronson S, et al. The use of topical hemostatic agents in cardiothoracic surgery. Ann Thorac Surg. 2017;104:353–360.
- Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135.
- Govaert JA, Fiocco M, van Dijk WA, et al. Costs of complications after colorectal cancer surgery in the Netherlands: building the business case for hospitals. Eur J Surg Oncol. 2015;41:1059–1067.
- Samudrala S. Topical hemostatic agents in surgery: a surgeon’s perspective. Aorn J. 2008;88:S2–S11.
- Corral M, Ferko N, Hogan A, et al. A hospital cost analysis of a fibrin sealant patch in soft tissue and hepatic surgical bleeding. Clinicoecon Outcomes Res. 2016;8:507–519.
- Ikeme S, Weltert L, Lewis K, et al. Cost-effectiveness analysis of a sealing hemostat patch (HEMOPATCH) vs standard of care in cardiac surgery. J Med Econ. 2018;21(3):273-281.
- Frantz VK, Lattes R. Oxidized cellulose-absorbable gauze (cellulosic acid). Jama. 1945;129(12):798–801.
- Jenkins HP, Janda R, Clarke J. Clinical and experimental observations on the use of gelatin sponge or foam. Surgery. 1946;20(1):124–132.
- Engvall E, Ruoslahti E, Miller E. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978;147(6):1584–1595.
- Winters KJ, Walsh J, Rubin B, et al. Platelet interactions with fibronectin: divalent cation-independent platelet adhesion to the gelatin-binding domain of fibronectin. Blood. 1993;81(7):1778–1786.
- Xu J, Mosher D. Fibronectin and other adhesive glycoproteins. In: Mecham R, editor. The Extracellular Matrix: An Overview. Berlin, Springer Science & Business Media; 2011. pp. 41–75.
- Miller J, Jackson D, Collier C. An investigation of the chemical reactions of oxidized regenerated cellulose. Exp Med Surg. 1961;19:196.
- Hickerson WL, Nur I, Meidler R. A comparison of the mechanical, kinetic, and biochemical properties of fibrin clots formed with two different fibrin sealants. Blood Coagul Fibrinolysis. 2011;22(1):19–23.
- Gugerell A, Schossleitner K, Wolbank S, et al. High thrombin concentrations in fibrin sealants induce apoptosis in human keratinocytes. J Biomed Mater Res A. 2012;100(5):1239–1247.
- Dhillon S. Fibrin sealant (evicel® [quixil®/crosseal™]): a review of its use as supportive treatment for haemostasis in surgery. Drugs. 2011;71(14):1893–1915.
- Renkens KL Jr, Payner TD, Leipzig TJ, et al. A multicenter, prospective, randomized trial evaluating a new hemostatic agent for spinal surgery. Spine (Phila Pa 1976). 2001;26(15):1645–1650.
- Oz MC, Cosgrove DM 3rd, Badduke BR, et al. Controlled clinical trial of a novel hemostatic agent in cardiac surgery. The Fusion Matrix Study Group. Ann Thorac Surg. 2000;69(5):1376–1382.
- Fischer CP, Bochicchio G, Shen J, et al. A prospective, randomized, controlled trial of the efficacy and safety of fibrin pad as an adjunct to control soft tissue bleeding during abdominal, retroperitoneal, pelvic, and thoracic surgery. J Am Coll Surg. 2013;217(3):385–393.
- Öllinger R, Mihaljevic AL, Schuhmacher C, et al. A multicentre, randomized clinical trial comparing the Veriset™ haemostatic patch with fibrin sealant for the management of bleeding during hepatic surgery. Hpb. 2013;15(7):548–558.
- Corral M, Ferko N, Hollmann S, et al. Health and economic outcomes associated with uncontrolled surgical bleeding: a retrospective analysis of the premier perspectives database. Clinicoecon Outcomes Res. 2015;7:409–421.
- Koea JB, Batiller J, Aguirre N, et al. A multicentre, prospective, randomized, controlled trial comparing EVARREST™ fibrin sealant patch to standard of care in controlling bleeding following elective hepatectomy: anatomic versus non-anatomic resection. HPB (Oxford). 2016;18(3):221–228.
- Glineur D, Hendrikx M, Krievins D, et al. A randomized, controlled trial of Veriset™ hemostatic patch in halting cardiovascular bleeding. Med Devices (Auckl). 2018;8(11):65–75.
- Howk K, Fortier J, Poston R. A novel hemostatic patch that stops bleeding in cardiovascular and peripheral vascular procedures. Ann Vasc Surg. 2016;31:186–195.
- Schuhmacher C, Pratschke J, Weiss S, et al. Safety and effectiveness of a synthetic hemostatic patch for intraoperative soft tissue bleeding. Med Devices (Auckl). 2015;8:167–174.
- Lewis KM, Spazierer D, Slezak P, et al. Swelling, sealing, and hemostatic ability of a novel biomaterial: a polyethylene glycol-coated collagen pad. J Biomater Appl. 2014;29:780–788.
- Lewis KM, Kuntze CE, Gulle H. Control of bleeding in surgical procedures: critical appraisal of HEMOPATCH (Sealing Hemostat). Med Devices (Auckl). 2016;9:1–10.
- Böhm S, Strauß C, Stoiber S, et al. Impact of source and manufacturing of collagen matrices on fibroblast cell growth and platelet aggregation. Materials. 2017;10(9):1086.
- Lewis KM, McKee J, Schiviz A, et al. Randomized, controlled comparison of advanced hemostatic pads in hepatic surgical models. ISRN Surg. 2014;2014:930803.
- Lewis KM, Schiviz A, Hedrich H-C, et al. Hemostatic efficacy of a novel, PEG-coated collagen pad in clinically relevant animal models. Int J Surg. 2014;12:940–944.
- Baumgartner B, Draxler W, Lewis KM. Treatment of severe aortic bleeding using hemopatch in swine on dual antiplatelet therapy. J Invest Surg. 2016;29:343–351.
- Lewis KM, Sweet J, Wilson ST, et al. Safety and efficacy of a novel, self-adhering dural substitute in a canine supratentorial durotomy model. Neurosurgery. 2018;82(3):397–406.
- Imkamp F, Tolkach Y, Wolters M, et al. Initial experiences with the Hemopatch® as a hemostatic agent in zero-ischemia partial nephrectomy. World J Urol. 2015;33:1527–1534.
- Fingerhut A, Uranues S, Ettorre G, et al. European initial hands-on experience with HEMOPATCH, a novel sealing hemostatic patch: application in general, gastrointestinal, biliopancreatic, cardiac, and urologic surgery. Surg Technol Int. 2014;25:29–35.
- Jainandunsing JS, Al-Ansari S, Woltersom BD, et al. Novel hemostatic patch achieves sutureless epicardial wound closure during complex cardiac surgery, a case report. J Cardiothorac Surg. 2015;10:12.
- Prestipino F, Nenna A, Casacalenda A, et al. Ventricular perforation by pacemaker lead repaired with two hemostatic devices. Int J Surg Case Rep. 2014;5:906–908.
- Sandrio S, Purbojo A, Cesnjevar RA, et al. Hemopatch application for ventricular wall laceration in redo cardiac surgical procedures. Ann Thorac Surg. 2016;101:752–753.
- Novotný R, LesensKy J, Hrubý J, et al. Chondrosarcoma resection followed by a branched crural revascularization of the right calf: case report. Cor Et Vasa. 2016;58:e470–e473.
- U.S. Prevetative Services Task Force. Guide to clinical preventative services: report of the U.S. Preventative Services Task Force. [place unknown]: DIANE Publishing, 1989. Appendix A, Task Force Ratings; p.263.
- Weltert L, D’Aleo S, Chirichilli I, et al. Prospective randomized clinical trial of HEMOPATCH topical sealant in cardiac surgery. Surg Technol Int. 2016; XXIX. pii: sti29/756. [Epub ahead of print].
- Scheurer S, Schultz N, Storkholm J, et al. Comparison of two hemostatic agents (Hemopatch™ vs. TachoSil™) in liver resection, A clinical randomised trial. Hpb. 2016;18:e268.
- Ruggiero R, Docimo L, Tolone S, et al. Effectiveness of an advanced hemostatic pad combined with harmonic scalpel in thyroid surgery, A prospective study. Int J Surg. 2016;28:S17–S21.
- Serradilla M, Palomares A, Paterna S, et al. Sealing with NHS-PEG patch to prevent postoperative pancreatic fistula after pancreatojejunostomy. Poster presented at: 12th Biennial Congress of the European–African Hepato-Pancreato-Biliary Association; Mainz, Germany 2017.
- Imkamp F, Von Klot C, Wolters M, et al. The role of Hemopatch® in zero ischemia laparoscopic partial nephrectomy. Eur Urol Supplements. 2016;15:e858.
- Torres Melero J, Vargas Fernández M, Lorenzo L, et al. Use of the hemopatch resorbable collagen-based sealing hemostat to reinforce bowel anastomoses made in patients with peritoneal carcinomatosis treated with curative intent by complete cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy (CRS + HIPEC). Poster presented at: XXXI Congreso Nacional de Cirugia Asociación Española de Cirgujanos; Madrid, Spain 2016.
- González Pombo M, Rivero Garvia M, Rocha Romero S, et al. Dural reconstruction with heterologous plastia of collagen coated with NHS-PEG (pentaerythritol polyethylene glycol ether tetrasuccinimidyl glutarate). Lessons learned and experience in 200 cases. Oral presented at: XXI Congreso de la Sociedad Española de Neurocirugía; Barcelona, Spain 2017.
- Tardáguila Serrano M, Gonçalves Ramírez FJ, Rimbau Muñoz J, et al. Use of hemostatic patterns as dural seals. Poster presented at: XXI Congreso de la Sociedad Española de Neurocirugía; Barcelona, Spain 2017.
- Vuylsteke A, Pagel C, Gerrard C, et al. The papworth bleeding risk score: a stratification scheme for identifying cardiac surgery patients at risk of excessive early postoperative bleeding. Eur J Cardiothorac Surg. 2011;39:924–930.
- Biancari F, Tauriainen T, Perrotti A, et al. Bleeding, transfusion and the risk of stroke after coronary surgery: A prospective cohort study of 2357 patients. Int J Surg. 2016;32:50–57.
- Karthik S, Grayson AD, McCarron EE, et al. Reexploration for bleeding after coronary artery bypass surgery: risk factors, outcomes, and the effect of time delay. Ann Thorac Surg. 2004;78:527–534, discussion 534
- Vivacqua A, Koch CG, Yousuf AM, et al. Morbidity of bleeding after cardiac surgery: is it blood transfusion, reoperation for bleeding, or both? Ann Thorac Surg. 2011;91:1780–1790.
- Redžek A, Mironicki M, Gvozdenović A, et al. Predictors for hospital readmission after cardiac surgery. J Card Surg. 2015;30:1–6.
- Murphy GJ, Angelini GD. Indications for blood transfusion in cardiac surgery. Ann Thorac Surg. 2006;82:2323–2334.
- Ulrich F, Ettorre GM, Weltert L, et al. Intra-operative use of hemopatch®: interim results of a nationwide European survey of surgeons. Surg Technol Int. 2016;28:19–28.
- ClinicalTrials.gov [Internet]. Identifier NCT02364791, A Phase II Prospective, Single Blinded, Randomized Trial of Hemopatch Compared to Standard Techniques to Achieve Air Leak Control After Complex Thoracic Surgical Procedures (Hemopatch) Bethesda (MD): National Library of Medicine (US); 2015 [updated 2017 Mar 29; cited 2017 Mar 29]. Available from: https://clinicaltrials.gov/show/NCT02364791
- ClinicalTrials.gov [Internet]. Identifier NCT02633670, Hemopatch Versus No Hemopatch (Renal Transplant) Bethesda (MD): National Library of Medicine (US); 2015 [updated 2017 Oct 4; cited 2017 Oct 4]. Available from: https://clinicaltrials.gov/show/NCT02633670
- ClinicalTrials.gov [Internet]. Identifier NCT02589483, Reinforcement of Rectal Anastomosis-RORA Bethesda (MD): National Library of Medicine (US); 2015 [updated 2017 Apr 13 cited 2017 Apr 13]. Available from: https://clinicaltrials.gov/show/NCT02589483
- ClinicalTrials.gov [Internet]. Identifier NCT02491671, Effectiveness Novel Tissue Sealant, Prevention Prolonged Air Leak (PAL) After Lung Resection Bethesda (MD): National Library of Medicine (US); 2015 [updated 2017 Oct 10; cited 2017 Oct 10]. Available from: https://clinicaltrials.gov/show/NCT02491671
- ClinicalTrials.gov [Internet]. Identifier NCT02769754, Effectiveness of the Use of the New Hemostatic Patch Hemopatch ® in Patients Undergoing Surgical Liver Resection (HEMOPATCH) Bethesda (MD): National Library of Medicine (US); 2016 [updated 2016 Sep 7; cited 2016 Sep 7]. Available from: https://clinicaltrials.gov/show/NCT02769754
- ClinicalTrials.gov [Internet]. Identifier NCT02777307, Demonstrate the Effectiveness to Hemopatch in Controlling Postoperative Bleeding After Laparoscopic Cholecistectomy and in Reducing of Morbidity and Postoperative Hospital Stay Bethesda (MD): National Library of Medicine (US); 2016 [updated 2016 May 19; cited 2016 May 19]. Available from: https://clinicaltrials.gov/show/NCT02777307
- ClinicalTrials.gov [Internet]. Identifier NCT02668978, SEALLS (Sealing Evaluation of Air Leaks After Lung Surgery) Trial Using HEMOPATCH Bethesda (MD): National Library of Medicine (US); 2016 [updated 2016 Feb 1; cited 2016 Feb 1]. Available from: https://clinicaltrials.gov/show/NCT02668978
- ClinicalTrials.gov [Internet]. Identifier NCT03340090, Assessment of the Usefulness of Hemopatch in Coronary Artery Bypass Graft Surgery (Hemopatch) Bethesda (MD): National Library of Medicine (US); 2017 [updated 2017 Nov 14; cited 2017 Nov 14]. Available from: https://clinicaltrials.gov/show/NCT03340090
- ClinicalTrials.gov [Internet]. Identifier NCT03323359, Efficacy and Tolerability of Hemopatch After Hepatic Resection Bethesda (MD): National Library of Medicine (US); 2017 [updated 2017 Nov 8; cited 2017 Nov 8]. Available from: https://clinicaltrials.gov/show/NCT03323359
- ClinicalTrials.gov [Internet]. Identifier NCT03166683, Standard of Care Versus Hemopatch® During Liver Resection (IBERLIVER) Bethesda (MD): National Library of Medicine (US); 2017 [updated 2017 Nov 9; cited 2017 Nov 9]. Available from: https://clinicaltrials.gov/show/NCT03166683
- Baratti D, Scivales A, Balestra MR, et al. Cost analysis of the combined procedure of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Eur J Surg Oncol. 2010;36:463–469.
- Daskalaki D, Butturini G, Molinari E, et al. A grading system can predict clinical and economic outcomes of pancreatic fistula after pancreaticoduodenectomy: results in 755 consecutive patients. Langenbecks Arch Surg. 2011;396:91–98.
- Jamous N, Socievole G, Ferko N, et al. Economic analysis of evarrest(R) sealant matrix compared with standard of care in severe soft tissue surgical bleeding: an Italian hospital perspective. Value Health. 2015;18(7):A369.
- Jamous N, Martini O, Ferko N, et al. Economic analysis of evarrest(R) sealant matrix compared with standard of care in severe soft tissue surgical bleeding: a German hospital perspective. Value Health. 2015;18(7):A369.
- Jamous N, Carter S, Ferko N, et al. Economic analysis of evarrest(R) sealant matrix compared with standard of care in severe soft tissue surgical bleeding: a United Kingdom hospital perspective. Value Health. 2015;18(7):A368.
- Martyn D, Kocharian R, Lim S, et al. Reduction in hospital costs and resource consumption associated with the use of advanced topical hemostats during inpatient procedures. J Med Econ. 2015;18:474–481.
- Tackett SM, Calcaterra D, Magee G, et al. Real-world outcomes of hemostatic matrices in cardiac surgery. J Cardiothorac Vasc Anesth. 2014;28(6):1558–1565.
- Price JS, Tackett S, Patel V. Observational evaluation of outcomes and resource utilization from hemostatic matrices in spine surgery. J Med Econ. 2015;18(10):777–786.
- Makhija D, Rock M, Ikeme S, et al. Cost-consequence analysis of two different active flowable hemostatic matrices in spine surgery patients. J Med Econ. 2017;20:606–613.
- Ferraris VA, Brown JR, Despotis GJ, et al. 2011 update to the society of thoracic surgeons and the society of cardiovascular anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91(3):944–982.
- Goodnough LT, Shander A. Patient blood management. Anesthesiology. 2012;116(6):1367–1376.
- American Society of Anesthesiologists Task Force on Perioperative Blood Management. Practice guidelines for perioperative blood management an updated report by the American society of anesthesiologists task force on perioperative blood management. Anesthesiology. 2015;122(2):241–275.
