ABSTRACT
Introduction: Patients with lumbar disc herniation and associated sciatica are often referred for lumbar discectomy. The surgical defect in the annulus fibrosus is typically left unrepaired after lumbar discectomy. Patients with large postsurgical annular defects (≥6 mm width) have a higher risk of symptom recurrence and reoperation compared to those with small defects. In these high-risk patients, a treatment gap exists due to the lack of effective treatments for durable annulus fibrosus repair.
Areas covered: This article highlights the therapeutic need and summarizes the clinical results of a bone-anchored annular closure device (Barricaid) that was designed to fill the treatment gap in patients with large postsurgical annular defects. Clinical results were summarized by means of a systematic review with meta-analysis of two randomized and two nonrandomized controlled studies.
Expert opinion: Professional societal recommendations and clinical study results support the adoption of bone-anchored annular closure for use in properly selected patients undergoing lumbar discectomy who are at high-risk for reherniation due to a large postsurgical defect in the annulus fibrosus. The risks of symptomatic reherniation and reoperation are approximately 50% lower in patients treated with lumbar discectomy and the Barricaid device compared to lumbar discectomy only, representing a clinically effective treatment strategy.
1. Introduction
Sciatica affects 10% of adults in the United States each year [Citation1] and is a leading cause of disability [Citation2]. Affected individuals experience severe radiating pain along the distribution of the sciatic nerve through the buttocks and leg. Herniation of the nucleus pulposus from within the lumbar intervertebral disc space to the extradiscal space is the primary instigating event in sciatica. Extrusion or sequestration of disc contents may cause these radicular symptoms by direct impingement of adjacent nerve roots or induction of local inflammatory processes. The prognosis of individuals with sciatica is generally favorable with approximately two-thirds of patients reporting complete symptom resolution after 1 year [Citation3,Citation4]. However, bothersome symptoms that are refractory to conservative treatments persist in the remaining one-third of patients who are often referred for lumbar disc surgery to remove the offending herniated disc contents. While continued conservative treatment may also be considered, increasing evidence suggests that surgery is more effective than continued conservative care for chronic sciatica symptoms [Citation5].
Lumbar discectomy is the preferred method of surgical management for persistent sciatica arising from lumbar intervertebral disc herniation. Lumbar discectomy involves surgical removal of the intervertebral disc material that herniated through the annulus fibrosus into the extradiscal space. Traditional aggressive (or subtotal) lumbar discectomy involves near-complete excision of the intervertebral disc. While this procedure effectively reduces the risk of future reherniation, complete disc excision alters spinal kinematics and increases the risk of painful degenerative disc disease [Citation6,Citation7]. Consequently, lumbar disc surgery techniques have evolved over time to favor limited discectomy (or sequestrectomy), which aims to remove only the herniated disc material while leaving the intradiscal contents intact. While a limited surgical approach maintains disc height and spinal kinematics, the risk of reherniation of residual disc material is increased [Citation8]. Reoperations for lumbar herniation are more costly [Citation9] and less effective [Citation10,Citation11] than primary procedures. Therefore, identification of patient characteristics and surgical techniques that contribute to this excess risk is crucial to minimize patient morbidity due to symptomatic reherniation and associated reoperations.
2. The therapeutic gap in surgical management of lumbar disc herniation
The risk of reherniation after lumbar discectomy is approximately 3% per year of postsurgical follow-up [Citation12,Citation13]. In addition, the 1- and 3-year cumulative risks of leg pain recurrence were 20% and 45%, respectively [Citation14]. However, this risk is considerably higher in certain subsets of patients. Regardless of whether an aggressive or limited disc excision surgery is performed, the residual defect in the annulus fibrosus is typically left unrepaired. Increasing evidence has accumulated over the last decade that recognizes the annular defect as a likely pathway for future disc reherniation, with this risk increasing in proportion to the size of the defect [Citation15]. Based on data derived from the meta-analysis of Miller and colleagues [Citation15], 6.6% of patients with a small (<6 mm width) annular defect experienced symptom recurrence within 3 years. This risk of recurrence is acceptably low and probably requires no preventative measures other than typical postsurgical activity restrictions and recommendations. However, 30% to 44% of patients have large (≥6 mm width) postsurgical defects [Citation15,Citation16] and this subgroup represents patients at very high risk for recurrence. Comparing patients with large versus small annular defects, patients with large defects have a 2.5-fold higher rate of symptom recurrence [Citation15]. Among these high-risk patients, there are limited options to reduce the risk of recurrent herniation.
The annulus fibrosus is largely avascular, which explains its poor innate healing potential after surgical annulotomy. During the postsurgical healing process, a thin layer of biomechanically inferior fibrous tissue develops over the defect, which serves as a constant potential location for reherniation occurrence [Citation17]. Increasing anatomical knowledge combined with advancements in medical technology over the last several decades has yielded several promising treatments for repair, replacement, or regeneration of the damaged annulus fibrosus. Candidate therapies have included suturing systems, fibrin glue, biologics, and tissue engineering [Citation18]. Unfortunately, none of these are clinically proven effective treatments for durable annulus fibrosus repair [Citation18].
3. Barricaid annular closure device for annulus fibrosus closure
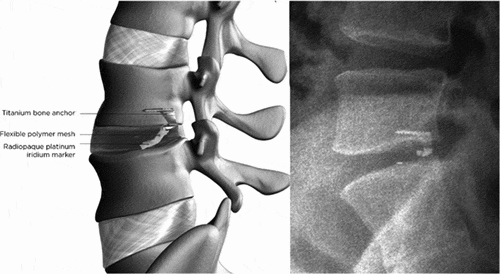
To the authors’ knowledge, one method of annular closure has reliably demonstrated efficacy and safety – the Barricaid Annular Closure Device (Intrinsic Therapeutics, Woburn, MA) (). The Barricaid is a Food and Drug Administration approved intervertebral biomechanical device that consists of a woven polyethylene terephthalate flexible fabric component that attaches to a titanium alloy (Ti-6Al-4 V ELI) intravertebral bone anchor. The flexible fabric component is designed to reconstruct the annulus at the site of the annular defect. The bone anchor component is used to secure the device to one of the adjacent vertebral bodies and ensure correct positioning of the flexible fabric component. The device is implanted following lumbar discectomy and is intended to physically occlude large annular defects and, consequently, lower the risk of reherniation in this high-risk subgroup of patients.
Figure 1. Schematic of the Barricaid Annular Closure Device (left) and lateral radiograph showing the Barricaid Annular Closure Device implanted at L4-L5 (right).
3.1. Indications
The intended use of the Barricaid device is to reduce the incidence of reherniation and reoperation in skeletally mature patients with radiculopathy (with or without back pain) attributed to a posterior or posterolateral herniation. Treatment involves closing a large annular defect (between 4 and 6 mm tall and between 6 and 10 mm wide) following a primary discectomy procedure at a single level between L4 and S1. The source of pain must be confirmed by history, physical examination, and imaging studies which demonstrate neural compression using magnetic resonance imaging (MRI). The rationale for this indication is that patients with annular defects at least 6 mm in width represent a high-risk subgroup that experiences reherniation and reoperation at more than twice the rate of those with smaller annular defects [Citation15].
3.2. Contraindications
The contraindications for the Barricaid device are generally comparable to those of other implantable spinal medical devices. The Barricaid device should not be implanted in patients with (a) active systemic or implantation site infection, (b) prior surgery at the index level, (c) indicated spinal surgery other than discectomy, (d) sensitivity to the device’s components, (e) low lumbar bone mineral density defined as a T-score ≤ −2.0, (f) back or non-radicular leg pain of unknown etiology, (g) scoliosis >10 degrees, (h) moderate/severe spondylolisthesis, (i) clinically compromised lumbosacral vertebral bodies, (j) posterior disc height <5 mm, (k) annular defect sizes smaller or larger than those able to be treated with the device, or (l) other conditions including insulin-dependent diabetes, peripheral neuropathy, arterial insufficiency, or body mass index over 40 kg/m2.
3.3. Procedural steps
At the completion of a lumbar discectomy procedure, the annular defect size is measured with a specialized tool. Patients with defect widths between 6 and 10 mm are eligible for implantation with the Barricaid device. A sizing assessment is performed under fluoroscopic guidance to establish the correct access trajectory and placement of the device. Next, the device is implanted under fluoroscopic guidance by placing the occlusion component in the annular defect to prevent future expulsion of disc material into the extradiscal space. The bone anchor is implanted in the adjacent vertebral body to provide a secure and durable repair. After fluoroscopic confirmation of correct device placement, the surgical site is inspected and standard wound closure is performed. Perioperative care is at the discretion of the implanting surgeon.
4. Systematic review with meta-analysis of Barricaid annular closure device efficacy
4.1. Systematic review and meta-analysis methodology
In order to evaluate the effectiveness of the Barricaid device when used in addition to a limited lumbar discectomy procedure, we performed a systematic review and meta-analysis that adhered to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation19].
4.1.1. Literature search
Medline, Embase, and the Cochrane Central Register were searched for randomized trials and prospective or retrospective nonrandomized controlled studies that reported on the clinical use of the Barricaid device in addition to a lumbar discectomy procedure for prevention of reherniation. We used the following keywords in our searches: Barricaid, anular closure, anulus closure, annular closure, and annulus closure. Additionally, manual searches were conducted using the Directory of Open Access Journals, Google Scholar, and the reference lists of included papers and relevant meta-analyses. The final search was performed on 31 October 2019.
4.1.2. Study selection
Study selection was performed by two independent reviewers. Study selection discrepancies between the reviewers were resolved by consensus. Titles and abstracts were initially screened to exclude review articles, commentaries, letters, case reports, and obvious irrelevant studies. Full texts of the remaining articles were retrieved and reviewed.
4.1.3. Data extraction
Data were independently extracted from eligible articles by the same two reviewers. Data extraction discrepancies between the reviewers were resolved by consensus. The types of data recorded in the standardized data extraction forms included general manuscript information, patient characteristics, study characteristics, risk of bias using the Cochrane Risk of Bias Tool for randomized and nonrandomized controlled studies [Citation20], and main outcomes including symptomatic reherniation and reoperation occurring during follow-up.
4.1.4. Data analysis
Random-effects meta-analysis models using inverse variance weighting were developed based on the a priori assumption that treatment effects may be heterogeneous among studies due to differences in patient characteristics and surgical techniques. The statistic of interest was the risk ratio and 95% confidence interval, where a risk ratio of less than 1 indicated lower risk with the Barricaid device and a risk ratio greater than 1 indicated higher risk with the Barricaid device. Forest plots were used to illustrate individual study findings and pooled meta-analysis results. We used the I2 statistic to estimate heterogeneity of effects across studies with values of ≤25%, 50%, and ≥75% representing low, moderate, and high inconsistency, respectively [Citation21]. A subgroup analysis was performed to assess the main outcomes derived from randomized trials only. Statistical analyses were performed using RevMan version 5.3 (Cochrane Collaboration, Copenhagen, Denmark).
4.2. Systematic review and meta-analysis results
4.2.1. Study selection
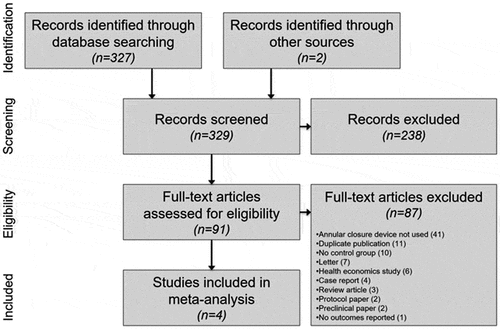
Among 329 papers identified in our searches, 91 full-text papers were reviewed including 50 published papers of the Barricaid device (). Ultimately, four controlled studies were identified and included in the meta-analysis [Citation22–Citation25]. A PRISMA flow diagram depicting the study identification and selection process is provided in .
Table 1. Compendium of 50 peer-reviewed manuscripts of the Barricaid device for annulus fibrosus closure.
4.2.2. Study and patient characteristics
The systematic review identified four controlled studies (two randomized) of the Barricaid device involving 801 patients – 381 treated with lumbar discectomy and the Barricaid device and 420 treated with lumbar discectomy only. The mean patient age in each study ranged from 40 to 44 years, body mass index ranged from 24 to 26 kg/m2, and a slight male preponderance (60% of patients) was identified. The follow-up duration was 2 years in three studies and 4 years in one study. We therefore extracted 2-year outcomes from all studies to ensure comparability of results among studies (). The main risks of bias in this analysis were attributable to the inclusion of nonrandomized studies ().
Table 2. Patient and study characteristics in controlled studies with the Barricaid device for annulus fibrosus closure.
Table 3. Risk of bias in controlled studies with the Barricaid device for annulus fibrosus closure.
4.2.3. Symptomatic reherniation and reoperations
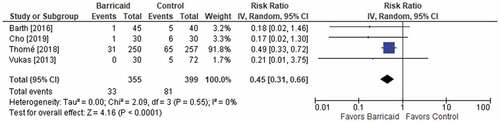
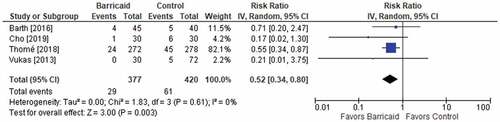
Among the four studies, the risk of symptomatic reherniation over 2 years of follow-up was 55% lower with the Barricaid device (9.3% vs. 20.3%, risk ratio = 0.45, p < 0.001). The results were consistent among studies since each study reported a risk ratio of less than 1, and no heterogeneity was observed (ICitation2 = 0%) (). Similar conclusions were derived for the risk of reoperation over 2 years of follow-up. The risk of reoperation was 48% lower with the Barricaid device (7.7% vs. 14.5%, risk ratio = 0.52, p = 0.003), all risk ratios were less than 1 (favoring the Barricaid device) in individual studies, and no heterogeneity was observed (ICitation2 = 0%) (). When isolating the analysis to only the results derived from randomized trials, the conclusions were unchanged with 53% reductions in symptomatic reherniation and reoperation risk noted in patients treated with the Barricaid device (each risk ratio = 0.47).
4.3. Systematic review conclusions
In four controlled studies involving 801 patients with large annular defects followed for 2 years after lumbar discectomy, the risks of symptomatic reherniation and reoperation were approximately 50% lower in patients who received additional treatment with the Barricaid device.
5. Evidence synthesis and future research directions
5.1. Evidence synthesis
Clinical data derived from randomized and nonrandomized controlled studies provide support for the adoption of the Barricaid device in well-selected patients undergoing limited lumbar discectomy who are at elevated risk for reherniation due to a large postsurgical defect in the annulus fibrosus.
A prior meta-analysis of four studies by Choy et al. [Citation62] evaluated the clinical utility of annular closure devices, where they reported lower rates of symptomatic reherniation in patients receiving these devices (odds ratio = 0.34, p < 0.001). Yet the review included results from suture-based and bone-anchored annular closure techniques, failed to include several relevant studies, included a study with only 90-day follow-up, and failed to report reoperation rates, which is arguably the most clinically relevant outcome in this patient population. In contrast, the current meta-analysis summarizes data on symptomatic reherniation and reoperations from four studies of bone-anchored annular closure with the Barricaid device, all with 2 years of patient follow-up.
The results of the meta-analysis of controlled studies are further supported by several prospective case series of patients with large annular defects who were treated with lumbar discectomy and the Barricaid device in real-world settings. Symptomatic reherniation rates in these studies were 1.4% through 2 years in the study of Ardeshiri et al. [Citation63], 3.2% through 15 months in the study of Kursumovic et al. [Citation64], and 1.4% through 2 years in the study of Bouma et al. [Citation65]. Notably, the study of Sanginov and colleagues [Citation66] provided the longest follow-up data with the Barricaid device thus far. In this study of 120 patients with up to 5-year follow-up, the symptomatic reherniation rate and the reoperation rate for reherniation were 1.7% each. For comparison, among patients with large annular defects treated with lumbar discectomy but without annular closure, symptomatic reherniation rates typically range from 15% to 30% [Citation68]. This finding demonstrates that the results of the current meta-analysis which included only controlled studies are congruent with the results from real-world noncontrolled studies of the Barricaid device in the same high-risk patient population.
Our systematic review identified several other clinically relevant findings regarding the Barricaid device that warrant further discussion. Despite the efficacy of the Barricaid device in reducing the risk of future reoperation, it is prudent to consider the possible influence of the implant in patients who require future lumbar surgery. In the study of Klassen et al. [Citation70], it was shown that among patients undergoing a reoperation, patients with an existing Barricaid device were treated with similar reoperation techniques, experienced comparable surgical risks, and reported comparable post-revision outcomes relative to patients without the Barricaid device. Further, there were no instances in which the presence of the Barricaid device was reported to complicate a reoperation or interfere with execution of the planned surgical approach. Overall, these results suggest that the Barricaid device does not interfere with standard revision strategies commonly used by spine surgeons.
Because lumbar disc herniation predominantly impacts middle-aged adults, relatively fewer older adults were treated in studies of the Barricaid device. Investigation of sciatica treatments in older adults is an important pursuit since these individuals suffer from disability levels comparable to those with a history of chronic obstructive pulmonary disease, stroke, or myocardial infarction [Citation71]. In order to address this question, a pooled analysis from multiple studies representing over 2,000 patient-years of follow-up identified several important clinical findings as it related to the Barricaid device and the aging adult [Citation72]. First, older patients with large post-surgical annular defects had a high risk of symptomatic reherniation and reoperation that was comparable to younger patients. This was a unique finding since it is commonly reported in the general discectomy literature that the relative risk of reherniation declines with advancing age [Citation10]. Second, additional implantation of the Barricaid device resulted in significant reductions in reherniation and reoperation risk that were independent of age. Finally, leg pain, disability, and quality of life greatly improved with lumbar disc surgery regardless of age, were durably maintained during follow-up, and favored patients treated with the Barricaid device. Overall, the results of this pooled analysis highlighted the clinical utility and patient-centric benefit of the Barricaid device in older, as well as younger, adults with large annular defects following lumbar disc surgery.
5.2. Directions for future research
In this review, we identified 50 published papers of the Barricaid device since 2012, which included two randomized trials and two nonrandomized controlled studies. Based in part on this compendium of clinical data, the International Society for the Advancement of Spine Surgery (ISASS) issued a policy supporting the use of the Barricaid device in patients with large annular defects [Citation73]. Several future research initiatives may further clarify the clinical utility of the Barricaid device. The majority of clinical data have been derived from the 8- and 10-mm Barricaid devices, yet the 12-mm device, which is typically reserved for massive defects, has been relatively less studied. It is plausible that patients with larger defects may convey increased reherniation risk and, therefore, additional study on the impact of exact defect sizing and its relationship to device sizing could be helpful to identify risk-stratified subgroups among patients already at elevated reherniation risk. Importantly, the Barricaid device is not suitable for the treatment of patients with annular defects less than 6 mm width because of the low risk of reherniation in this population. Additionally, this systematic review utilized 2-year follow-up data, but the publication of longer-term comparative data is encouraged. In the pivotal trial of the Barricaid device, clinical results through 4 years of follow-up confirm there are no excess clinically important patient risks with Barricaid treatment relative to lumbar discectomy only [Citation74] and that the Barricaid device does not interfere with reoperations should a patient require one in the future[Citation70]. The final 5-year follow-up data from the pivotal trial of the Barricaid device will be available in late 2020 and dissemination of these results will be helpful to clarify the long-term clinical outcome among patients with large annular defects treated with lumbar discectomy and the Barricaid device.
6. Conclusions
The Barricaid Annular Closure Device serves to bridge the treatment gap in the management of patients with large annular defects following lumbar discectomy and is a medically necessary treatment in this patient population. The Barricaid device has received final regulatory approval for use in the United States, and its use is supported by ISASS guidance. Currently, no other devices are approved for the repair of annular defects in this patient population. The scientific evidence derived from a meta-analysis of randomized and nonrandomized controlled studies demonstrates that the Barricaid device fundamentally improves the net health outcome of treated patients by lowering the risk for future symptomatic reherniation and reoperation by approximately 50%. Further, patient outcomes in real-world settings mimic those derived from controlled studies. Overall, results of clinical studies as well as ISASS recommendations provide support for the adoption of the bone-anchored annular closure device for use in well-selected patients undergoing lumbar discectomy for lumbar disc herniation who are high-risk for symptomatic reherniation due to a large postsurgical defect in the annulus fibrosus.
7. Expert opinion
A distinct therapeutic gap exists for the patient with a large defect in the annulus fibrosus following lumbar disc surgery. These patients are exposed to excess risk of reherniation and associated reoperation owing to the large unrepaired annular defect that may allow nuclear material to exit and cause symptom recurrence. Previous attempts at developing effective annular repair solutions have been largely unsuccessful. The Barricaid device was specifically developed to satisfy this unmet need. The device is comprised of a titanium bone anchor and a polymer occlusion component that blocks the defect in the annulus, thereby reducing the risk of reherniation. Midterm results from four controlled studies (two randomized) comprising over 800 patients have provided strong evidence that the Barricaid device effectively reduces the risks of symptomatic reherniation and reoperation following lumbar discectomy. Given the excess risk of reherniation among patients with large annular defects, the Barricaid device is a welcome treatment option for the high-risk lumbar discectomy patient.
7.1. Five-year view
Lumbar disc herniation will remain a major health concern and a leading cause of disability for affected patients. As more clinical study data continue to accrue demonstrating the positive long-term results of the Barricaid device, treatment of large defects in the annulus fibrosus during the index surgery may become the standard of care to prevent future symptomatic reherniations and associated reoperations.
Article Highlights
Among patients undergoing lumbar discectomy for disc herniation, those with large postsurgical defects in the annulus fibrosus (≥6 mm width) are more prone to reherniation and reoperation.
Surgery for reherniation is associated with inferior clinical outcome and increased complication risk compared to the index surgery.
Attempted treatments such as annular suturing systems or fibrin glue have been unable to modify this risk given their inability to durably withstand the chronic outward forces imposed upon the annulus fibrosus during physiological loading.
The Barricaid annular closure device is a permanent implant that repairs the annulus fibrosus and lowers the risk of reherniation of lumbar disc contents and associated reoperations via a flexible occlusion component that is anchored to a vertebral body.
A systematic review with meta-analysis of randomized and nonrandomized controlled studies demonstrated that the Barricaid annular closure device reduced the risks of symptomatic reherniation and reoperation by approximately 50% compared to lumbar discectomy only in high-risk patients with large annular defects.
Declaration of Interest
L Miller has received personal fees from Intrinsic Therapeutics. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
One peer reviewer was a coinvestigator in a randomized-controlled trial of the Barricaid device. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- U.S. Department of Health and Human Services. Use of complementary health approaches for musculoskeletal pain disorders among adults: United States, 2012. 2012 [cited 2019 Dec 17]. Available from: https://www.cdc.gov/nchs/data/nhsr/nhsr098.pdf
- GBD Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858.
- Vroomen PC, de Krom MC, Knottnerus JA. Predicting the outcome of sciatica at short-term follow-up. Br J Gen Pract. 2002;52:119–123.
- Vroomen PC, de Krom MC, Wilmink JT, et al. Lack of effectiveness of bed rest for sciatica. N Engl J Med. 1999;340:418–423.
- Arts MP, Kursumovic A, Miller LE, et al. Comparison of treatments for lumbar disc herniation: systematic review with network meta-analysis. Medicine (Baltimore). 2019;98:e14410.
- Goel VK, Goyal S, Clark C, et al. Kinematics of the whole lumbar spine. Effect of discectomy. Spine (Phila Pa 1976). 1985;10:543–554.
- Mariconda M, Galasso O, Attingenti P, et al. Frequency and clinical meaning of long-term degenerative changes after lumbar discectomy visualized on imaging tests. Eur Spine J. 2010;19:136–143.
- Carragee EJ, Spinnickie AO, Alamin TF, et al. A prospective controlled study of limited versus subtotal posterior discectomy: short-term outcomes in patients with herniated lumbar intervertebral discs and large posterior anular defect. Spine (Phila Pa 1976). 2006;31:653–657.
- Ambrossi GL, McGirt MJ, Sciubba DM, et al. Recurrent lumbar disc herniation after single-level lumbar discectomy: incidence and health care cost analysis. Neurosurgery. 2009;65:574–578; discussion 578.
- Abdu RW, Abdu WA, Pearson AM, et al. Reoperation for recurrent intervertebral disc herniation in the spine patient outcomes research trial: analysis of rate, risk factors, and outcome. Spine (Phila Pa 1976). 2017;42:1106–1114.
- Fritzell P, Knutsson B, Sanden B, et al. Recurrent versus primary lumbar disc herniation surgery: patient-reported outcomes in the Swedish spine register swespine. Clin Orthop Relat Res. 2015;473:1978–1984.
- Heindel P, Tuchman A, Hsieh PC, et al. Reoperation rates after single-level lumbar discectomy. Spine (Phila Pa 1976). 2017;42:E496–E501.
- Kim CH, Chung CK, Park CS, et al. Reoperation rate after surgery for lumbar herniated intervertebral disc disease: nationwide cohort study. Spine (Phila Pa 1976). 2013;38:581–590.
- Suri P, Pearson AM, Zhao W, et al. Pain recurrence after discectomy for symptomatic lumbar disc herniation. Spine (Phila Pa 1976). 2017;42:755–763.
- Miller LE, McGirt MJ, Garfin SR, et al. Association of annular defect width after lumbar discectomy with risk of symptom recurrence and reoperation: systematic review and meta-analysis of comparative studies. Spine (Phila Pa 1976). 2018;43:E308–E315.
- Ammerman J, Watters WC, Inzana JA, et al. Closing the treatment gap for lumbar disc herniation patients with large annular defects: a systematic review of techniques and outcomes in this high-risk population. Cureus. 2019;11:e4613.
- Fazzalari NL, Costi JJ, Hearn TC, et al. Mechanical and pathologic consequences of induced concentric anular tears in an ovine model. Spine (Phila Pa 1976). 2001;26:2575–2581.
- Bron JL, Helder MN, Meisel HJ, et al. Repair, regenerative and supportive therapies of the annulus fibrosus: achievements and challenges. Eur Spine J. 2009;18:301–313.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94.
- Higgins JP, Altman DG, Gotzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560.
- Barth M, Fontana J, Thome C, et al. Occurrence of discal and non-discal changes after sequestrectomy alone versus sequestrectomy and implantation of an anulus closure device. J Clin Neurosci. 2016;34:288–293.
- Cho PG, Shin DA, Park SH, et al. Efficacy of a novel annular closure device after lumbar discectomy in Korean patients: a 24-month follow-up of a randomized controlled trial. J Korean Neurosurg Soc. 2019;62:691–699.
- Thome C, Klassen PD, Bouma GJ, et al. Annular closure in lumbar microdiscectomy for prevention of reherniation: a randomized clinical trial. Spine J. 2018;18:2278–2287.
- Vukas D, Ledic D, Grahovac G, et al. Clinical outcomes in patients after lumbar disk surgery with annular reinforcement device: two-year follow up. Acta Clin Croat. 2013;52:87–91.
- Kienzler JC, Klassen PD, Miller LE, et al. Three-year results from a randomized trial of lumbar discectomy with annulus fibrosus occlusion in patients at high risk for reherniation. Acta Neurochir (Wien). 2019;161:1389–1396.
- Klassen PD, Bernstein DT, Kohler HP, et al. Bone-anchored annular closure following lumbar discectomy reduces risk of complications and reoperations within 90 days of discharge. J Pain Res. 2017;10:2047–2055.
- Kursumovic A, Kienzler JC, Bouma GJ, et al. Morphology and clinical relevance of vertebral endplate changes following limited lumbar discectomy with or without bone-anchored annular closure. Spine (Phila Pa 1976). 2018;43:1386–1394.
- van den Brink W, Fluh C, Miller LE, et al. Lumbar disc reherniation prevention with a bone-anchored annular closure device: 1-year results of a randomized trial. Medicine (Baltimore). 2019;98:e17760.
- Trummer M, Eustacchio S, Barth M, et al. Protecting facet joints post-lumbar discectomy: Barricaid annular closure device reduces risk of facet degeneration. Clin Neurol Neurosurg. 2013;115:1440–1445.
- Ardeshiri A, Miller LE, Synowitz M, et al. Surgical experience and complications in 50 patients treated with an anular closure device following lumbar discectomy. Orthop Surg. 2019;11:431–437.
- Kursumovic A, Rath SA. Effectiveness of an annular closure device in a “real-world” population: stratification of registry data using screening criteria from a randomized controlled trial. Med Devices (Auckl). 2018;11:193–200.
- Ledic D, Vukas D, Grahovac G, et al. Effect of anular closure on disk height maintenance and reoperated recurrent herniation following lumbar diskectomy: two-year data. J Neurol Surg A Cent Eur Neurosurg. 2015;76:211–218.
- Lequin MB, Barth M, Thome C, et al. Primary limited lumbar discectomy with an annulus closure device: one-year clinical and radiographic results from a prospective, multi-center study. Korean J Spine. 2012;9:340–347.
- Parker SL, Grahovac G, Vukas D, et al. Effect of an annular closure device (Barricaid) on same-level recurrent disk herniation and disk height loss after primary lumbar discectomy: two-year results of a multicenter prospective Cohort study. Clin Spine Surg. 2016;29:454–460.
- Martens F, Vajkoczy P, Jadik S, et al. Patients at the highest risk for reherniation following lumbar discectomy in a multicenter randomized controlled trial. JB JS Open Access. 2018;3:e0037.
- Barth M, Weiss C, Bouma GJ, et al. Endplate changes after lumbar discectomy with and without implantation of an annular closure device. Acta Neurochir (Wien). 2018;160:855–862.
- Bouma GJ, Barth M, Miller LE, et al. Challenges in the analysis of longitudinal pain data: practical lessons from a randomized trial of annular closure in lumbar disc surgery. Pain Res Treat. 2019;2019:3498603.
- Bouma GJ, van den Brink W, Miller LE, et al. Does patient blinding influence clinical outcomes after annular closure device implantation? A propensity score-matched analysis. Orthop Res Rev. 2019;11:177–182.
- Kursumovic A, Bouma GJ, Miller LE, et al. Clinical implications of vertebral endplate disruptions after lumbar discectomy: 3-year results from a randomized trial of a bone-anchored annular closure device. J Pain Res. 2020;13. In press.
- Ament J, Thaci B, Yang Z, et al. Cost-effectiveness of a bone-anchored annular closure device versus conventional lumbar discectomy in treating lumbar disc herniations. Spine (Phila Pa 1976). 2019;44:5–16.
- Ament JD, Thaci B, Yang Z, et al. Postoperative direct healthcare costs of lumbar discectomy are reduced with the use of a novel annular closure device in high-risk patients. Spine J. 2019;19:1170–1179.
- Bostelmann R, Petridis A, Meder A, et al. Who benefits from medical technical innovations? A medical and medical economic analysis using the example of lumbar disc surgery. Orthopade. 2019;49:32–38.
- Klassen PD, Hsu WK, Martens F, et al. Post-lumbar discectomy reoperations that are associated with poor clinical and socioeconomic outcomes can be reduced through use of a novel annular closure device: results from a 2-year randomized controlled trial. Clinicoecon Outcomes Res. 2018;10:349–357.
- Parker SL, Grahovac G, Vukas D, et al. Cost savings associated with prevention of recurrent lumbar disc herniation with a novel annular closure device: a multicenter prospective cohort study. J Neurol Surg A Cent Eur Neurosurg. 2013;74:285–289.
- Thaci B, McGirt MJ, Ammerman JM, et al. Reduction of direct costs in high-risk lumbar discectomy patients during the 90-day post-operative period through annular closure. Clinicoecon Outcomes Res. 2019;11:191–197.
- Barth M, Weiss C, Bouma GJ, et al. Reply to the letter to the editor of E. Shiban and B. Meyer regarding “Endplate changes after lumbar discectomy with and without implantation of an annular closure device” by Barth M et al., (Acta Neurochir (Wien) 2018 Apr;160(4):855-862). Acta Neurochir (Wien). 2018;160:1611–1612.
- Bouma GJ. Answer to the letter to the editor of Dr. Yusuf Izci entitled “anular closure device: is it necessary after discectomy?” concerning “the high-risk discectomy patient: prevention of reherniation in patients with large anular defects using an anular closure device” by G. J. Bouma, M. Barth, D. Ledic, M. Vilendecic (2013) Eur Spine J; 22(5):1030-1036. Eur Spine J. 2014;23:485.
- Grasso G. Reoperations after first lumbar disk herniation surgery with or without implantation of mechanical annular closure device. World Neurosurg. 2019;131:217–219.
- Izci Y. Anular closure device: is it necessary after discectomy? Eur Spine J. 2014;23:483–484.
- Klassen PD, Bernstein DT, Kohler HP, et al. Erratum: bone-anchored annular closure following lumbar discectomy reduces risk of complications and reoperations within 90 days of discharge [Corrigendum]. J Pain Res. 2017;10:2739.
- Lange N, Meyer B, Shiban E. Low-grade infection due to annular closure device. Acta Neurochir (Wien). 2018;160:1867.
- Shiban E, Meyer B. Letter to the editor of Acta Neurochirurgica: endplate changes after lumbar discectomy with and without implantation of an annular closure device. Acta Neurochir (Wien). 2018;160:1609.
- Gautschi OP, Corniola MV, Schaller K. Risk of recurrence and postoperative intervertebral disc degeneration after lumbar intervertebral disc operation - is an anulus closure prosthesis the solution?. Praxis (Bern 1994). 2014;103:775–779.
- Hahn BS, Ji GY, Moon B, et al. Use of annular closure device (Barricaid(R)) for preventing lumbar disc reherniation: one-year results of three cases. Korean J Neurotrauma. 2014;10:119–122.
- Krutko AV, Baykov ES, Sadovoy MA. Reoperation after microdiscectomy of lumbar herniation: case report. Int J Surg Case Rep. 2016;24:119–123.
- Lange N, Meyer B, Shiban E. Symptomatic annulus-repair-device loosening due to a low-grade infection. Acta Neurochir (Wien). 2018;160:199–203.
- Klassen PD, Hes R, Bouma GJ, et al. A multicenter, prospective, randomized study protocol to demonstrate the superiority of a bone-anchored prosthesis for anular closure used in conjunction with limited discectomy to limited discectomy alone for primary lumbar disc herniation. Int J Clin Trials. 2016;3:120–131.
- Strenge KB, DiPaola CP, Miller LE, et al. Multicenter study of lumbar discectomy with Barricaid annular closure device for prevention of lumbar disc reherniation in US patients: a historically controlled post-market study protocol. Medicine (Baltimore). 2019;98:e16953.
- Bostelmann R, Steiger HJ, Cornelius JF. Effect of annular defects on intradiscal pressures in the lumbar spine: an in vitro biomechanical study of diskectomy and annular repair. J Neurol Surg A Cent Eur Neurosurg. 2017;78:46–52.
- Wilke HJ, Ressel L, Heuer F, et al. Can prevention of a reherniation be investigated? Establishment of a herniation model and experiments with an anular closure device. Spine (Phila Pa 1976). 2013;38:E587–593.
- Choy WJ, Phan K, Diwan AD, et al. Annular closure device for disc herniation: meta-analysis of clinical outcome and complications. BMC Musculoskelet Disord. 2018;19:290.
- Ardeshiri A, Miller LE, Thome C. Two-year real-world results of lumbar discectomy with bone-anchored annular closure in patients at high risk of reherniation. Eur Spine J. 2019;28:2572–2578.
- Kursumovic A, Rath S. Performance of an annular closure device in a ‘real-world’, heterogeneous, at-risk, lumbar discectomy population. Cureus. 2017;9:e1824.
- Bouma GJ, Barth M, Ledic D, et al. The high-risk discectomy patient: prevention of reherniation in patients with large anular defects using an anular closure device. Eur Spine J. 2013;22:1030–1036.
- Sanginov AJ, Krutko AV, Baykov ES, et al. Outcomes of surgical treatment of lumbar disk herniation using an annular closure device. Coluna/Columna. 2018;17:188–194.
- Carragee EJ, Han MY, Suen PW, et al. Clinical outcomes after lumbar discectomy for sciatica: the effects of fragment type and anular competence. J Bone Joint Surg Am. 2003;85-A:102–108.
- Kim KT, Lee DH, Cho DC, et al. Preoperative risk factors for recurrent lumbar disk herniation in L5-S1. J Spinal Disord Tech. 2015;28:E571–577.
- Zhou C, Tian YH, Zheng YP, et al. Mini-invasive transforaminal lumbar interbody fusion through wiltse approach to treating lumbar spondylolytic spondylolisthesis. Orthop Surg. 2016;8:44–50.
- Klassen PD, Lesage G, Miller LE, et al. Reoperation after primary lumbar discectomy with or without implantation of a bone-anchored annular closure device: surgical strategies and clinical outcomes. World Neurosurg. 2019;130:e926–e932.
- Jia H, Lubetkin EI, Barile JP, et al. Quality-adjusted Life Years (QALY) for 15 chronic conditions and combinations of conditions among US adults aged 65 and older. Med Care. 2018;56:740–746.
- Bouma GJ, Ardeshiri A, Miller LE, et al. Clinical performance of a bone-anchored annular closure device in older adults. Clin Interv Aging. 2019;14:1085–1094.
- Lorio M, Kim C, Araghi A, et al. ISASS policy 2019 - Surgical treatment of lumbar disc herniation with radiculopathy. Int J Spine Surg. 2020;14:1–7.
- Nanda D, Arts MP, Miller LE, et al. Annular closure device lowers reoperation risk 4 years after lumbar discectomy. Med Devices (Auckl). 2019;12:327–335.