ABSTRACT
Objective: Percutaneous breast and axillary core biopsy followed by marker placement are integral parts of a breast imager’s practice benefiting both patients and clinicians. Marker placement is the standard to facilitate future care. The purpose of this study is to characterize the safety and performance of MammoMARK, CorMARK, and HydroMARK biopsy markers by evaluating device-related adverse events, device deficiencies, and long-term safety.
Methods: A retrospective review of three radiology practices identified patients who underwent image-guided breast or axillary biopsies followed by marker placement between 1 January 2012 and 1 January 2017. Medical records were reviewed with adverse events related to marker placement and use recorded.
Results: 768 markers were placed with three (0.4%) events recorded. Two device deficiencies and one non-serious adverse event occurred in three patients. Device deficiency events involved user errors deploying the markers, one to inability to locate the marker on post-biopsy imaging, and the second to misplacement relative to biopsy target. One non-serious adverse event involved inability to locate/retain the marker in a surgically resected specimen. No serious adverse events were reported.
Conclusion: Placement of breast biopsy markers is safe with minimal associated risks. Issues related to device malfunction, durability, reliability, safety, or performance were not reported.
1. Introduction
Minimally invasive image-guided breast core biopsy using stereotactic, sonographic, or magnetic resonance imaging has become an integral component of clinical practice replacing the need for surgical excision for initial tissue evaluation [Citation1]. Placement of biopsy site markers, sometimes referred to as biopsy clips, is routine practice after image-guided biopsy of suspicious findings to facilitate future localization of the biopsy site. The US Food and Drug Administration first approved biopsy markers for use in soft tissues in 1995 [Citation2]. Before that time, targeted lesions were localized by identifying residual disease on imaging or using surrounding landmarks with associated disadvantages including inability to identify the target and the necessity of resecting larger volume of breast tissues at the time of surgery to increase the chances of excising the target. Higher rates of positive margins were also found in the excised specimens without marker placement compared to those that had marker placement following breast biopsy [Citation3]. Thus, marker placement following biopsy became the mainstay of clinical practice as it allowed for reliable re-identification of the biopsy target, precise localization of biopsied lesions, and it facilitates patient care among radiologists, surgeons, and pathologists [Citation2]. For the radiologist, marker placement provides many advantages including distinguishing multiple biopsied lesions within the same breast, preventing unnecessary re-biopsy of a previously sampled lesion, providing sonographic and mammographic correlation, and providing a target for pre-operative localization. Marking a malignant tumor or lymph node prior to neoadjuvant chemotherapy facilitates monitoring treatment response and allows for successful localization even following complete imaging response, meaning complete absence of residual imaging findings of malignancy [Citation4]. Biopsy markers permit image-guided localization procedures to more precisely mark the margins of disease extent helping to guide precise intraoperative tumor resection. Finally, the retrieval of biopsy marker within a specimen confirms that the original biopsied site of interest has been removed and may serve to identify the region of interest in a mastectomy specimen [Citation5].
Biopsy markers have proven to be generally safe with very few incidences of adverse events (AE) documented in the literature. Reports of AEs have been mostly centered on marker migration/displacement, disappearance, and allergic reactions [Citation4,Citation6–11]. Most of the studies done to evaluate adverse effects involved smaller sample sizes and case reports [Citation11,Citation12]. To our knowledge, no study in the literature has gone beyond device-related adverse effects to investigate device deficiencies (DD) or malfunction. This study focused on the MammoMARK, CorMARK, and HydroMARK biopsy site markers (Mammotome, Devicor Medical Products, Inc., part of Leica Biosystems, USA (Global Headquarters). The purpose of this study is to characterize the safety and performance of these three specific breast biopsy site markers by evaluating all device-related adverse events and device-related serious adverse events, and device deficiencies. This study was performed to comply with European Union Medical Device Regulation requirements with regards to implantable medical devices [Citation13].
2. Materials and methods
Approval for the study was obtained by the Western IRB with a waiver of informed consent. Three radiology practices participated in the multi-site retrospective review and included two community private radiology practices (Orlando Health and OhioHealth) and one academic practice (Ohio State University Wexner Medical Center). Breast imaging care at the community-based private practice groups was provided by a mixture of fellowship trained breast imagers and focused generalists while the academic practice consisted of entirely sub-specialist breast imagers. Seven Hundred Sixty-Eight (768) subjects were included and their medical records served as the basis for review and collection of applicable study data. In patients with multiple biopsy markers, only one marker was evaluated per patient to assure that each observation was independent. Biopsy markers were deployed at the time of image-guided biopsy and post-biopsy mammography or ultrasound obtained to confirm placement. The decision to use a particular marker during a biopsy was made by individual radiologists as part of routine clinical care.
Inclusion criteria included implantation of a MammoMARK or CorMARK or HydroMARK biopsy site marker during a biopsy procedure on or between the dates of 1 January 2012 and 1 January 2017 in female patients aged 18 or older. Patients were excluded if their medical records indicated that the anticipated site to biopsy was initially infected or suspected to be infected at the time of biopsy marker deployment and/or the patient has or had a hypersensitivity or immune response to bovine products, collagen, and/or collagen products. To avoid bias in subject selection, subjects meeting the eligibility criteria were enrolled consecutively, in reverse chronological order. Enrollment was started with the most recent patients who underwent biopsy marker placement, on or before 1 January 2012 and 1 January 2017 thereby assessing the most current, real-world post-implant data at each of the investigative clinical study sites. Assessment for AEs and DDs was made by chart review of all subsequent encounters after marker placement. Patients were considered to have completed the study upon device explantation, having undergone 1–5 years of follow-up, or at patient death. Sample size calculations were based on a target acceptable rate of 1% and the upper bound of the two-sided 95% confidence interval of 2% for device-related AEs, device-related serious AEs and DD, for each MammoMARK/CorMARK and HydroMARK group. The target acceptable rates were based on the results of prior studies on implantable devices and their complication rates [Citation14–16]. All analyses are descriptive and include counts and percentages to summarize the data.
For the purposes of this study, non-serious AEs are defined as any unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of an investigational product, whether or not related to the investigational product. Serious AEs were defined as any untoward medical occurrence that led to death or led to serious deterioration in the health of the subject, that either resulted in a life-threatening illness or injury, or a permanent impairment of a body structure or a body function, or in-patient or prolonged hospitalization, or medical or surgical intervention to prevent life-threatening illness or injury or permanent impairment to a body structure or a body function, led to fetal distress, fetal death, or congenital abnormality or birth defect. Evidence of potential allergic or hypersensitivity reactions was assessed and classified based on severity. DDs in this study were defined as the inadequacy of a medical device with respect to its identity, quality, durability, reliability, safety, or performance. Biopsy marker migration was not specifically considered as an AE or DD and was not assessed in this study.
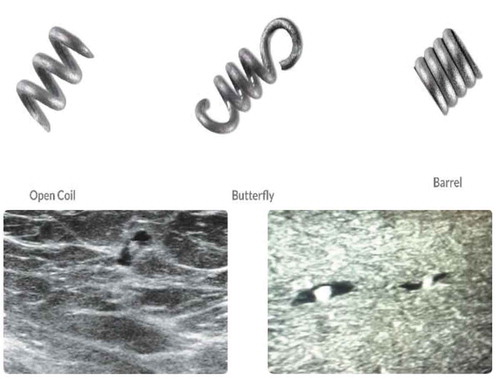
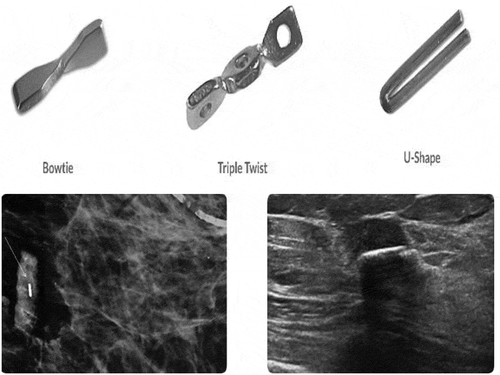
The HydroMARK device is a two-component marker that is made of a highly expandable solid cylinder of polymerized and desiccated polyethylene glycol (PEG) based hydrogel which expands with fluid contact and is visible under ultrasound for at least 6 weeks and up to 12 months [Citation17]. The marker also contains an embedded metallic component, either titanium or stainless steel, which is permanently visible by x‐ray and MR imaging (). HydroMARK was initially cleared for market in the USA by the FDA in 2006 under 510(k) K060769. The most recent 510(k) filing, K170803, was approved by the FDA in 2017.
The MammoMARK/CorMARK device is comprised of a bioresorbable collagen plug that is visible under ultrasound for at least 28 days and up to 8 weeks, with an embedded non-resorbable radiopaque titanium marker which is permanently visible by x-ray and MR imaging [Citation18] (). MammoMARK/CorMARK markers were initially cleared for market in the USA by the FDA in 2001 under 510(k) K003777 and most recently in 2008 under K082278. Both the MammoMark/CorMARK devices are available in a variety of shapes and introducer sizes ().
Table 1. Complete listing of available marker shapes, material composition, and introducer sizes in products studied
3. Results
A total of 768 MammoMARK/CorMARK and HydroMARK breast biopsy site markers were placed after image-guided biopsy. In total, 668 (87%) of the markers were placed during ultrasound-guided biopsies, 81 (10.5%) were placed during stereotactic biopsies, and 19 (2.5%) at the time of MRI-guided biopsy. The MammoMARK biopsy marker was deployed in 115 (15%) patients of which 19 (17%) were placed during MRI-guided biopsies, 30 (26%) were placed at stereotactic biopsy, and 66 (57%) using sonographic guidance. Of the 269 (35%) patients who received the CorMARK biopsy marker, all 269 (100%) were placed using sonographic guidance. Of the 384 (50%) patients who had the HydroMARK marker deployed after breast biopsy, 51 (13%) were placed using stereotactic guidance and 333 (87%) were placed using ultrasound guidance (). Of the 768 patients enrolled in the study, 404 (52.6%) markers were explanted through surgical excision, 358 (46.6%) patients completed between 1 and 5 years of clinical follow-up, and 6 (0.8%) exited at their death.
Table 2. Highlighting the types of image-guided biopsies performed. Note: Percentages are among each marker
Of the 768 biopsy site markers placed, 2 DDs and 1 non-serious device AE were recorded in 3 patients (0.4%) (). All reported events occurred at one of the community radiology practice sites with three different radiologists, all described as having significant experience in breast imaging and intervention. All reported events occurred in patients with markers placed under ultrasonographic guidance. In the first patient (DD), a placed CorMARK marker was not identifiable on post-biopsy mammography requiring placement of a second marker. In a second patient (DD), a HydroMARK marker was misplaced relative to its target after biopsy requiring placement of a second marker. In a third patient (non-serious AE), post-biopsy mammography demonstrated appropriate placement of a HydroMARK marker; however, the marker was missing from the resected surgical specimen. The marker was directly visualized at the time of ultrasound-guided localization in preparation for lumpectomy and pathology confirmed resection of the carcinoma.
Table 3. Summary of identified device deficiencies and adverse events
4. Discussion
Our study was done to characterize the safety and performance of MammoMARK/CorMARK and HydroMARK breast biopsy site markers. The current literature on breast biopsy markers focuses on indications for use, sonographic visibility, post-procedure migration, and assessment of MRI susceptibility artifacts and safety [Citation10]. There are very few reports of device-related AEs displacement or allergic reactions. We further investigated the safety of breast biopsy markers by carrying out a retrospective analysis on all device-related adverse effects of the MammoMARK/CorMARK and HydroMARK breast biopsy site markers for 768 patients across three clinical sites. To our knowledge, no study of this sample size has collectively looked at device-related serious and non-serious AEs in addition to DD or malfunction in characterizing the safety and performance of MammoMARK, CorMARK, and HydroMARK breast biopsy markers.
The safety and reliability of medical devices are regulated through organizations such as the US Food and Drug Administration (FDA) Center for Devices and Radiological Health and European Medicines Agency (EMA) with recommendations of best practice by the Global Harmonization Task Force (GHTF) and the International Organization for Standardization [Citation19]. The FDA mandates manufacturers, importers, and device user facilities, such as hospitals, surgery centers, and nursing homes, to report any incidence of adverse effects, device failures, and deficiencies. Health-care providers, patients, and consumers are also encouraged to make voluntary reports. Reports are then entered into the Manufacturer and User Facility Device Experience (MAUDE) or MedSun Databases which are publicly accessible [Citation20]. Similarly, the EU mandates manufacturers to report device-related serious AEs, device failures/malfunction, or labeling inaccuracies that might lead to or might have led to death or serious injury, to their competent authority (CA) in the nation of occurrence. These data are submitted by the CAs to the European Databank on Medical Devices (EUDAMED). This database is not currently accessible to the public [Citation21].
Reporting of AEs is of great importance as it allows for tracking and evaluation of adverse effects, including the cause, severity, and risk mitigation. Over the last 5 years (2016–2020), 16 incidences of non-serious AEs and DDs involving MammoMARK and HydroMARK breast biopsy markers in the FDA’s MAUDE database [Citation22]. These reports while valuable in identifying safety concerns might be limited by reporting quality, under-reporting, delayed/biased report, and lack of denominator [Citation23,Citation24]. In this light, we retrospectively investigated the safety of MammoMARK, CorMARK, and HydroMARK biopsy markers of 768 patients that had biopsy clip implanted between January 2012 and 2017. We report one non-serious AE and two DDs that occurred in three patients.
The first case of DD recorded was due to a failure to correctly deploy a CorMARK biopsy marker. The absence of the marker was noted in the post-biopsy mammography which required placement of an additional biopsy marker. Marker absence on post-biopsy mammographic imaging has been previously reported. A similar situation was documented by Orel et al. [Citation10] in which the marker appeared to be deployed but was not identified on follow-up mammography. It has been proposed that failure of marker deployment after biopsy may occur when clip adheres to the retained tissue fragments in the trough of the biopsy needle rather than deploying in the breast [Citation25]. Failure of marker deployment can have potential consequences such as inability to localize the lesion at the time of surgery if not prospectively recognized. Thus, the need for post-biopsy imaging cannot be overstated so a new marker can be deployed while the patient is still available and the biopsy site identifiable.
In the second case of DD, due to user error, a HydroMARK biopsy marker was misplaced relative to target under ultrasound guidance, requiring replacement. Though breast biopsy markers are intended to mark biopsy sites, research suggests that the position of the markers may sometimes differ from the location of the biopsy site. Some studies have suggested that this might be due to the accordion effect when breast compression is released [Citation2,Citation25]. In such instances, it has been proposed that releasing compression prior to marker deployment may minimize the accordion effect. This was not a likely cause of any DD or AE in this study as all recorded events occurred with ultrasound-guided biopsies which are not performed with the breast in compression. Other hypothesized causes of displacement include errors related to deploying the marker, either from partial deployment of the plug or deploying the plug beyond the biopsy cavity [Citation26,Citation27]. Lastly, marker displacement may result when there is hematoma formation or excess air in the biopsy site. This is particularly true for stereotactic biopsies and MRI-guided biopsies which employ larger gauge needles [Citation6]. Clinicians should be aware of the possibility of marker misplacement as this can affect patient care. In cases of marker misplacement/displacement, the radiologist should either replace the biopsy marker or document in the procedure report and post-biopsy mammogram the relative distance of the marker to the target site to facilitate accurate localizations.
In the third recorded event, a non-serious AE involving a HydroMARK biopsy marker occurred where post-biopsy mammography and sonography at the time of localization demonstrated appropriate placement of the marker; however, it was missing from the resected surgical specimen. Complete disappearance of markers following biopsy has also been documented in the literature [Citation7–9]. Shah et al. [Citation28] hypothesized that non-visualization of the marker at post-biopsy mammogram might be due to failed deployment or extrusion of the marker out of the breast. They went further to state that extrusion of the marker usually occurs when the biopsy site is located superficially and/or when there is excessive bleeding. Our study recorded one event of marker disappearance from all 768 clips that were deployed. The disappearance of a HydroMARK marker in our study is an interesting occurrence given that intraoperative difficulties and marker disappearance specific to HydroMARK markers have been documented in the literature. For example, Klein et al. [Citation29] reported problems with surgical excision as the biopsy tract was transected in 51.6% (16/31) of HydroMARK markers. They suggested this might be due to poor tissue adherence of the hydrogel marker to breast tissue surrounding the biopsy cavity and slickness of the hydrogel marker during excision. Shah et al. [Citation28] also highlighted their experiences with HydroMARK markers and found them to be susceptible to displacement and extrusion during surgery. While this might be plausible, it appears to be a more rare event than previously reported as there are no reports of HydroMARK marker disappearance in the FDA’s MAUDE database over the last 5 years and our study documented only one such instance. However, future studies could potentially look at the intrinsic characteristics of the HydroMARK marker that increases intraoperative difficulties and consider ways to mitigate this effect.
Cases of allergic reaction to breast biopsy markers are exceedingly rare in the current medical literature. However, of the 16 reported AEs in the FDA’s MAUDE database in the last 5 years, 6 (37.5%) were related to allergic reactions [Citation22]. Allergic reactions to titanium, a component of both HydroMARK, MammoMARK, and CorMARK biopsy markers, have been reported in dental and orthopedic implants as well as titanium-based breast surgical clips [Citation30–33]. The clinical presentation of titanium allergy varies to include contact dermatitis, severe itching skin rash, and inflammatory granulomatous reactions [Citation31–34]. Though epidemiological studies have not been carried out to assess the rate of titanium allergy in the general population, a study conducted by Sicilia et al. [Citation35] found the estimated prevalence of titanium in dental implants to be 0.6%.
The presence of titanium in biopsy markers raises the possibility of allergic reactions during or after placements. A 2019 case report by Wegner et al. [Citation12] highlights an allergic reaction to HydroMARK titanium-based biopsy marker in a patient who presented with pain, discomfort, and itching. To investigate the possibility of allergic reactions to titanium-based biopsy markers, we excluded patients who have had a hypersensitivity or an immune response to bovine products, collagen, and/or collagen products. Patients with titanium allergies were not excluded from our study and all 768 charts were reviewed for evidence of hypersensitivity Type I–Type 4 reactions as well as evidence of foreign body reaction. Of the 768 marker placements, no records of hypersensitivity or foreign body reactions were documented supporting the rarity of allergic events in the literature. However, obtaining a thorough history of past hypersensitivity reaction may be warranted before biopsy marker placement.
With only three cases of non-serious AEs or DDS, our study supports current literature that biopsy markers are safe for routine patients use. However, this study was limited by its retrospective nature and it also focused on MammoMARK, CorMARK, and HydroMARK breast biopsy markers, not including other types of markers that are commercially available that may differ in their rates of DDs or AEs. The study was performed across three clinical sites with different personnel participating in biopsy and post-procedure care. While this could have introduced a confounding variable if techniques used varied between personnel it reflects a real-world clinical experience. As all adverse events occurred at one specific community practice radiology site, there may be some intrinsic practice-related factor affecting the frequency of events as well. Lastly, while post-biopsy imaging was performed to confirm marker placement, subsequent imaging studies were not specifically reviewed by research coordinators to confirm retention of the markers. The absence of a marker on subsequent imaging or prior to surgery, after confirmation of appropriate placement at biopsy, would be unexpected and rare occurrence based on the current literature as well as our own practice experience.
5. Conclusion
Breast biopsy markers, specifically, the MammoMARK, CorMARK, and HydroMARK biopsy clips, are safe with rare occurrences of non-serious AEs or DDs. Physicians and their patients should be reassured that such devices are safe and their routine use is justified based upon their safety profile and significant clinical benefits.
Author contributions
E Kanevsky was responsible for study design. S Smith, C Taylor, E Kanevsky, S Povoski, and J Hawley were responsible for analysis and interpretation of the data, drafting of the paper or revising it critically for intellectual content. J Hawley was responsible for the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Declaration of interest
E Kanevsky is employed by Leica Biosystems. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Parker SH, Burbank F. A practical approach to minimally invasive breast biopsy. Radiology. 1996 July;200(1):11–20.
- Burbank F, Forcier N. Tissue marking clip for stereotactic breast biopsy: initial placement accuracy, long-term stability, and usefulness as a guide for wire localization. Radiology. 1997 Nov;205(2):407–415.
- Nurko J, Mancino AT, Whitacre E, et al. Surgical benefits conveyed by biopsy site marking system using ultrasound localization. Am J Surg. 2005 Oct;190(4):618–622.
- Dash N, Chafin SH, Johnson RR, et al. Usefulness of tissue marker clips in patients undergoing neoadjuvant chemotherapy for breast cancer. AJR Am J Roentgenol. 1999 Oct;173(4):911–917.
- Thomassin-Naggara I, Lalonde L, David J, et al. A plea for the biopsy marker: how, why and why not clipping after breast biopsy? Breast Cancer Res Treat. 2012 Apr;132(3):881–893.
- Yen P, Dumas S, Albert A, et al. Post-vacuum-assisted stereotactic core biopsy clip displacement: a comparison between commercially available clips and surgical clip. Can Assoc Radiol J. 2018 Feb;69(1):10–15.
- Esserman LE, Cura MA, DaCosta D. Recognizing pitfalls in early and late migration of clip markers after imaging-guided directional vacuum-assisted biopsy. Radiographics. 2004 Jan-Feb;24(1):147–156.
- Bourke AG, Peter P, Jose CL. The disappearing clip: an unusual complication in MRI biopsy. BMJ Case Rep. 2014 Aug 19; 2014. DOI:10.1136/bcr-2014-204092
- DiPiro PJ. Disappearance of a localizing clip placed after stereotactic core biopsy of the breast. AJR Am J Roentgenol. 1999 Oct;173(4):1134.
- Orel SG, Rosen M, Mies C, et al. MR imaging-guided 9-gauge vacuum-assisted core-needle breast biopsy: initial experience. Radiology. 2006 Jan;238(1):54–61.
- Portnow LH, Thornton CM, Milch HS, et al. Biopsy marker standardization: what’s in a name? AJR Am J Roentgenol. 2019;11:1–6.
- Wegner U, Rainford S. Adverse reaction regarding titanium-based marker clip: case report of a potential complication. Int Med Case Rep J. 2019;12:291–295.
- Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC. Available from: https://eur-lex.europa.eu/eli/reg/2017/745/oj
- Weiss R, Knight BP, Gold MR, et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation. 2013 Aug;128(9):944–953.
- Scheffes IJ, Kroon AA, Schmidli J, et al. Novel baroreflex activation therapy in resistant hypertension: results of a European multi-center feasibility study. J Am Coll Cardiol. 2010 Oct;56(15):1254–1258.
- Sarr MG, Bilington CJ, Brancatisano R, et al. The EMPOWER study: randomized, prospective, double-blind, multicenter trial of vagal blockade to induce weight loss in morbid obesity. Obes Surg. 2012 Nov;22(11):1771–1782.
- Sakamoto N, Fukuma E, Tsunoda Y, et al. Evaluation of the dislocation and long-term sonographic detectability of a hydrogel-based breast biopsy site marker. Breast Cancer. 2018 Sept;25(5):575–582.
- Corsi F, Sorrentino L, Sartani A, et al. Localization of nonpalpable breast lesions with sonographically visible clip: optimizing tailored resection and clear margins. Am J Surg. 2015 June;209(6):950–958.
- Meeting international standard for medical device reliability and risk management. Available from: https://3hti.com/wp-content/uploads/documents/Medical-Device-Reliability-White-Paper.pdf
- Medical Device Reporting (MDR): How to Report Medical Device Problems. [ cited 2020 June 20]. Available from: https://www.fda.gov/medical-devices/medical-device-safety/medical-device-reporting-mdr-how-report-medical-device-problems
- European Commission Guidance Document-Guidance on Medical Device Vigilance System. [ cited 2020 June 20]. Available from: file:///C:/Users/shash/Downloads/2_%2012-1_rev8_en%20(1).pdf
- MAUDE - Manufacturer and User Facility Device Experience. [ cited 2020 June 20]. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/TextSearch.cfm
- Rajan PV, Kramer DB, Kesselheim AS. Medical device postapproval safety monitoring: where does the United States stand? Circ Cardiovasc Qual Outcomes. 2015 Jan;8(1):124–131.
- Shuren J, Califf RM. Need for a national evaluation system for health technology. JAMA. 2016 Sept 20;316(11):1153–1154.
- Liberman L, Dershaw DD, Morris EA, et al. Clip placement after stereotactic vacuum-assisted breast biopsy. Radiology. 1997 Nov;205(2):417–422.
- Pinkney DM, Mychajlowycz M, Shah BA. A prospective comparative study to evaluate the displacement of four commercially available breast biopsy markers. Br J Radiol. 2016 Sept;89(1065):20160149.
- Rosen EL, Baker JA, Soo MS. Accuracy of a collagen-plug biopsy site marking device deployed after stereotactic core needle breast biopsy. AJR Am J Roentgenol. 2003 Nov;181(5):1295–1299.
- Shah AD, Mehta AK, Talati N, et al. Breast tissue markers: why? What’s out there? How do I choose? Clin Imaging. 2018 Nov - Dec;52:123–136.
- Klein RL, Mook JA, Euhus DM, et al. Evaluation of a hydrogel based breast biopsy marker (HydroMARK®) as an alternative to wire and radioactive seed localization for non-palpable breast lesions. J Surg Oncol. 2012 May;105(6):591–594.
- Ko N, Mine A, Egusa H, et al. Allergic reaction to titanium-made fixed dental restorations: a clinical report. J Prosthodont. 2014 Aug;23(6):501–503.
- Lalor PA, Gray AB, Wright S, et al. Contact sensitivity to titanium in a hip prosthesis? Contact Dermatitis. 1990 Sept;23(3):193–194.
- Motton S, Gardinal I, Soulé-Tholy M, et al. Hurt eczematiforme column (chronicle) of the breast after implementation of a surgical clip. J Gynecol Obstet Biol Reprod (Paris). 2011 Apr;40(2):174–177.
- Tamai K, Mitsumori M, Fujishiro S, et al. A case of allergic reaction to surgical metal clips inserted for postoperative boost irradiation in a patient undergoing breast-conserving therapy. Breast Cancer. 2001;8(1):90–92. .
- Yamauchi R, Morita A, Tsuji T. Pacemaker dermatitis from titanium. Contact Dermatitis. 2000 Jan;42(1):52–53.
- Sicilia A, Cuesta S, Coma G, et al. Titanium allergy in dental implant patients: a clinical study on 1500 consecutive patients. Clin Oral Implants Res. 2008 Aug;19(8):823–835.