ABSTRACT
Introduction: Patients with severe aortic stenosis and regurgitation who are inoperable or at high-risk for surgery can be treated with transcatheter aortic valve replacement (TAVR). The aim of this study was to provide a comprehensive overview of the literature of TAVR and reported clinical and performance outcomes.
Areas covered: A total of 16 devices, described in 204 articles describing clinical and performance outcomes, were included. The most frequently observed outcome was 30-day mortality, ranging between 0-23%. Other commonly reported clinical outcomes were 30-day stroke, ranging between 0–14.3% and pacemaker implantation, ranging from 0–44.9%. The most common valve performance outcome was aortic valve regurgitation, however, mostly reported at 7 days follow-up. Next to a follow-up period of 30 days, numerous articles reported outcomes at 6 months and 1 year. The numbers of articles describing outcomes with a longer follow-up as well as including intermediate and low-risk patients were limited.
Expert commentary: This literature review provided a clear overview of the reported clinical and performance outcomes of TAVR devices. Despite the frequently used VARC-2 definitions, we identified a huge variation across studies. Future studies using standardized definitions of study set-ups and outcomes are essential and might lead to better insights of TAVR.
1. Transcatheter aortic valve replacement
Aortic stenosis (AoS) and aortic regurgitation (AoR) can be treated with the replacement of the failing valve by a prosthesis. In 1960, Harken et al. reported surgical aortic valve replacement (SAVR) which has been the standard procedure for patients with severe, symptomatic AoS for many years [Citation1]. The procedure showed a 2-year survival rate of 78%, compared to 40% for patients who were managed medically [Citation2]. Unfortunately, about 33% of the AoS patients referred to SAVR are rejected, because of their high surgical risk [Citation3,Citation4].
Over the past decade, an alternative approach using transcatheter aortic valve implantation (TAVI) – also known as transcatheter aortic valve replacement (TAVR) – has emerged for the treatment of inoperable patients and is a preferred treatment option for patients considered at high surgical risk. After its first-in-man implantation in 2002 [Citation5], the first two transcatheter heart valves (THVs), the balloon-expandable SAPIEN and the self-expandable CoreValve ReValving, received a Conformité Européenne (CE) mark, i.e. European market approval, in 2007. Since then, more devices have become available, and more than 300,000 procedures have been performed worldwide [Citation6]. The ‘first generation’ THVs () showed promising outcomes, however, various TAVR associated complications were observed. Nowadays, several ‘second generation’ THVs () have been developed, in order to further improve outcomes and reduce complications. Moreover, recent studies showed that TAVR might be a valid option for intermediate and low-risk patients. THVs can be implanted using several access routes, with transfemoral (TF) and transapical (TA) approaches the most frequently used. Various manufacturers have THVs on the market with a range of structures and sizes, intended for various patient populations, with their own TAVR delivery systems and previously described clinical and performance outcomes (). Knowledge of these factors is of utmost importance for the heart team that is responsible for selecting the best treatment strategy for each patient. Every patient is unique, with his or her own patients’ characteristics including comorbidities, risk scores and potential of improving quality of life. In order to increase the quality of clinical research and to enable comparisons between clinical trials, the Valve Academic Research Consortium (VARC) published standardized consensus definitions for TAVR endpoints, which will be updated (VARC-3) soon [Citation7,Citation8]. Consensus was reached for proper endpoints reflecting device success, combined safety and efficacy endpoint [Citation7,Citation8].
Table 1. Overview of old and new generation THVs.
In this review, we aimed to provide an overview of the VARC clinical and performance outcomes available in the scientific literature of all THVs that had CE-mark approval in or before June 2017.
2. Materials and methods
2.1. Article selection
PubMed (National Center for Biotechnology Information, US National Library of Medicine) and Scopus (Elsevier BV, the Netherlands) were searched to identify articles describing TAVR. The following search terms were used: name of THV (Acurate neo, Acurate TA, Allegra, CoreValve, CoreValve Evolut, CoreValve Evolut R, CoreValve ReValving, Direct Flow Medical, Engager valve, Jena Valve, Lotus Edge, Lotus Valve System, Portico valve, Edwards SAPIEN, Edwards SAPIEN 3, Edwards SAPIEN XT), in combination with transcatheter aortic valve implantation, TAVI, transcatheter aortic valve replacement, TAVR, clinical study, trial or registry. The THVs were selected based on a previous web-based search in which all THVs were identified that were CE-marked or were in clinical investigation using the public internet [Citation9]. For the current review, CE-marked THVs were selected from this list.
Only articles describing clinical and performance outcomes in relation to TAVR or TAVI were included in this review. The search language was limited to English. Excluded were reviews, case reports, articles describing valve-in-valve implantation, articles describing no procedural or clinical endpoints, articles with a follow-up below 30 days and articles in which devices were grouped (without distinguishing results for specific devices). Additionally, studies were identified by analyzing the reference lists of selected articles. After removing duplicate studies, potentially relevant articles were reviewed.
2.2. Data extraction
For all identified relevant articles, the study set-up as well as procedural and clinical endpoints were extracted per THV. Regarding study set-up, we identified follow-up time, patient population (AoS or AoR), number of patients, access route, size of the valve, and whether the study was a comparison of several THVs. Concerning clinical and device performance outcomes, we selected follow-up periods of 30 days, 6 months, 1 year and every year up to 5 years, as far as available. In addition, based on the VARC [Citation7] and VARC-2 [Citation8] clinical endpoint definitions, the in listed information was extracted from each article.
Table 2. Overview of information extracted from each article.
Several articles graded paravalvular or AoR without providing details like none, trivial/trial, mild, moderate or severe AoR. In those cases, the following classification was used: grade 0 = none AoR; grade 1 = trivial/trace AoR; grade 2 = mild AoR; grade 3 = moderate AoR; grade 4 = severe AoR [Citation10].
Interesting observations on study characteristics, clinical and valve performance endpoints were described in the results part of this review and discussed in the discussion part of this review. Number of patients and follow-up time were shown as mean ± standard error of the mean (SEM). Clinical endpoints, like mortality, stroke and TIA, major vascular complication, pacemaker implantation, major and life threatening bleeding, myocardial infarction, acute kidney injury and valve performance outcomes were described as median with range. A meta-analysis was not performed because many variations in study characteristics were identified. Clinical and performance outcomes are presented using a descriptive approach.
3. Results
3.1. Transcatheter heart valves
From a previous list of THVs [Citation9], publications related to 14 aortic THVs were identified; articles describing the CoreValveTM EvolutTM and Lotus Edge Valve System were not identified. In addition, 4 THVs, Evolut PRO, Edwards Centera, SAPIEN 3 Ultra and Myval, received CE mark approval in 2017, 2018, Citation2018 and 2019 respectively. Clinical endpoints are reviewed in a few articles [Citation11–Citation13] and studies showed 30-day mortality rates of 1.7% (Evolut PRO [Citation14,Citation15]) and 1% (Centera [Citation14,Citation15]) and 9.1% 1-year mortality rate for Centera [Citation11–Citation15]. Articles describing clinical endpoints of Myval were not identified. provides an overview of THV devices including valve design characteristics like type of frame, deployment and valve size. Images of THVs can be found in other articles [Citation13–Citation17].
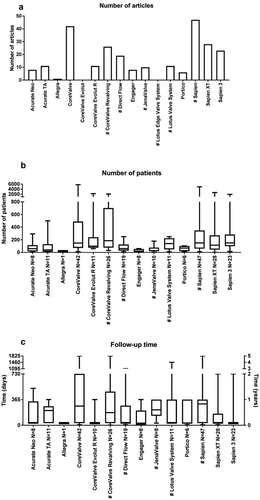
This review identified 2639 publications that were potentially relevant. Of these articles, 591 were found using PubMed and 2048 using Scopus. Finally, 204 articles met the inclusion criteria. Three reviewers were involved in selecting and reviewing the articles. An overview of the number of articles per THV showed that most articles identified focused on CoreValveTM and SAPIEN ()). For each investigated THV, complete overviews can be found in S1 and S2 Tables (10.6084/m9.figshare.7964363).
Figure 1. Overview of study characteristics. This figure shows the number of articles per valve identified in this review (a), the median number of patients included per valve (b) and the median follow-up time per valve (c). Data of Figure 1(b,c) are expressed as box plots, the upper and lower limits of the boxes indicate the 5th and 95th percentiles, and the lines inside the boxes the median. # indicates that the THV is not on the European market anymore. N indicates the number of articles that reported this study characteristic.
3.2. Study characteristics
From all 204 articles, the overall follow-up was 262 days ± 24 days. This included 116 articles with a follow-up of 30 days and 47 articles with a follow-up of 1 year. Four articles describing the outcomes of THVs that entered the European market first (SAPIEN, CoreValve ReValvingTM and CoreValveTM) had a follow-up of 5 years ().
Table 3. Overview of mortality data from 1 to 5 year follow-up.
All 204 articles together included in total 80,009 patients, of which 20,374 patients were included in articles concerning SAPIEN and 19,789 patients concerning CoreValveTM. Most articles included patients with severe AoS, however, we found a total of 7 articles that included patients with AoR. Most articles included patients that were inoperable or were at high-risk for surgery, however, some included intermediate-risk patients. TF and TA were the most frequently used access routes. About 60% of patients underwent TAVR with TF access route, 18% of patients TA and 22% underwent other access approaches including transaortic (TAo), subclavian (SC), direct aortic or were not specified or unknown.
In this review, we identified a huge variation across articles in terms of numbers of patients varying from 4 to 5790 patients ()). We identified one study that reported clinical outcomes after TAVR using the Allegra THV (CE-mark approval in 2017) and this study included 21 patients [Citation18]. In addition, we identified 8 articles reporting the Engager in which 28 ± 9 patients were included. In contrast to this, the studies describing clinical endpoints after TAVR using the valves, Acurate TATM (11 articles), CoreValveTM (42 articles), CoreValve Evolut RTM (11 articles), CoreValve ReValvingTM (26 articles), LotusTM Valve System (11 articles), SAPIEN (47 articles), SAPIEN XT (28 articles) and SAPIEN 3 (23 articles) included on average more than 100 patients in their studies. Articles describing, CoreValve ReValvingTM included on average most patients, 516 ± 118 patients, CoreValveTM included 471 ± 145 patients and SAPIEN included 428 ± 122 patients (all mean ± SEM).
Substantial differences in follow-up time were identified for the various THVs, ranging from 1 to 1825 days ()). Three valves were described with short follow-up times of maximal 90 days: Allegra (CE-mark approval in 2017, 1 article, 30-day follow-up), CoreValve Evolut RTM (CE-mark approval in 2012, 10 articles with a follow-up of 64 ± 34 days) and SAPIEN 3 (CE-mark approval in 2014, 23 articles with a follow-up of 90 ± 35 days). In contrast, articles describing the earlier THVs followed their patients for a longer period: CoreValve ReValvingTM 408 ± 100 days, SAPIEN 384 ± 69 days and CoreValveTM 356 ± 67 days.
Differences were observed in the descriptions of patient populations. For example, patients were categorized as inoperable, high-risk, intermediate-risk, inoperable to high-risk or intermediate to high-risk. Several articles used a scoring system for calculating predictive operative mortality, such as the logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) >20%, Society of Thoracic Surgeons (STS) score >10% or specified logistic EuroSCORE ranging from 10.8 ± 4.8% to 31.7 ± 1.4%, STS score ranging from 4.05 ± 4.9% to 7.8 ± 3.6% or EuroSCORE II from 5.2 ± 5.7% to 8.1 ± 6.3%. Therefore, it was not possible to differentiate patient populations into various risk groups for the description of the various endpoints below.
3.3. Clinical endpoints
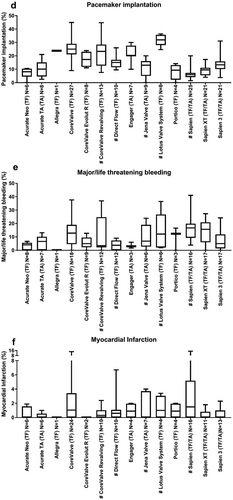
Regularly, data concerning clinical endpoints was described. The clinical endpoints 30-day mortality, pacemaker implantation, all stroke/TIA and major vascular complications were most often described. Mortality was the most frequently reported clinical endpoint at 30-days. The median mortality rates of all identified articles ranged between 1.8% (0–4) (CoreValve Evolut RTM) and 11.1% (0–30) (Jena Valve). CoreValve Evolut RTM, LotusTM Valve System, SAPIEN 3 and Portico reported lowest mortality rates. Jena Valve, Engager, SAPIEN and CoreValve ReValvingTM reported higher mortality rates. Interestingly, the first generation SAPIEN had a median 30-day mortality of 8.5% (0–18.8), the newer device SAPIEN XT 4.2% (0–14) and SAPIEN 3, the most recent device, 2.6% (0–11.1) ()).
Figure 2. Overview of mortality. This figure shows per valve the median identified mortality at 30 days (A), 6 months (B) and 1 year (C). Data are expressed as box plots, the upper and lower limits of the boxes indicate the 5th and 95th percentiles, and the lines inside the boxes the median. # indicates that the THV is not on the European market anymore. TA indicates transapical access route, TF indicates transfemoral access route, N indicates the number of articles that reported 30 day, 6 months and 1 year mortality, respectively.
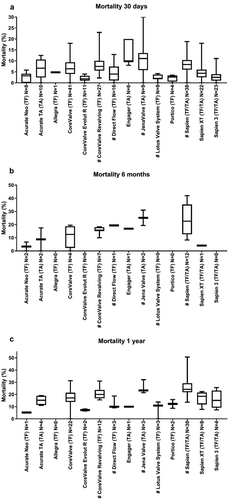
In total, only 32 articles reported 6 months mortality rates, ranging from 0% to 42%. For most THVs, 0, 1 or 2 articles were identified. For CoreValve 4 articles were identified, describing a median 6 months mortality of 12.6% (0–19.6). More articles were identified focusing on CoreValve ReValving (7 articles, mortality rate 15.8% (10–18.4)) and on the SAPIEN (12 articles, mortality rate 22.6% (8.3–42); )).
The number of articles that reported 1-year mortality data was small (in total 47 articles). The number of articles describing 1-year mortality for SAPIEN3, Acurate TATM Direct Flow Medical, Jena Valve, LotusTM Valve System, CoreValve Evolut RTM, PorticoTM, EngagerTM and Acurate NeoTM were small and varied from 1 to 4 articles per valve. Most articles describing 1-year mortality rates were related to CoreValveTM, CoreValve ReValvingTM, SAPIEN and SAPIEN XT. These showed that SAPIEN had the highest median 1-year mortality rate of 24.2% (13.6–50.7), CoreValve ReValvingTM had 20% (15.6–31), SAPIEN XT had 18.4% (7.8–22.3) and CoreValveTM showed the lowest median mortality of 17.2% (0–31.2) ( and )). The number of articles describing mortality at a longer follow-up period was limited ().
Other clinical endpoints were primarily reported at 30 days follow-up. Stroke can be categorized into ‘all stroke’ and ‘major/disabling stroke’. Reported all stroke and TIA rates ranged from 0% to 14.3%. The stroke and TIA rates of all THVs from which more than 4 articles were identified varied from 1.8% (0–5) (CoreValve ReValving) to 4.2% (2.9–9.1) (Lotus Valve System; )). Major/disabling stroke was not frequently reported. The median reported major/disabling stroke ranged between 0% (0–1.8) (Engager) and 2.9% (0–10) (SAPIEN XT; )). The major vascular complication rate ranged from 0% to 33.3%. The median major vascular complication from all THVs from which more than 4 articles were identified ranged from 2.2% (0–6)(Direct Flow Medical) to 9% (0–17) (SAPIEN XT; )). Pacemakers were frequently implanted after TAVR ranging from a median of 6% (1.5–20) (SAPIEN) to 31.9% (24.7–36.4) (LotusTM Valve System). Interestingly, pacemaker implantation rate of the newer device SAPIEN XT was 9.5% (4–17.3) and the most recent, SAPIEN 3, was 13.3% (3.8–31) ()). The major and/or life-threatening bleeding rate ranged from 0% to 41%. The median major and/or life threatening bleeding rate from all THVs from which more than 4 articles were identified ranged from 3.5% (0–36.9) (CoreValve ReValving) to 16.7% (3.9–41) (SAPIEN; )). The median myocardial infarction rate was relatively low ranging from 0% (0–1) (Acurate TA) to 1.1% (0–14.3) (CoreValve; )).
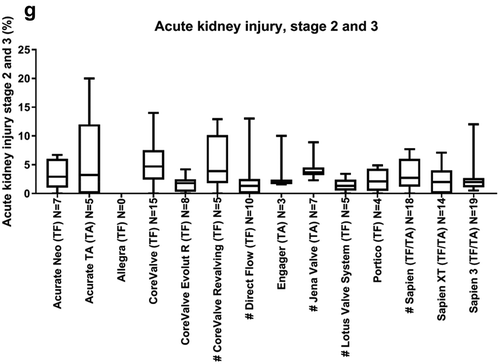
Figure 3. Overview of clinical endpoints. This figure shows per valve the median identified clinical outcomes. All stroke and TIA (a), major/disabling stroke (b), major vascular complications (c), pacemaker implantation (d), major/life threatening bleeding (e), myocardial infarction (MI; f), and stage 2 and 3 kidney injury (g). Data are expressed as box plots, the upper and lower limits of the boxes indicate the 5th and 95th percentiles, and the lines inside the boxes the median. # indicates that the THV is not on the European market anymore. TA indicates transapical access route, TF indicates transfemoral access route, N indicates the number of articles that reported this clinical endpoint.
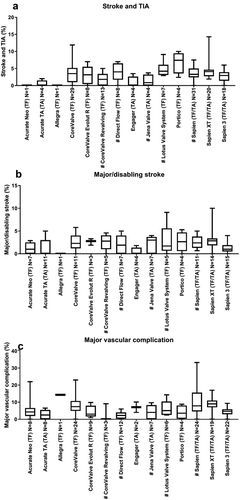
As recommended by VARC-2, acute kidney injury can be categorized into stages 1 to 3, with stage 3 as the most serious stage. Since stage 1 was not frequently described in the literature, we only extracted data on stage 2 and 3. Overall acute kidney injury stage 2/3 ranged from 0% to 20%. The median acute kidney injury stage 2/3 was relatively low, ranging from 1.3% (0–3.4) (Lotus Valve System) to 4.7% (0–14) (CoreValve; )).
3.4. Valve performance outcomes
Data concerning valve performance outcomes were limited. These outcomes are often only reported post-procedure or at discharge and not at longer follow-up moments. Additionally, many patients are lost during follow-up, even in a follow-up period of 7 days. Aortic valve gradient is one of the THV performance endpoints, providing evidence of prosthetic aortic valve stenosis. A mean gradient below 20 mm Hg is normal, while a mean gradient between 20–40 mm Hg is mild stenosis [Citation8]. Most articles that described this outcome reported a decreased aortic valve gradient after TAVR to normal. In more detail, all articles reported a mean or median aortic valve gradient below 20 mm Hg, ranging from 3.2 ± 5.2 mm Hg (CoreValve ReValvingTM) [Citation19] to 19.6 ± 5.7 mm Hg (Direct Flow Medical; S1 and S2 Tables; 10.6084/m9.figshare.7964363) [Citation20]. Bijuklic et al. described a mean ± SD of 19.6 ± 5.7 mm Hg; this indicated that there were patients without a normal mean gradient below 20 mm Hg [Citation20].
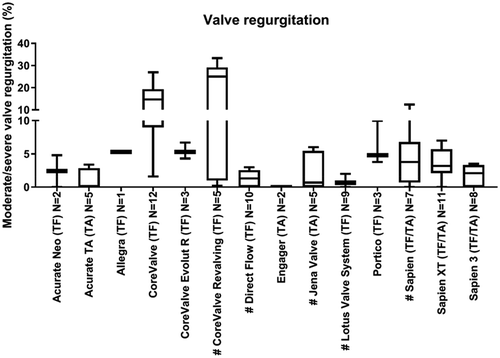
AoR is another important THV performance endpoint and is the consequence of poor apposition of the THV with the aortic annulus. We observed that this endpoint was not uniformly described; articles described either paravalvular aortic regurgitation, aortic regurgitation, paravalvular leakage, central or the combined total aortic regurgitation. For six THVs just 1 to 4 articles describing AoR were identified. Eight THVs with more than 4 articles describing AoR were identified. Several of these showed a low rate of patients with moderate/severe AoR, e.g. 0.6% (0–2) (LotusTM Valve System), while other devices showed rates up to 25% (0.2–33.3) (CoreValve ReValvingTM; ).
Figure 4. Valve regurgitation. This figure indicates per valve the median valve regurgitation after TAVR. Data are expressed as box plots, the upper and lower limits of the boxes indicate the 5th and 95th percentiles, and the lines inside the boxes the median. # indicates that the THV is not on the European market anymore. TA indicates transapical access route, TF indicates transfemoral access route, N indicates the number of articles that reported valve regurgitation.
4. Expert opinion
To our knowledge, this is currently the most comprehensive review analyzing outcomes after TAVR. It includes data from over 80,000 patients in total. Previously, several reviews focused on main TAVR studies [Citation17], TAVI registries [Citation21], newer generation THVs [Citation22] or repositionable self-expandable THV systems [Citation23]. In this review, all data identified in 204 articles is available (S1 and S2 Tables 10.6084/m9.figshare.7964363). These can be used for example by the heart teams that assess a tailored and individual approach for every unique patient with AoS or AoR. It is important to note that THVs showing apparently weak outcomes on certain endpoints in this review are not necessarily of poor quality. There might simply be a lack of sufficient evidence in the scientific literature. Furthermore, the THVs may have favorable outcomes on other endpoints. A certain THV might be the most appropriate device for a patient based on the patient, THV or TAVR characteristics.
We observed that 60% of patients underwent TF approach TAVR; this approach is usually the first option for TAVR [Citation24]. If TF access route is not suitable, other access routes can be used, like TA. However, from literature it is known that compared to TF, TA is associated with an increased risk of myocardial injury, bleeding and an overall increased risk of mortality [Citation25,Citation26]. For the interpretation of clinical and performance outcomes, the access route needs to be taken into account. In addition to the access route, also other factors influence clinical and performance outcomes. Examples are the design of the THV, the delivery system, complex clinical settings, comorbidities of the patient and knowledge and experience of the surgeons. Operating centers and surgeons have a progressive learning curve and improvement of expertise and high annual volume is associated with decreased patient mortality [Citation27].This literature review indicated that SAPIEN and CoreValveTM are the most studied THVs. This is not surprising because these were among the first devices entering the European market. Our overview also showed that 30-day mortality decreased with the newer generation THVs. This can partly be explained by the developed experience of the surgeon. Many articles did not describe all endpoints, did not include a decent number of patients and did not follow the patients for a longer time than 30 days. Furthermore, the VARC-2 clinical endpoint ‘new atrial fibrillation’ was only observed in a limited number of articles. Therefore, results on this complication were only incorporated into the S1 and S2 Tables (10.6084/m9.figshare.7964363). VARC-2 also described the composite endpoint ‘device success’, which is assembled of various factors including absence of procedural mortality, correct positioning of a single THV into the proper location and intended performance of the THV [Citation8]. We frequently observed that articles did not describe all aspects of this composite endpoint, however, only described absence of procedural mortality. Therefore, we only included device success into the S1 and S2 Tables (10.6084/m9.figshare.7964363). Another VARC-2 endpoint to determine THV functioning is AoR. AoR should be evaluated including central and paravalvular measurements, with a combined measurement of total aortic regurgitation [Citation8]. Frequently articles described either paravalvular aortic regurgitation, aortic regurgitation or paravalvular leakage, however, also central, paravalvular and the combined total aortic regurgitation were described. We showed that this endpoint was not uniformly described in the literature and we observed numerous articles that even did not determine this endpoint, or determined the parameter in smaller groups or only at 1 time point (post-procedural/at discharge). Therefore, it is difficult to draw conclusions from these results. In order to determine THV functioning over time it is necessary to increase the amount of data of total valve regurgitation at several time points.
A heart team is in charge of selecting the most suitable treatment option (SAVR or TAVR and which THV) for each patient. This team is critical to ensure the best outcome for a patient. In order to select the most suitable THV for each individual patient, it is important to consider all relevant factors. In addition to patient specific characteristics, these include the TAVR and THV specific characteristics, like THV design, access route or delivery system. Therefore, it is important to increase the evidence, the amount of literature and data on THVs. This review can provide valuable input for the selection process. In addition, this review also provides important information for ethics committees, regulators, patient associations and manufacturers. Ethics committees can use the data when evaluating a proposed clinical study on TAVR. Patient associations can use them to provide detailed information on THVs to their members. Manufacturers are required to perform post market surveillance of their products. Under the recently published new medical device regulations in Europe, this includes a comparison with similar devices available on the market [Citation28]. Regulators can use the information to guide their market surveillance activities. For all of them, it is important that articles report outcomes in a standardized method based on VARC-2 definitions to provide a clear overview and to make comparisons possible.
Here we showed that except for the THVs that entered the market first, SAPIEN, CoreValveTM and CoreValve ReValving, articles describing follow-up periods longer than 1 year were very limited ( and ). This is an important issue also previously pointed out by Tarantini et al.; long follow-up studies are necessary to provide insight regarding potential limitations concerning THV durability and dysfunctioning, concerns about repeated valve-in-valve procedures and the risk of a future surgical repair [Citation29]. To assess long-term durability of valves standardized definitions have been developed [Citation30,Citation31]. Several studies reported long-term valve performance outcomes of SAPIEN, SAPIEN XT and CoreValve. The Notion trial has a follow-up of up to 6 years assessing valve durability comparing TAVR and SAVR [Citation32]. These studies demonstrated no alarm concerning transcatheter durability [Citation33], 3.3% of patients had undergone valve implantation again due to paravalvular leakage [Citation34] and a majority of patients showed mild/trivial paravalvular leakage during long-term follow-up [Citation35]. Results on valve performance with a follow-up of 5 years are promising, however, the number of studies is limited and assessment in younger and lower-risk patients is required [Citation33]. Since the newer generation TAVR devices showed an improvement in clinical performance, TAVR is now also performed in younger and lower-risk patients. It is important that data on the outcomes in these new groups of patients become available as soon as possible. One consequence might be that the number of patients that require repeated valve-in-valve procedures will increase in the future [Citation36]. Therefore, it will become more and more important that THVs can be repositionable, retrievable, are highly durable and have a long device lifetime [Citation24].
This review has several limitations. First, we focused on outcomes described in VARC-2 definitions and did not incorporate other complications. For example, neurological complications might be of importance, however, literature describing this complication was limited. An example related to TAVR is cerebral micro-embolization, which might have long-term effects on cognitive function, however, thus far literature is limited [Citation37]. Second, we did not include all articles, like case reports or articles in which several THVs were grouped together. When THVs are grouped together, distinguishing outcomes per THV separately is not possible, therefore these articles were excluded. Next, we had to group inoperable, high risk and intermediate risk patients together. Frequently, articles described outcomes in a mixed patients risk population including intermediate to high-risk patients. In addition, articles described the risk scores as median/mean with a range/SD, which included a combination of several risk groups, therefore distinguishing patients risk groups separately was not possible. In order to compare various THVs in a patient group like intermediate or low-risk, it is essential to have standardized definitions, as well as uniformity in study set-up and endpoints. Recently, several studies properly selected a group of low-risk patients and implanted CoreValve Evolut R, CoreValve Evolut PRO or SAPIEN 3 by TAVR or SAVR [Citation38,Citation39].
Previous literature showed that, compared to SAVR, TAVR is a safe, reproducible and effective treatment option with improved clinical and performance outcomes and extending the lives of patients who are inoperable or for patients considered to be at high risk for surgery [Citation37]. SAVR remains the golden standard for low-risk and intermediate-risk patients. However, recently TAVR has been expanded to intermediate [Citation40] and low-risk patients [Citation29]. For example, the SAPIEN 3 and CoreValve Evolut RTM received CE-mark approval in 2016 to treat intermediate-risk patients with TAVR. Furthermore, the randomized PARTNER 3, Evolut R low risk and NOTION 2 trials determine all-cause mortality in low-risk patients after TAVR or SAVR with a follow-up of 5 to 10 years [Citation37]. Results look promising, for example, the rate of mortality and stroke at 1-or 2-year follow-up was lower with TAVR than with surgery [Citation38,Citation39]. In detail, results showed decreased 30-day and 1-year mortality in TAVR compared to surgery using CoreValve Evolut R, or Evolut PRO (30-day: 0.5% and 1.3%; 1 year: 2.4% and 3.0% respectively) [Citation38]. In addition, SAPIEN 3 also showed decreased 30-day and 1 year mortality rates in TAVR compared to surgery (30-day: 0.4% and 1.1%; 1 year: 1.0% and 2.5%, respectively) [Citation39]. Tarantini et al. concluded that TAVI is already everyday clinical practice for elderly patients with low surgical risk, despite the lack of robust evidence [Citation29]. A European study, including 301 centers, reported that 45% of the centers performed TAVI in intermediate-risk patients and 10% in low-risk patients [Citation41]. We observed variation in the description and analysis of the various risk group populations. Articles included intermediate and high-risk patients grouped together, therefore, we were not able to analyze and compare outcomes after TAVR for risk groups separately. TAVR has become an attractive alternative approach for intermediate- and low-risk patients including younger patients. Therefore, it is important that long-term durability of TAVR devices is demonstrated. For this, stratification of data in homogeneous subgroups is essential.
We observed that the use of VARC-2 definitions is gradually increasing. However, we still observed a huge variation across studies in terms of follow-up, numbers of patients and assessed endpoints. This was previously shown by Zhang et al. systematically reviewing TAVI registries reporting VARC-2 definitions [Citation21]. However, since the introduction in 2011 [Citation7], the definitions are more and more widely used [Citation21,Citation37]. In order to be able to compare TAVR outcomes, it is very important to describe endpoints uniformly; therefore, the use of standardized definitions like VARC-2 is of utmost importance. Incorporation of these endpoint definitions into studies will provide the possibility of performing a precise and systematic analysis including all THVs. This will provide better insights comparing and distinguishing THVs, access routes and various patient populations.
Article highlights
Despite the frequently used VARC-2 definitions, we identified a huge variation across studies in terms of follow-up, number of patients, patients’ risk groups, clinical and performance outcomes.
30-day mortality decreased with the newer generation THVs.
Except for the THVs that entered the market first, articles describing follow-up periods longer than 1 year were very limited.
More data specific for intermediate- and low-risk patients, including younger patients are necessary.
Author contributions
JWPM van Baal study concept and design, collection, analysis and interpretation of data, statistical analysis and drafting of the manuscript. B Roszek collection, analysis and interpretation of data, critical revision. M van Elk analysis of data, critical revision. RE Geertsma study supervision, critical revision.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
One reviewer is a consultant for Medtronic, Abbott, and Boston Scientific. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Acknowledgments
This investigation has been performed by order and for the account of Dutch Health and Youth Care Inspectorate, within the framework of project V080168 Supporting the Dutch Health and Youth Care Inspectorate on Medical Technology.
Data availability statement
All data obtained in this literature review (regarding VARC clinical and performance outcomes) are incorporated as Supplemental material (https://doi.org/10.6084/m9.figshare.7964363 DOI: 10.6084/m9.figshare.7964363).
References
- Harken DESH, Taylor WF, Lefimine AA. Partial and complete prosthese in aortic insufficiency. J Thorac Cardiovasc Surg. 1960;40:744–762.
- Varadarajan P, Kapoor N, Bansal RC, et al. Survival in elderly patients with severe aortic stenosis is dramatically improved by aortic valve replacement: results from a cohort of 277 patients aged≥ 80 years. Eur J Cardiothorac Surg. 2006;30(5):722–727.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro heart survey on valvular heart disease. Eur Heart J. 2003;24(13):1231–1243.
- Bach DS, Siao D, Girard SE, et al. Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk. Circ Cardiovasc Qual Outcomes. 2009;2(6):533–539.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106(24):3006–3008.
- Puri R, Chamandi C, Rodriguez-Gabella T, et al. Future of transcatheter aortic valve implantation—evolving clinical indications. Nat Rev Cardiol. 2018;15:57–65.
- Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the valve academic research consortium. Eur Heart J. 2010;32(2):205–217.
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium-2 consensus document. Eur Heart J. 2012;33(19):2403–2418.
- RIVM. Overview of CE marked TAVR. 2018. [cited 2017 Jun]. Available from: http://www.rivm.nl/Onderwerpen/M/Medische_hulpmiddelen/Hartkleppen/Overview_of_transcatheter_heart_valve_systems_CE_marked_and_in_Clinical_Investigations
- Gupta SD, Das S, Ghose T, et al. Controlled transient respiratory arrest along with rapid right ventricular pacing for improving balloon stability during balloon valvuloplasty in pediatric patients with congenital aortic stenosis-A retrospective case series analysis. Ann Card Anaesth. 2010;13(3):236.
- Brennan PF, Spence M. Self-expanding CENTERA valve for the treatment of severe, symptomatic aortic stenosis. Future Cardiol. 2019;15(2):79–84.
- Choudhury T, Solomonica A, Bagur R. The Evolut R and Evolut PRO transcatheter aortic valve systems. Expert Rev Med Devices. 2019;16(1):3–9.
- Solomonica A, Choudhury T, Bagur R. Newer-generation of Edwards transcatheter aortic valve systems: SAPIEN 3, Centera, and SAPIEN 3 ultra. Expert Rev Med Devices. 2019;16(2):81–87.
- Forrest JK, Mangi AA, Popma JJ, et al. Early outcomes with the Evolut PRO repositionable self-expanding transcatheter aortic valve with pericardial wrap. JACC Cardiovasc Interv. 2018;11(2):160–168.
- Reichenspurner H, Schaefer A, Schäfer U, et al. Self-expanding transcatheter aortic valve system for symptomatic high-risk patients with severe aortic stenosis. J Am Coll Cardiol. 2017;70(25):3127–3136.
- Athappan G, Gajulapalli RD, Tuzcu ME, et al. A systematic review on the safety of second-generation transcatheter aortic valves. EuroIntervention. 2016;11(9):1034–1043.
- del Val D, Ferreira-Neto AN, Asmarats L, et al. Transcatheter aortic valve replacement: relative safety and efficacy of the procedure with different devices. Expert Rev Med Devices. 2019;16(1):11–24.
- Wenaweser P, Stortecky S, Schutz T, et al. Transcatheter aortic valve implantation with the NVT Allegra transcatheter heart valve system: first-in-human experience with a novel self-expanding transcatheter heart valve. EuroIntervention. 2016;12(1):71–77.
- Piazza N, Grube E, Gerckens U, et al. Procedural and 30-day outcomes following transcatheter aortic valve implantation using the third generation (18 Fr) corevalve revalving system: results from the multicentre, expanded evaluation registry 1-year following CE mark approval. EuroIntervention. 2008;4(2):242–249.
- Bijuklic K, Tuebler T, Reichenspurner H, et al. Midterm stability and hemodynamic performance of a transfemorally implantable nonmetallic, retrievable, and repositionable aortic valve in patients with severe aortic stenosis. Up to 2-year follow-up of the direct-flow medical valve: a pilot study. Circ Cardiovasc Interventions. 2011;4(6):595–601.
- Zhang S, Kolominsky-Rabas PL. How TAVI registries report clinical outcomes—A systematic review of endpoints based on VARC-2 definitions. PLoS One. 2017;12(9):e0180815.
- Gatto L, Biondi-Zoccai G, Romagnoli E, et al. New-generation devices for transcatheter aortic valve implantation. Minerva Cardioangiol. 2018;66(6):747–761.
- Gomes B, Geis NA, Chorianopoulos E, et al. Improvements of procedural results with a new‐generation self‐expanding transfemoral aortic valve prosthesis in comparison to the old‐generation device. J Interv Cardiol. 2017;30(1):72–78.
- Wiegerinck EM, Van Kesteren F, Van Mourik MS, et al. An up-to-date overview of the most recent transcatheter implantable aortic valve prostheses. Expert Rev Med Devices. 2016;13(1):31–45.
- Herrmann HC, Thourani VH, Kodali SK, et al. One-year clinical outcomes with SAPIEN 3 transcatheter aortic valve replacement in high-risk and inoperable patients with severe aortic stenosis. Circulation. 2016;134(2):130–140.
- Bapat V, Frank D, Cocchieri R, et al. Transcatheter aortic valve replacement using transaortic access: experience from the multicenter, multinational, prospective ROUTE registry. JACC Cardiovasc Interv. 2016;9(17):1815–1822.
- Wassef AW, Rodes-Cabau J, Liu Y, et al. The learning curve and annual procedure volume standards for optimum outcomes of transcatheter aortic valve replacement: findings from an international registry. JACC Cardiovasc Interv. 2018;11(17):1669–1679.
- Regulation (EU). 2017/745 of the European Parliament and of the council of 5 April 2017 on medical devices, amending directive 2001/83/EC, regulation (EC) No 178/2002 and regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC.
- Tarantini G, Nai Fovino L, Gersh BJ. Transcatheter aortic valve implantation in lower-risk patients: what is the perspective? Eur Heart J. 2018;39:658–666.
- Dvir D, Bourguignon T, Otto CM, et al. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation. 2018;137(4):388–399.
- Capodanno D, Petronio AS, Prendergast B, et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg. 2017;52(3):408–417.
- Søndergaard L, Ihlemann N, Capodanno D, et al. Durability of transcatheter and surgical bioprosthetic aortic valves in patients at lower surgical risk. J Am Coll Cardiol. 2019;73(5):546–553.
- Eltchaninoff H, Durand E, Avinee G, et al. Assessment of structural valve deterioration of transcatheter aortic bioprosthetic balloon-expandable valves using the new European consensus definition. EuroIntervention. 2018;14(3):e264–e271.
- Holy EW, Kebernik J, Abdelghani M, et al. Long-term durability and haemodynamic performance of a self-expanding transcatheter heart valve beyond five years after implantation: a prospective observational study applying the standardised definitions of structural deterioration and valve failure. EuroIntervention. 2018;14(4):e390–e396.
- Panico R, Giannini C, De MC, et al. Long-term results and durability of the CoreValve transcatheter aortic bioprosthesis: outcomes beyond five years. EuroIntervention. 2019;14(16):1639–1647.
- Barbanti M, Tamburino C. Late degeneration of transcatheter aortic valves: pathogenesis and management. EuroIntervention. 2016;12(Y):Y33–6.
- Durko AP, Osnabrugge RL, Kappetein AP. Long-term outlook for transcatheter aortic valve replacement. Trends Cardiovasc Med. 2018;28:174–183.
- Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. NEJM. 2019;380(18):1706–1715.
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. NEJM. 2019;380(18):1695–1705.
- Terré JA, George I, Smith CR. Pros and cons of transcatheter aortic valve implantation (TAVI). Ann Cardiothorac Surg. 2017;6(5):444–452.
- Petronio AS, Capranzano P, Barbato E, et al. Current status of transcatheter valve therapy in Europe: results from an EAPCI survey. EuroIntervention. 2016;12(7):890–895.