ABSTRACT
Introduction
In transcatheter aortic valve implantation (TAVI), assessment of aortic valve calcification is not as standardized as aortic annulus measurement. Aortic valve calcification is important for stable anchoring of the prosthesis to the aortic annulus. However, excessive aortic valve calcification is related to procedural complications.
Areas covered
This review covers the methods to assess aortic valve calcification and the implications of aortic valve calcium burden for TAVI outcomes. We performed a systematic review of the literature in Pubmed and secondary sources. Furthermore, future perspectives on how to integrate aortic valve calcification assessment in the management of patients with aortic stenosis is discussed.
Expert opinion
Thorough assessment of the aortic valve and aortic root components including aortic valve calcification is key in the planning of TAVI. Aortic valve calcification load, location and extension are important contributors to paravalvular regurgitation. Asymmetric calcification burden with greater calcification of the left-coronary cusp related to higher need of permanent pacemaker implantation. Patients with moderate and severe left ventricular outflow tract/subannular calcification are more susceptible to aortic annular rupture. Periprocedural dislodgement of calcium form cusps and commissures is one of the main reasons of coronary artery ostial occlusion during transcatheter aortic valve implantation.
Abbreviations
Ao, aorta; LA, left atrium; LAA, left atrial appendage; LV, left ventricle; LVOT, left ventricular outflow tract; THV, transcatheter heart valve
1. Introduction
Transcatheter aortic valve implantation (TAVI) has been a therapeutic breakthrough for patients with symptomatic severe aortic stenosis (AS) who have contraindications for surgery or have high operative risk, demonstrating to provide better outcomes as compared to medical therapy or surgical aortic valve replacement [Citation1,Citation2]. Subsequent randomized trials in patients with symptomatic severe AS and intermediate or low operative risk have also shown that TAVI is non-inferior to surgical aortic valve replacement [Citation3–5]. However, procedural-related complications still remain a concern due to its impact on morbidity and mortality.
Paravalvular aortic regurgitation and conduction disturbances are the most frequent complications. In the Placement of Aortic Transcatheter Valves (PARTNER) trials, moderate-to-severe paravalvular regurgitation was observed in 12% of patients at 30 days and 11% at 1 year in patients not suitable for surgery, and in 12.2% at 30 days and 6.8% at 1 year in patients with high surgical risk [Citation1,Citation2]. A fast learning curve and new generation TAVI prostheses led to a considerable decrease in the incidence of moderate-to-severe paravalvular regurgitation, and in the PARTNER 2 trial (including intermediate-risk patients), this complication was only observed in 3.8% at 30 days and 3.4% at 1 year follow-up [Citation3]. The most frequent conduction disturbances after TAVI are left bundle branch block (LBBB) and high-degree atrioventricular block. The 30-day rate of new-onset LBBB after TAVI is around 27% (ranging from 4% to 57%) and the rate of high-degree atrioventricular block requiring permanent pacemaker implantation is 17% (ranging from 2% to 51%) [Citation6,Citation7]. These rates vary according to the type of prosthesis (balloon or self-expandable), depth of implantation (deeper into the left ventricular outflow tract [LVOT]), and the presence of conduction abnormalities prior to the procedure.
In contrast, aortic annulus rupture and coronary ostia occlusion are infrequent but are the most life-threatening complications. Aortic annulus rupture encompasses a spectrum of lesions with very low prevalence (about 0.5% to 1.0% of TAVI procedures) but with a reported mortality of 75% and usual need of open-heart surgery [Citation8]. The incidence of coronary ostial occlusion is <1% and the left main coronary artery is the most frequently affected (88.6%). The 30-day mortality rate of this complication reaches 40% [Citation9].
One of the factors that has been involved in all these complications is the burden of aortic valve calcification. Asymmetric accumulation of calcium predominantly in one cusp, the extent into the aortic root and/or LVOT, and the total amount of calcium in the region where the prosthesis is deployed are the parameters that have been related to complications. However, the evidence is controversial, and it is well accepted that some degree of calcification is needed to ensure stable implantation of the prosthesis. Therefore, the assessment of aortic valve calcification burden is not yet standardized and systematically included in the preprocedural evaluation.
This review will focus on the methodology to assess aortic valve calcium burden, the evidence showing how aortic valve calcification can influence the outcomes of TAVI and what are the potential solutions. We performed a systematic review of the literature on Pubmed including the terms ‘aortic valve calcification,’ ‘transcatheter aortic valve implantation,’ ‘paravalvular leakage,’ ‘conduction disturbances,’ ‘coronary artery occlusion,’ and ‘annular rupture.’ Further studies were sought by means of a manual search of secondary resources, including references from primary papers.
2. How to assess aortic valve calcification
Pre-procedural assessment of aortic valve anatomy and AS severity can be performed with 2-dimensional echocardiography in combination with 3-dimensional imaging methods such as 3-dimensional transoesophageal echocardiography (TOE) or multidetector row computed tomography (MDCT) [Citation10]. MDCT is the best imaging tool for procedural planning because it provides information on the dimensions of the aortic annulus, location, and quantification of calcification of the aortic valve, LVOT, and aortic root and ascending aorta, and distance of coronary arteries ostia from aortic valve annulus plane [Citation11]. In addition, MDCT is key to decide on the procedural access by assessing the caliber of the femoral arteries.
Assessment of aortic valve calcification is accomplished by calculating the computed tomography aortic valve calcium (CT-AVC) score on non-contrast enhanced data. This parameter has a good correlation with the measurement of calcium in explanted native valves and with the valve hemodynamics [Citation12]. It is important to note that there are sex-specific CT-AVC thresholds that indicate severe AS (≥1,275 arbitrary units (AU) in women and ≥2,065 AU in men), which are independent predictors of all-cause mortality, with higher values such as ≥1,600 AU for women and ≥3,000 AU for men making the diagnosis of severe AS very likely [Citation13,Citation14]. CT-AVC score quantification requires good visualization of the aortic valve and should be done in multiple planes to include only the calcium of the aortic valve and to exclude the nonvalvular calcification of the LVOT and aortic sinus (). On contrast-enhanced images, measurement of the CT-AVC load relies on the degree of opacification which results from the interaction of factors related to the patient, the scanner, and contrast material. Moreover, the threshold for CT-AVC derived from these scans, in order to determine AS severity, has not been standardized [Citation12].
Figure 1. Evaluation of aortic valve calcification with computed tomography
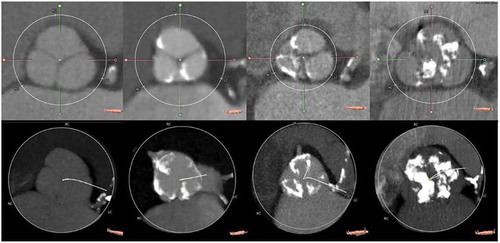
The aortic valve cusps, aortic annulus, and LVOT are the components of the so-called device landing zone, and a high burden calcification of this region is linked to TAVI complications. Estimation of annular and sub-annular (upper 4 to 5 mm of LVOT) calcification with MDCT is based on the circumferential extent, the depth into the LVOT, and the thickness of calcification projecting radially into the LVOT. Annular and sub-annular calcification should be described (as crescent/flat/adherent or protruding) as well as its relation to the aortic cusps. The estimation is performed in a subjective and qualitative manner as none, mild (single, adherent, non-protruding focus of calcification), moderate (two or more nodules of calcification or a single nodule with limited protrusion into annular/sub-annular lumen) and severe (single or multiple nodules of calcification, protruding into the annular lumen, and/or extending into the LVOT) [Citation15].
3. Aortic valve calcification and paravalvular regurgitation
Rates of significant paravalvular regurgitation after surgical aortic valve replacement are considerably low and stable throughout the years. This can be explained by calcium excision and suture of the prosthesis directly to the aortic annulus, with more control when sealing potential areas for regurgitation [Citation16]. Implantation of transcatheter heart valve in a very calcified aortic valve may prevent full and symmetric prosthesis expansion, contributing to post-procedural residual leakage (). The association between the presence of moderate or greater paravalvular regurgitation and increased morbidity and mortality after TAVI was reported in several studies and meta-analyses [Citation17–23]. The pooled estimate for moderate to severe paravalvular regurgitation post-TAVI was 11.7%, with higher rates observed for self-expandable prostheses as compared to balloon-expandable prostheses [Citation24].
Figure 2. Association between aortic valve calcification and paravalvular regurgitation after transcatheter aortic valve implantation
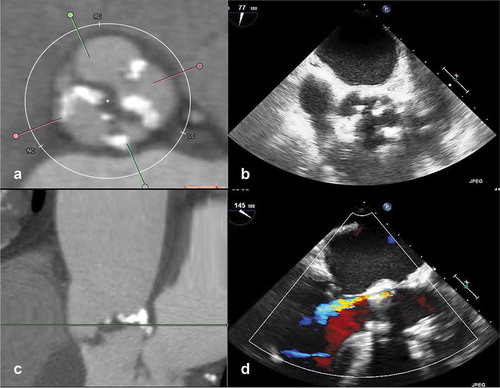
Several studies showed significant associations between aortic valve calcification and paravalvular regurgitation after TAVI () [Citation25–38]. Ewe et al. correlated aortic valve calcification volume and location of the bulkiest calcifications on CT with the location of paravalvular regurgitation on transesophageal echocardiography in 79 patients who underwent TAVI with a balloon-expandable prosthesis and showed that patients with greater aortic valve calcification volume at baseline had a greater prevalence of paravalvular regurgitation [Citation25]. It is important to acknowledge that besides aortic valve calcification load, the location and extension of the calcification are important contributors to significant paravalvular regurgitation. The amount of calcium at the aortic wall was the main determinant of paravalvular regurgitation at the corresponding site. In addition, Feuchtner et al. found that the presence of aortic annulus calcification greater than mild and protruding annulus calcification greater than 4 mm, particularly at the left and non-coronary cusps, predicted subsequent development of significant paravalvular regurgitation [Citation26]. Patients with extremely high aortic valve calcification (AVC score > 8,000 Agatston units) who underwent balloon-expandable TAVI had significantly lower device success rates (80% vs 95.3%) and more significant paravalvular regurgitation (15% vs 1.8%) [Citation39].
Table 1. Aortic valve calcification and incidence of paravalvular regurgitation after transcatheter aortic valve implantation
First-generation transcatheter heart valves were used in the majority of the aforementioned studies. The impact of aortic calcification on postprocedural paravalvular regurgitation was also assessed for new generation prosthesis, either balloon-expandable and/or self-expandable. Akodad et al. compared the incidence of paravalvular leakage between patients who underwent TAVI with first-generation and new generation balloon- and self-expandable prostheses and found lower rates of residual regurgitation in the new generation group (8.3% vs 22.9%). Aortic valve calcium score was strongly associated with the occurrence of paravalvular regurgitation in first-generation patients but not in the new generation group [Citation40]. Guimaraes et al. assessed the impact of calcium score on the performance of a balloon-expandable prosthesis with an outer skirt aiming at better sealing and found similar rates of paravalvular regurgitation between patients with high and low indexed calcium score. There was also no impact of aortic valve calcification on residual regurgitation in the patients with extreme indexed AV calcium score [Citation41].
4. Aortic valve calcification and conduction abnormalities
The occurrence of conduction disturbances in patients with AS is explained by the intimate relationship of the aortic valve and the membranous portion of the interventricular septum. The His bundle pierces the membranous septum in the right atrium and exits in the central fibrous body on the left side, which comprises the membranous septum and the fibrous triangle limited by the leaflets of the right and non-coronary cusps, and is in close proximity to the right-coronary cusp [Citation7]. The association between the presence of severe calcifications in the device landing zone and the occurrence of conduction abnormalities due to damage of the conduction system has not been systematically demonstrated.
In a study with 300 patients who underwent TAVI (81.3% balloon-expandable valves), aortic valve calcium quantification was not predictive of the 30-day pacemaker implantation rate (odds ratio for 500 unit increase: 1.05, p = 0.275) [Citation42]. Conversely, in a study with 81 patients who underwent TAVI with self-expandable valves (CoreValve ReValving system), the device landing zone calcification was predictive of permanent pacemaker implantation (odds ratio 1.06, p = 0.004) [Citation43] (). It is important to point out that the total rate of permanent pacemaker implantations was 19% in the former study versus 47% in the latter and that these differences may be related to other factors than the calcification of the device landing zone and type of prosthesis implanted.
Figure 3. Implications of aortic valve calcification and conduction abnormalities after transcatheter aortic valve implantation
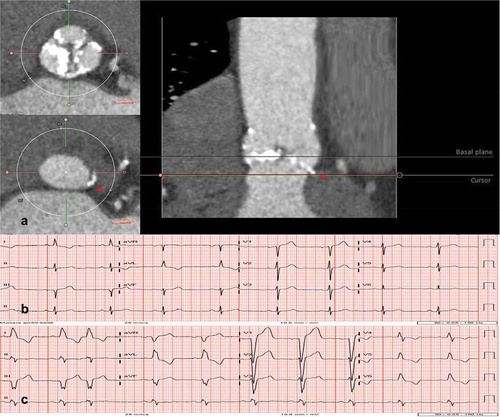
Although the association between the CT-AVC and the need for permanent pacemaker implantation is controversial, the asymmetric calcific burden is a relevant variable. Fujita et al. showed that greater calcification of the left-coronary cusp was related to a higher incidence of complete atrioventricular block and the need for permanent pacemaker implantation [Citation44]. This asymmetric pattern of calcification leads to the increased radial force exerted on the non-coronary and right-coronary cusps and adjacent structures (including the His bundle and the left-ventricular bundle branch). Mauri et al. found that LVOT calcium volume in the left-coronary cusp sector above a threshold of 41.4 mm3 was a risk factor for pacemaker implantation, but the device landing zone calcium volume and calcification of the aortic cusps were similar in patients with and without need for permanent pacemaker implantation [Citation31]. In addition, the asymmetry in calcification between the left-coronary cusp (high) and the non-coronary cusp (low) was significantly higher in patients requiring pacemaker implantation.
5. Aortic valve calcification and aortic annulus rupture
Aortic annulus rupture is a life-threatening complication that comprises uncontained and contained aortic annulus rupture (), periaortic hematoma, and ventricular septal rupture, resulting from disruption of the aortoventricular junction after balloon valvuloplasty, valve implantation, or valve redilatation because of residual paravalvular regurgitation [Citation45–47]. Subannular damage is also of importance. The LVOT has a fibrous component and a muscular, more vulnerable, portion. The fibrous component of the LVOT is composed of the aortomitral continuity and the membranous portion of the interventricular septum, formed by strong and distensible tissue. Injury of this region may result in acute mitral regurgitation or perimembranous ventricular septal defect. The muscular portion has a small segment located below the commissure between the left and right-coronary cusps and left fibrous trigone which has no external support, and injury to this site represents an extreme risk of fatal bleeding [Citation8]. Clinical manifestations usually occur during TAVI or in the first 3 hours after the procedure; rarely, small injuries remain silent and are diagnosed later when the rupture progresses leading to paravalvular leak or peri-aortic root hematoma ().
Figure 4. Aortic annulus rupture in an 88 year-old patient receiving a balloon-expandable transcatheter aortic valve prosthesis
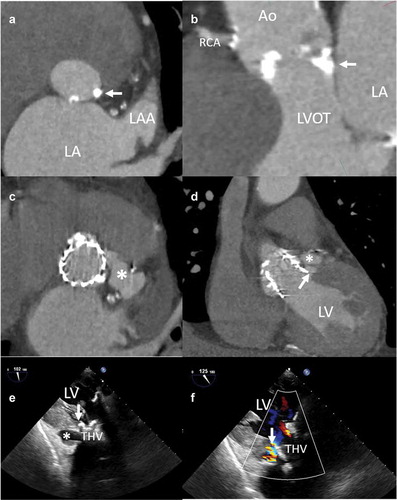
A high calcification burden of the aortic valve cusps, annular calcification (especially circular), presence of calcification adjacent to the annulus (wall of sinuses of Valsalva and LVOT), and a heavily calcified bicuspid valve are anatomic characteristics that may predispose annular rupture [Citation48,Citation49]. Hansson et al. reported on the effect of aortic valve calcium volume and distribution on 33 patients experiencing aortic root injury during TAVI with balloon-expandable valve and compared them to a control group without aortic root injury [Citation49]. Upper LVOT (2 mm distance down from the annulus plane) calcium volume compared to LVOT (10 mm distance down from the annulus plane), and upper LVOT calcium below noncoronary cusp were the strongest predictors of aortic root injury. Barbanti et al. identified MDCT anatomical predictors of aortic root rupture in 31 patients who underwent TAVI with balloon-expandable devices compared to a matched sample of 31 patients without aortic root rupture [Citation50]. Burden of LVOT/subannular calcification was higher in patients with aortic root rupture, but no difference was found in the rate of moderate/severe aortic valve cusp calcification. Calcification of the LVOT below the left and non-coronary cusps was comparable between groups. However, calcification of the LVOT below the right-coronary cusp was present only in patients experiencing annular rupture, which might be explained by a higher radial force in the contralateral susceptible portion of the muscular LVOT and injury to this unprotected region. Moderated and severe LVOT/subannular calcification (odds ratio 10.92, p < 0.001) and prosthesis oversizing ≥20% (odds ratio 8.38, p < 0.001) were associated with aortic root rupture.
6. Aortic valve calcification and coronary ostia occlusion
Coronary artery ostial obstruction during or after TAVI is a life-threatening situation and must be considered when periprocedural hemodynamic deterioration, electrocardiographic changes, and ventricular arrhythmias occur. The left main coronary ostium is the most frequently affected and the obstruction is mainly the consequence of dislocated calcium from the cusps or commissures after balloon dilatation and/or prosthesis expansion [Citation51].
In a large multicenter registry including 6,688 patients from 81 centers worldwide, the incidence of coronary obstruction was 0.66%, with higher rates in patients who received balloon-expandable valves (0.81% versus 0.34% in self-expandable devices) and in patients with a previous aortic bioprosthesis who underwent a valve-in-valve procedure (incidence of 2.4%) [Citation9]. Narrow aortic root (<28 mm at the sinuses of Valsalva) and short distance between the aortic annulus and the coronary ostia (<10 mm) increase the likelihood of obstruction of the coronary artery ostia by native valve leaflets when the aortic bioprosthesis is deployed [Citation52,Citation53]. This complication is more frequently observed in women (>80%) probably related to the smaller sinus of Valsalva dimensions and lower coronary ostia height as compared to men. Global aortic valve calcium load, however, was not associated with the occurrence of coronary obstruction in this registry, although the role of calcium nodules was not assessed.
7. Aortic valve calcification and stroke
Cerebrovascular events (CVE) are potential periprocedural complications of surgical or transcatheter aortic valve replacement. Independent risk factors of acute periprocedural CVE comprise a new-onset of atrial fibrillation and calcification of the aortic arch [Citation54]. Calcification of aortic valve was also demonstrated to be associated with emboli [Citation55–57]. Analysis of aortic valve calcification/degeneration by 2D TOE showed that patients in the upper quartile of the aortic valve calcification score had significantly more acute CVE detected in diffusion-weighted brain magnetic resonance imaging compared to the ones in the lower quartile [Citation56]. Moreover, calcium load in LVOT beneath the right-coronary cusp, measured by MDCT, was significantly associated with stroke. This could be explained by the fact that the calcium deposit on the interventricular septum could be more susceptible to embolization or that bloodstream reaching this region favors the supra-aortic branches as targets for emboli [Citation57].
Clinical trials comparing TAVI and surgical aortic valve replacement (SAVR) showed a significant higher incidence of stroke at 30 days in high surgical risk TAVI patients (5.5% TAVI vs 2.4% SAVR in PARTNER trial) and low surgical risk SAVR patients (0.6% TAVI vs 2.4% SAVR in PARTNER 3 trial) whereas data are controversial for intermediate-risk patients (5.5% TAVI vs 6.1% SAVR in PARTNER 2 trial – non-significant difference -, and 4.5% TAVI vs 6.5% SAVR in SURTAVI trial – significant difference) [Citation2–4,Citation58]. However, long-term follow-up rates of stroke are not significantly different between patients who underwent TAVI and SAVR, irrespective of surgical risk [Citation20,Citation59–61]. Nonetheless, TAVI patients who received balloon-expandable valves had a significantly higher incidence of stroke compared to the ones who received self-expandable valves [Citation62,Citation63]. The rate of new radiographic cerebral lesions after aortic valve replacement can be higher than 60%, and although only a minority of patients develop a focal neurological deficit, they have been shown to negatively affect cognitive function and increase the risk of future stroke [Citation64–68]. Aiming at reducing the load of emboli reaching cerebral vascular territory, embolic protection devices were developed. Embolic material was observed in 99% of patients from 2 prospective studies and included thrombi, valve tissue, arterial wall/necrotic core, calcification, foreign material, and myocardium [Citation69]. Despite a good rationale for their use and reduction of the burden of cerebral embolism during TAVI, the studies have not clearly demonstrated a decline in hard clinical outcomes, such as mortality and stroke [Citation64,Citation70,Citation71].
8. Expert opinion
The use of MDCT to evaluate patients with severe AS who may be candidates for TAVI is crucial. Aortic valve calcium load is an important parameter to identify patients with severe AS among those with discordant grading on echocardiography. In this scenario, according to the current European guidelines on valvular heart disease, calcium score by CT is recommended in patients with low-flow and low-gradient (LFLG) status: (1) with reduced LVEF and no flow reserve on dobutamine stress echocardiography, or (2) with preserved LVEF (≥50%) as part of an integrated approach to determine AS severity [Citation14]. Assessment of calcium load is also important to identify patients at increased risk for the development of paravalvular aortic regurgitation, conduction abnormalities following TAVI. The evidence showing the association between bulky, asymmetric calcifications of the device landing zone and the presence of significant residual paravalvular regurgitation has helped to modify the design of the transcatheter prostheses to decrease this complication rate [Citation72].
Discrepancies between the severity of AS by echocardiographic criteria and AV calcium score by CT can occur. A study reported that 16% of patients with severe AS who underwent TAVI had AV calcium score below the threshold determined for likely severe AS, mentioned in Section 2 [Citation73]. Comparison of outcomes between groups with high and low calcification showed similar device success rate and survival up to 18 months after TAVI, but significantly lower rates of paravalvular regurgitation in the group with low aortic valve calcium score. In this group of patients, performing TAVI with balloon-expandable valve was associated with higher device success and decreased rates of residual regurgitation.
The rates of post-TAVI permanent pacemaker implantation in patients who received self-expandable transcatheter heart valves remain higher compared to surgical aortic valve replacement [Citation58,Citation74,Citation75]. It might be explained by the extended frame length in the LVOT that comes in direct contact with the portion of the membranous interventricular septum where the conduction system is more superficial [Citation76]. In patients with conduction disturbances post-TAVI, the data on the role of aortic valve calcium score are conflicting, but calcification asymmetry is a relevant variable because it determines higher exertional forces in the central fibrous trigone with temporarily (edema, hematoma) or permanent damage of the conduction system and need of permanent pacemaker implantation. Aortic valve calcification is also related to life-threatening complications. The incidence of these entities is <1.0% and to minimize the risk, proper preprocedural measures regarding accurate annular sizing and prosthesis type selection must be assessed.
The impact of aortic valve calcification in patients with LFLG severe AS who underwent TAVI was analyzed using tertiles of AVCdensity, a ratio of total AVC load to the aortic annulus area measured by contrast-enhanced CT [Citation77]. There was no significant difference in permanent pacemaker implantation rates according to aortic valve calcification, whereas rates of more-than-mild paravalvular leakage were higher in patients with high AVCdensity compared to low AVCdensity (significant for classical LFLG and non-significant trend for paradoxical LFLG). The impact of aortic valve calcification on survival was present only in patients with classical LFLG: (1) patients of the high AVCdensity tertile had lower mortality rates at 1 and 3 years after TAVI compared to their counterparts, which may underscore a not beneficial effect of TAVI for patients with a lower degree of calcification in this subtype of severe AS, and (2) AVCdensity was the strongest independent predictor for survival after TAVI on multivariable analysis.
The majority of the data correlating the association of aortic valve calcification and these complications concern patients with tricuspid aortic valve. Extending this therapy to patients with intermediate and low risk may lead to a higher probability of bicuspid aortic valve since those patients are usually younger than patients with contraindications and high risk for surgery. Patients with bicuspid aortic valve were excluded from the main TAVI randomized clinical trials since they have peculiar anatomy of the valve and different distributions of calcification as compared to tricuspid aortic valve [Citation1–3]. The higher burden of calcification of the annulus and cusps, abnormal cusp fusion with asymmetric orifice, and presence of raphe are postulated to affect transcatheter heart valve expansion which can lead to less optimal results [Citation78–80]. In this population, MDCT is useful to provide information on leaflet morphology, symmetry of valve leaflets, presence of raphe, and location of calcification [Citation81]. Data on aortic valve calcium score in this population are scarce and conflicting. Ferda et al. analyzed 37 patients with severe AS, 13 with bicuspid aortic valve and 24 with tricuspid aortic valve, who had non-contrast MDCT prior to valve surgery and showed that aortic valve calcium score was not significantly different between groups (1,168 ± 717 AU in bicuspid aortic valve vs 795 ± 530 AU in tricuspid aortic valve, p-value = 0.093) [Citation82]. On the other hand, Watanabe et al. analyzed retrospectively 67 patients from a population of high-risk patients with severe AS treated with TAVI. Of 67 patients, 11 had bicuspid aortic valve morphology. Patients with bicuspid aortic valve had significantly higher aortic valve calcification volume and calcification index as compared to patients with tricuspid aortic valve (1,263 ± 396 AU vs 556 ± 462 AU, p < 0.01 and 744 ± 218 AU vs 313 ± 251 AU, p < 0.01, respectively) [Citation83]. The reported rates of permanent pacemaker implantation after TAVI were similar for balloon- and self-expandable valves in patients with bicuspid aortic valves in two different studies: Mylotte et al. reported rates of 16.7% for balloon-expandable and 26.7% for self-expandable devices, while Jilaihawi et al. reported 25.5% for balloon-expandable and 26.9% for self-expanding devices [Citation81,Citation84,Citation85].
Recent randomized trials on low-risk patients treated with TAVI have reported a reduction of these complications and similar rates to those of surgical aortic valve replacement [Citation5]: significant paravalvular regurgitation was 0.8% in the TAVI group vs 0.0% in the surgical aortic valve replacement group at 30 days, and respective 0.6% vs 0.5% at 1 year; and new pacemaker implantation rate was 6.6% in the TAVI arm vs 4.1% in surgical arm at 30 days and respective 7.5% vs 5.5% at 1 year. However, patients treated with TAVI had a higher prevalence of new left bundle branch block as compared to the surgically treated patients (22.0% vs 8.0% at 30 days and 23.7% vs 8.0% at 1 year, respectively). It is postulated that the annular calcification burden might play a role in these results but further analysis is needed to identify the determinants of outcomes in the low-surgical risk patients [Citation86].
The relevance of assessing the aortic valve calcification prior to TAVI will be demonstrated in ongoing studies including patients in whom a watchful waiting strategy and medical therapy are currently the standard of care, unless there are other concomitant conditions that need cardiac surgery (for example, coronary revascularization). While accurate measurement of the aortic valve annulus to select the prosthesis size and accurate assessment of the femoral artery anatomy remain key to ensure successful transfemoral TAVI, the amount of aortic valve calcification may impact on the results of this therapy. It is worthy to point out that current guidelines on the management of valvular heart disease recommend that SAVR must be preferred in patients with acceptable surgical risk when valve morphology characteristics, such as degree of calcification and calcification pattern, are unfavorable to TAVI [Citation14].
The Evaluation of Transcatheter Aortic Valve Replacement Compared to Surveillance for Patients with Asymptomatic Severe Aortic Stenosis (EARLY-TAVR ClinicalTrials.gov number, NCT03042104) randomizes patients with asymptomatic severe AS and preserved left ventricular ejection fraction to TAVI or to a watchful waiting strategy. As per current guidelines, these patients can follow a watchful waiting strategy and do not need valve replacement. The association between aortic valve calcium load and all-cause mortality has been demonstrated [Citation13]. However, it will be very interesting to know whether the aortic valve calcification load impacts on patient outcomes allocated to the TAVI arm and how it compares to medically treated patients in terms of outcomes.
In addition, the Transcatheter Aortic Valve Replacement to Unload the Left Ventricle in Patients with Advanced Heart Failure (TAVR-UNLOAD) trial randomizes patients with moderate AS and reduced left ventricular ejection fraction to TAVI or to medical therapy [Citation87]. These patients usually undergo aortic valve replacement if an associated intervention is needed (particularly coronary revascularization). The hypothesis driving the trial is the fact that the stenotic aortic valve can superimpose a pressure overload to a dysfunctioning left ventricle and by replacing the valve, the hemodynamics of the left ventricle improve leading to better outcomes as compared to patients treated medically. Patients with moderate AS may have less calcium load of the aortic valve than the patients with severe AS and this may impact on the outcomes of TAVI. The prosthesis size may be larger to ensure stable implantation and reduce paravalvular leakage.
The long-term impact of new valve technology, such as fully retrievable devices, on outcomes, is still to be determined. Repositionable and fully retrievable self-expandable transcatheter valves were designed to achieve accurate positioning and minimize paravalvular regurgitation. Short-term outcomes studies showed high success rate with this device, with 30-day all-cause mortality ranging from 2.6% to 4.2%, overall stroke rates ranging from 3.0% to 5.9%, moderate/severe paravalvular regurgitation from 0.3% to 1.0%, and permanent pacemaker implantation from 28.6% to 30.0%, with a good safety profile although with a pacemaker need similar to the first generation prostheses [Citation88,Citation89].
Furthermore, data on the safety and efficacy of TAVI in bicuspid aortic valve are growing and will provide additional evidence on how aortic valve calcification may influence on the outcomes. It could be speculated that the specific location of the aortic valve calcification (affecting the fusion raphe or the asymmetric calcium load of one cusp) may be more relevant than the total calcium load.
The results of the trials and registries will help to standardize the assessment of the aortic valve calcium and to understand when and how we need to quantify this aspect.
Article highlights
Transcatheter aortic valve implantation has expanded from non-operable, to high-, intermediate, and low-surgical risk patients with severe aortic stenosis.
Aortic valve calcification load and location are related to adverse outcomes during and after TAVI.
Aortic valve calcium load and distribution and extension in the landing zone are quantified by MDCT. It is worth noticing that there are different cut-off values of calcification for men and women with severe aortic stenosis, with lower values for women despite the same aortic stenosis severity compared to men.
Aortic valve calcification load plays an important role in PVL, as well as location and extension in the device landing zone, asymmetric calcification is relevant for conduction disturbances, and extension to LVOT/subannular region impacts on the chances of aortic annular rupture.
Patients with bicuspid aortic valve have distinct calcification characteristics compared to patients with tricuspid aortic valve.
Assessment of calcification burden may aid in the choice of prosthesis type aiming to prevent life-threatening complications.
Declaration of interest
SM Pio has received funding from the European Society of Cardiology in the form of an ESC training grant (T-2018-17405). V Delgado has received speaker fees from Abbott Vascular, Edwards Lifesciences, MSD, Medtronic and GE Healthcare. JJ Bax has received speaker fees from Abbott Vascular. The department of Cardiology of the Leiden University Medical Center receives unrestricted research grants from Abbott Vascular, Bayer, Biotronik, Bioventrix, Boston Scientific, Edwards Lifesciences, GE Healthcare and Medtronic. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they are a consultant for Japan Lifeline. Another reviewer has disclosed that they are a Transcatheter valve proctor for Edwards Life Sciences. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010 Oct 21;363(17):1597–1607.
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011 Jun 9;364(23):2187–2198.
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016 Apr 28;374(17):1609–1620.
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019 May 2;380(18):1695–1705.
- Waksman R, Rogers T, Torguson R, et al. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis. J Am Coll Cardiol. 2018 Oct 30;72(18):2095–2105.
- Bax JJ, Delgado V, Bapat V, et al. Open issues in transcatheter aortic valve implantation. Part 2: procedural issues and outcomes after transcatheter aortic valve implantation. Eur Heart J. 2014 Oct 7;35(38):2639–2654.
- Barbanti M, Gulino S, Costa G, et al. Pathophysiology, incidence and predictors of conduction disturbances during transcatheter aortic valve implantation. Expert Rev Med Devices. 2017 Feb;14(2):135–147.
- Girdauskas E, Owais T, Fey B, et al. Subannular perforation of left ventricular outflow tract associated with transcatheter valve implantation: pathophysiological background and clinical implications. Eur J Cardio-thorac Surg. 2017 Jan;51(1):91–96.
- Ribeiro HB, Webb JG, Makkar RR, et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol. 2013 Oct 22;62(17):1552–1562.
- Zamorano J, Goncalves A, Lancellotti P, et al. The use of imaging in new transcatheter interventions: an EACVI review paper. Eur Heart J Cardiovasc Imaging. 2016 Aug;17(8):835–835af.
- Achenbach S, Delgado V, Hausleiter J, et al. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr. 2012 Nov-Dec;6(6):366–380.
- Pawade T, Sheth T, Guzzetti E, et al. Why and how to measure aortic valve calcification in patients with aortic stenosis. JACC Cardiovasc Imaging. 2019 Sep;12(9):1835–1848.
- Clavel MA, Pibarot P, Messika-Zeitoun D, et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol. 2014 Sep 23;64(12):1202–1213.
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017 Sep 21;38(36):2739–2791.
- Blanke P, Weir-McCall JR, Achenbach S, et al. Computed Tomography Imaging in the Context of Transcatheter Aortic Valve Implantation (TAVI)/Transcatheter Aortic Valve Replacement (TAVR): an expert consensus document of the society of cardiovascular computed tomography. JACC Cardiovasc Imaging. 2019 Jan;12(1):1–24.
- Dvir D, Barbash IM, Ben-Dor I, et al. Paravalvular regurgitation after transcatheter aortic valve replacement: diagnosis, clinical outcome, preventive and therapeutic strategies. Cardiovasc Revascularization Med incl Mol Interventions. 2013 May-Jun;14(3):174–181.
- Sinning JM, Werner N, Nickenig G, et al. Transcatheter aortic valve implantation: upcoming new devices. Interv Cardiol Clin. 2012 Jan;1(1):37–43.
- Webb JG, Wood DA. Current status of transcatheter aortic valve replacement. J Am Coll Cardiol. 2012 Aug 7;60(6):483–492.
- Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011 Jan 25;123(3):299–308.
- Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012 May 3;366(18):1686–1695.
- Gilard M, Eltchaninoff H, Iung B, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med. 2012 May 3;366(18):1705–1715.
- Hayashida K, Lefevre T, Chevalier B, et al. Impact of post-procedural aortic regurgitation on mortality after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2012 Dec;5(12):1247–1256.
- Gotzmann M, Korten M, Bojara W, et al. Long-term outcome of patients with moderate and severe prosthetic aortic valve regurgitation after transcatheter aortic valve implantation. Am J Cardiol. 2012 Nov 15;110(10):1500–1506.
- Athappan G, Patvardhan E, Tuzcu EM, et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol. 2013 Apr 16;61(15):1585–1595.
- Ewe SH, Ng AC, Schuijf JD, et al. Location and severity of aortic valve calcium and implications for aortic regurgitation after transcatheter aortic valve implantation. Am J Cardiol. 2011 Nov 15;108(10):1470–1477.
- Feuchtner G, Plank F, Bartel T, et al. Prediction of paravalvular regurgitation after transcatheter aortic valve implantation by computed tomography: value of aortic valve and annular calcification. Ann Thorac Surg. 2013 Nov;96(5):1574–1580.
- Koos R, Mahnken AH, Dohmen G, et al. Association of aortic valve calcification severity with the degree of aortic regurgitation after transcatheter aortic valve implantation. Int J Cardiol. 2011 Jul 15;150(2):142–145.
- Haensig M, Lehmkuhl L, Rastan AJ, et al. Aortic valve calcium scoring is a predictor of significant paravalvular aortic insufficiency in transapical-aortic valve implantation. Eur J Cardio-thorac Surg. 2012 Jun;41(6):1234–1240. discussion 1240-1.
- John D, Buellesfeld L, Yuecel S, et al. Correlation of device landing zone calcification and acute procedural success in patients undergoing transcatheter aortic valve implantations with the self-expanding corevalve prosthesis. JACC Cardiovasc Interv. 2010 Feb;3(2):233–243.
- Schultz CJ, Tzikas A, Moelker A, et al. Correlates on MSCT of paravalvular aortic regurgitation after transcatheter aortic valve implantation using the Medtronic CoreValve prosthesis. Catheter Cardiovasc Interv. 2011 Sep 1;78(3):446–455.
- Mauri V, Deuschl F, Frohn T, et al. Predictors of paravalvular regurgitation and permanent pacemaker implantation after TAVR with a next-generation self-expanding device. Clin Res Cardiol. 2018 Aug;107(8):688–697.
- Pollari F, Grossmann I, Vogt F, et al. Risk factors for atrioventricular block after transcatheter aortic valve implantation: a single-centre analysis including assessment of aortic calcifications and follow-up. Europace. 2019 Jan 9;21(5):787–795.
- Unbehaun A, Pasic M, Dreysse S, et al. Transapical aortic valve implantation: incidence and predictors of paravalvular leakage and transvalvular regurgitation in a series of 358 patients. J Am Coll Cardiol. 2012 Jan 17;59(3):211–221.
- Colli A, D’Amico R, Kempfert J, et al. Transesophageal echocardiographic scoring for transcatheter aortic valve implantation: impact of aortic cusp calcification on postoperative aortic regurgitation. J Thorac Cardiovasc Surg. 2011 Nov;142(5):1229–1235.
- Pavicevic J, Nguyen TD, Caliskan E, et al. Aortic valve calcium score is a significant predictor for the occurrence of post-interventional paravalvular leakage after transcatheter aortic valve implantation - Results from a single center analysis of 260 consecutive patients. Int J Cardiol. 2015 Feb 15;181:185–187.
- Delgado V, Ng AC, van de Veire NR, et al. Transcatheter aortic valve implantation: role of multi-detector row computed tomography to evaluate prosthesis positioning and deployment in relation to valve function. Eur Heart J. 2010 May;31(9):1114–1123.
- Koh EY, Lam KY, Bindraban NR, et al. Aortic valve calcification as a predictor of location and severity of paravalvular regurgitation after transcatheter aortic valve implantation. Interact Cardiovasc Thorac Surg. 2015 Mar;20(3):345–350.
- Mihara H, Shibayama K, Berdejo J, et al. Impact of device landing zone calcification on paravalvular regurgitation after transcatheter aortic valve replacement: a real-time three-dimensional transesophageal echocardiographic study. J Am Soc Echocardiogr. 2015 Apr;28(4):404–414.
- Abramowitz Y, Jilaihawi H, Chakravarty T, et al. Balloon-expandable transcatheter aortic valve replacement in patients with extreme aortic valve calcification. Catheter Cardiovasc Interv. 2016 May;87(6):1173–1179.
- Akodad M, Lattuca B, Agullo A, et al. Prognostic impact of calcium score after transcatheter aortic valve implantation performed with new generation prosthesis. Am J Cardiol. 2018 May 15;121(10):1225–1230.
- Guimarães L, Ferreira-Neto AN, Urena M, et al. Transcatheter aortic valve replacement with the balloon-expandable SAPIEN 3 valve: impact of calcium score on valve performance and clinical outcomes. Int J Cardiol. 2020 May 1;306:20–24.
- Al-Azzam F, Greason KL, Krittanawong C, et al. The influence of native aortic valve calcium and transcatheter valve oversize on the need for pacemaker implantation after transcatheter aortic valve insertion. J Thorac Cardiovasc Surg. 2017 May;153(5):1056–1062.e1.
- Latsios G, Gerckens U, Buellesfeld L, et al. “Device landing zone” calcification, assessed by MSCT, as a predictive factor for pacemaker implantation after TAVI. Catheter Cardiovasc Interv. 2010 Sep 1;76(3):431–439.
- Fujita B, Kutting M, Seiffert M, et al. Calcium distribution patterns of the aortic valve as a risk factor for the need of permanent pacemaker implantation after transcatheter aortic valve implantation. Eur Heart J Cardiovasc Imaging. 2016 Dec;17(12):1385–1393.
- Leipsic J, Yang TH, Min JK. Computed tomographic imaging of transcatheter aortic valve replacement for prediction and prevention of procedural complications. Circ Cardiovasc Imaging. 2013 Jul;6(4):597–605.
- Pasic M, Unbehaun A, Dreysse S, et al. Rupture of the device landing zone during transcatheter aortic valve implantation: a life-threatening but treatable complication. Circ Cardiovasc Interventions. 2012 Jun;5(3):424–432.
- Tsuru Y, Miura M, Shirai S, et al. Aortic complex rupture after transcatheter aortic valve implantation. Int Heart J. 2019 May;60(3):772–777.
- Pasic M, Unbehaun A, Buz S, et al. Annular rupture during transcatheter aortic valve replacement: classification, pathophysiology, diagnostics, treatment approaches, and prevention. JACC Cardiovasc Interv. 2015 Jan;8(1 Pt A):1–9.
- Hansson NC, Norgaard BL, Barbanti M, et al. The impact of calcium volume and distribution in aortic root injury related to balloon-expandable transcatheter aortic valve replacement. J Cardiovasc Comput Tomogr. 2015 Sep-Oct;9(5):382–392.
- Barbanti M, Yang TH, Rodes Cabau J, et al. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation. 2013 Jul 16;128(3):244–253.
- Kiriyama H, Kaneko H, Itoh H, et al. Left main coronary artery obstruction by huge noncoronary cusp calcification after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019 Jul;12(13):1285–1287.
- Ribeiro HB, Nombela-Franco L, Urena M, et al. Coronary obstruction following transcatheter aortic valve implantation: a systematic review. JACC Cardiovasc Interv. 2013 May;6(5):452–461.
- Akinseye OA, Jha SK, Ibebuogu UN. Clinical outcomes of coronary occlusion following transcatheter aortic valve replacement: A systematic review. Cardiovasc Revascularization Med incl Mol Interventions. 2018 Mar;19(2):229–236.
- Nombela-Franco L, Webb JG, de Jaegere PP, et al. Timing, predictive factors, and prognostic value of cerebrovascular events in a large cohort of patients undergoing transcatheter aortic valve implantation. Circulation. 2012 Dec 18;126(25):3041–3053.
- Aggarwal SK, Rn N D, Menezes LJ, et al. Patterns of solid particle embolization during transcatheter aortic valve implantation and correlation with aortic valve calcification. J Interv Cardiol. 2018 Oct;31(5):648–654.
- Doerner J, Kupczyk PA, Wilsing M, et al. Cerebral white matter lesion burden is associated with the degree of aortic valve calcification and predicts peri-procedural cerebrovascular events in patients undergoing transcatheter aortic valve implantation (TAVI). Catheter Cardiovasc Interv. 2018 Mar 1;91(4):774–782.
- Pollari F, Hitzl W, Vogt F, et al. Aortic valve calcification as a risk factor for major complications and reduced survival after transcatheter replacement. J Cardiovasc Comput Tomogr. 2019 Dec 7. DOI:10.1016/j.jcct.2019.12.001.
- Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017 Apr 6;376(14):1321–1331.
- Makkar RR, Thourani VH, Mack MJ, et al. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med. 2020 Feb 27;382(9):799–809.
- Mc Morrow R, Kriza C, Urbán P, et al. Assessing the safety and efficacy of TAVR compared to SAVR in low-to-intermediate surgical risk patients with aortic valve stenosis: an overview of reviews. Int J Cardiol. 2020 Apr 11;314:43–53.
- Kolkailah AA, Doukky R, Pelletier MP, et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis in people with low surgical risk. Cochrane Database Syst Rev. 2019 Dec 20;12(12):Cd013319.
- Elgendy IY, Gad MM, Mahmoud AN, et al. Meta-analysis comparing outcomes of self-expanding versus balloon-expandable valves for transcatheter aortic valve implantation. Am J Cardiol. 2020 May 16. DOI:10.1016/j.amjcard.2020.05.007.
- Thiele H, Kurz T, Feistritzer HJ, et al. Comparison of newer generation self-expandable vs. balloon-expandable valves in transcatheter aortic valve implantation: the randomized SOLVE-TAVI trial. Eur Heart J. 2020 May 21;41(20):1890–1899.
- Kapadia SR, Kodali S, Makkar R, et al. Protection against cerebral embolism during transcatheter aortic valve replacement. J Am Coll Cardiol. 2017 Jan 31;69(4):367–377.
- Ghanem A, Müller A, Nähle CP, et al. Risk and fate of cerebral embolism after transfemoral aortic valve implantation: a prospective pilot study with diffusion-weighted magnetic resonance imaging. J Am Coll Cardiol. 2010 Apr 6;55(14):1427–1432.
- Kahlert P, Knipp SC, Schlamann M, et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study. Circulation. 2010 Feb 23;121(7):870–878.
- Fairbairn TA, Mather AN, Bijsterveld P, et al. Diffusion-weighted MRI determined cerebral embolic infarction following transcatheter aortic valve implantation: assessment of predictive risk factors and the relationship to subsequent health status. Heart. 2012 Jan;98(1):18–23.
- Spaziano M, Francese DP, Leon MB, et al. Imaging and functional testing to assess clinical and subclinical neurological events after transcatheter or surgical aortic valve replacement: a comprehensive review. J Am Coll Cardiol. 2014 Nov 4;64(18):1950–1963.
- Schmidt T, Leon MB, Mehran R, et al. Debris heterogeneity across different valve types captured by a cerebral protection system during transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2018 Jul 9;11(13):1262–1273.
- Van Mieghem NM, van Gils L, Ahmad H, et al. Filter-based cerebral embolic protection with transcatheter aortic valve implantation: the randomised MISTRAL-C trial. EuroIntervention. 2016 Jul 20;12(4):499–507.
- Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI randomized clinical trial. Jama. 2016 Aug 9;316(6):592–601.
- Hamm CW, Arsalan M, Mack MJ. The future of transcatheter aortic valve implantation. Eur Heart J. 2016 Mar 7;37(10):803–810.
- Abramowitz Y, Jilaihawi H, Pibarot P, et al. Severe aortic stenosis with low aortic valve calcification: characteristics and outcome following transcatheter aortic valve implantation. Eur Heart J Cardiovasc Imaging. 2017 Jun 1;18(6):639–647.
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014 May 8;370(19):1790–1798.
- Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019 May 2;380(18):1706–1715.
- Boskovski MT, Nguyen TC, McCabe JM, et al. Outcomes of transcatheter aortic valve replacement in patients with severe aortic stenosis: a review of a disruptive technology in aortic valve surgery. JAMA Surg. 2019 Nov 27. doi: 10.1001/jamasurg.2019.4449
- Ludwig S, Goßling A, Waldschmidt L, et al. TAVR for low-flow, low-gradient aortic stenosis: prognostic impact of aortic valve calcification. Am Heart J. 2020 Jul;225:138-148.
- Zegdi R, Blanchard D, Azarine A, et al. Elliptical shape of a SAPIEN XT prosthesis deployed in a patient with bicuspid aortic valve stenosis. J Heart Valve Dis. 2012 Nov;21(6):764–766.
- Zegdi R, Ciobotaru V, Noghin M, et al. Is it reasonable to treat all calcified stenotic aortic valves with a valved stent? Results from a human anatomic study in adults. J Am Coll Cardiol. 2008 Feb 5;51(5):579–584.
- Mahadevia R, Barker AJ, Schnell S, et al. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation. 2014 Feb 11;129(6):673–682.
- Jilaihawi H, Chen M, Webb J, et al. A bicuspid aortic valve imaging classification for the TAVR era. JACC Cardiovasc Imaging. 2016 Oct;9(10):1145–1158.
- Ferda J, Linhartova K, Kreuzberg B. Comparison of the aortic valve calcium content in the bicuspid and tricuspid stenotic aortic valve using non-enhanced 64-detector-row-computed tomography with prospective ECG-triggering. Eur J Radiol. 2008 Dec;68(3):471–475.
- Watanabe Y, Chevalier B, Hayashida K, et al. Comparison of multislice computed tomography findings between bicuspid and tricuspid aortic valves before and after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2015 Aug;86(2):323–330.
- Das R, Puri R. Transcatheter treatment of bicuspid aortic valve disease: imaging and interventional considerations. Front Cardiovasc Med. 2018;5:91.
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol. 2014 Dec 9;64(22):2330–2339.
- Thyregod HG, Steinbruchel DA, Ihlemann N, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers NOTION randomized clinical trial. J Am Coll Cardiol. 2015 May 26;65(20):2184–2194.
- Spitzer E, Ren B, Kroon H, et al. Moderate aortic stenosis and reduced left ventricular ejection fraction: current evidence and challenges ahead. Front Cardiovasc Med. 2018;5:111.
- Meredith Am IT, Walters DL, Dumonteil N, et al. Transcatheter aortic valve replacement for severe symptomatic aortic stenosis using a repositionable valve system: 30-day primary endpoint results from the REPRISE II study. J Am Coll Cardiol. 2014 Sep 30;64(13):1339–1348.
- Falk V, Wöhrle J, Hildick-Smith D, et al. Safety and efficacy of a repositionable and fully retrievable aortic valve used in routine clinical practice: the RESPOND study. Eur Heart J. 2017 Dec 1;38(45):3359–3366.
