ABSTRACT
Objectives
To report outcomes of patients who underwent carpal tunnel release with ultrasound guidance (CTR-US) in routine clinical practice.
Methods
This was a multicenter post-market registry of patients treated with CTR-US. Main outcomes included the Quick Disabilities of Arm, Shoulder, and Hand Questionnaire (QDASH), Boston Carpal Tunnel Questionnaire Symptom Severity Scale (BCTQ-SSS) and Functional Status Scale (BCTQ-FSS), return to normal activities, return to work, and complications.
Results
Of 535 patients who provided follow-up data, 373 (70%) were followed for 6 months post-treatment. Among these 373 patients (427 hands, mean age 55 years, 71% female), QDASH scores decreased by 30.8 points, BCTQ-SSS scores decreased by 1.6 points, and BCTQ-FSS scores decreased by 1.0 points at 6 months (all p < 0.001). The median time to return to normal activities was 3 days and time to return to work was 5 days. Subgroup analysis revealed consistent outcomes regardless of age group, sex, body mass index, diabetes, tobacco use, worker compensation status, or procedure type (unilateral/bilateral simultaneous). No major neurovascular complications were reported.
Conclusion
Patients treated with CTR-US reported clinically meaningful improvements in symptoms and function, rapid return to normal activities, and minimal work absenteeism, with an excellent safety profile.
1. Introduction
Carpal tunnel syndrome (CTS) is the most common peripheral neuropathy, affecting approximately 5% of the population [Citation1]. Initial treatment for CTS is typically nonoperative and consists of activity modification, physical therapy, splinting, nonsteroidal anti-inflammatory drugs, or corticosteroid injections [Citation2]. Carpal tunnel release (CTR) may be indicated in patients with symptoms that are nonresponsive to conservative measures. Approximately 600,000 CTR procedures are performed each year in the United States alone [Citation1,Citation3]. The goal of CTR is to transect the transverse carpal ligament (TCL) while avoiding iatrogenic injury to surrounding neurovascular structures. Most patients experience symptomatic relief after CTR regardless of the technique which may include open, mini-open, limited incision or endoscopic procedures [Citation4]. Because long-term outcomes and complication profiles are generally equivalent among CTR types [Citation5], factors such as time to return to normal activities and work absenteeism are important considerations that may assist in shared decision-making conversations between physicians and patients.
CTR with US guidance (CTR-US) is a minimally invasive technique performed with a small (generally < 5 mm) incision that uses ultrasound to continuously visualize the relevant anatomy during the procedure, including division of the TCL. Several small studies of CTR-US have reported clinically meaningful improvements in symptom severity and functional outcomes with low complication rates [Citation6–11]. However, data regarding time to return to normal activities and work absenteeism following CTR-US remain limited [Citation7,Citation8]. The purpose of this study was to report changes in symptom severity and functional status, time to return to normal activities, work absenteeism, and complications using a large database of patients who underwent CTR-US in real-world clinical practice.
2. Methods
2.1. Study design
This was a multicenter, observational post-market registry that enrolled patients treated with CTR-US in routine clinical practice in the United States. The device used for CTR-US (UltraGuideCTR, Sonex Health, Eagan, MN, United States) received FDA 510(k) clearance in 2019. Data collection in this registry began after FDA clearance to collect postmarket safety and effectiveness information on the device. The research methods adhered to the guidelines set forth in the Declaration of Helsinki. This study was granted a waiver of consent exemption from WCG IRB (Puyallup, WA, United States) under 45 CFR § 46.104(d)(4) because the identity of the human subjects could not readily be ascertained given the data collection methods.
2.2. Participants and eligibility criteria
The eligibility criteria for this observational study were purposely broad to reflect a heterogenous sample of CTS patients treated in routine clinical practice. Patient diagnosis was determined according to the practice patterns of each participating physician, all of whom were experienced in the diagnosis and management of CTS. Carpal tunnel syndrome was diagnosed primarily on clinical grounds, with ancillary testing such as electrophysiological studies ordered at the discretion of the physician. Eligible patients were adults (age ≥18 years) who were treated with CTR-US and demonstrated a willingness to participate in the registry and participate in specified follow-up activities. The decision to receive CTR-US was determined on a case-by-case basis considering physician and patient preferences. No limitations were imposed on maximum patient age, medical or surgical history, or clinical presentation.
2.3. Carpal tunnel release with ultrasound-guidance
All patients were treated with CTR-US using a single-use commercially available hand-held device (UltraGuideCTR) that is inserted into the carpal tunnel through a small (typically < 5 mm) wrist incision using real-time ultrasound guidance (). The device working tip consists of two inflatable balloons that border a centrally located, retractable retrograde cutting blade. When inflated with sterile saline, the balloons increase the diameter of the tip from 4 mm to 8 mm. After the tip is positioned within the carpal tunnel, the balloons are inflated to create space in the carpal tunnel, the centrally-located blade is activated, and the TCL is transected in a retrograde manner. Following TCL transection, the blade is recessed, the balloons are deflated, the device is removed, and the TCL is probed to ensure a complete release (Supplemental Video). The physicians in this study represented a variety of specialties and procedure experience and completed a formal cadaver-based training program prior to performing CTR-US in clinical practice. Factors such as patient selection, anesthesia, and postoperative care were determined by practice-specific preferences. Face-to-face follow-up visits varied according to the usual practice patterns of each participating physician and were not dictated by the study. There was no requirement for return follow-up visits as all data were collected via text message, e-mail, or chart review.
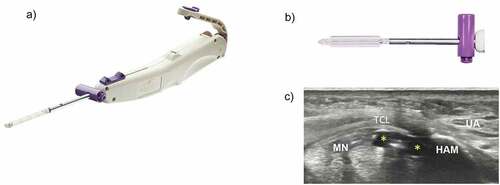
Figure 1. Carpal tunnel release with ultraGuideCTR and real-time ultrasound guidance. A. ultraGuideCTR device, consisting of hand piece and distal working tip. B. close-up view of device tip from ‘bird’s eye view’ demonstrating inflated balloons which increase the device diameter from 4 mm to 8 mm to create space within the carpal tunnel. C. cross-sectional view of the distal carpal tunnel with the device placed directly below the transverse carpal ligament (TCL) and between the median nerve (MN) and the hook of the hamate bone (HAM). the balloons (yellow asterisks) have been inflated to create space between the MN and the ulnar artery (UA). top = superficial, left = radial.
2.4. Outcome measures
Patients completed a preoperative questionnaire, daily postoperative text message questions for up to 14 days post-procedure, and emailed questionnaires at 2 weeks, 1 month, 3 months, and 6 months postoperatively. Pre-treatment patient assessments included demographic data, medical and surgical history, work status, and patient-reported outcomes including the Quick Disabilities of the Arm, Shoulder, and Hand Questionnaire (QDASH), and the Boston Carpal Tunnel Questionnaire Symptom Severity Scale (BCTQ-SSS) and Functional Status Scale (BCTQ-FSS). Return to normal activities and return to work were collected via daily text messages for the first 14 days. Thereafter, postoperative outcomes were collected via e-mail or text message and included QDASH, BCTQ-SSS, BCTQ-FSS, return to normal activities, return to work, and patient satisfaction.
Co-primary endpoints of this study were QDASH, BCTQ-SSS, and BCTQ-FSS. The QDASH is an 11-item patient-reported questionnaire that has been validated for CTS where the total score ranges from 0 (indicating no disability) to 100 (indicating most severe disability) [Citation12]. The BCTQ is a CTS-specific questionnaire consisting of 11 symptom severity questions and 8 functional status questions. Scoring for the BCTQ-SSS and BCTQ-FSS ranges from 1 to 5, with higher scores indicating more severe symptoms [Citation13]. Minimal clinically important differences (MCIDs) for the postoperative change in patient-reported outcomes were considered to be 15 points for QDASH [Citation14], 1.14 points for BCTQ-SSS [Citation15], and 0.74 points for BCTQ-FSS [Citation15]. Return to normal activities was ascertained by asking patients when they had returned to normal daily activities outside of work. Return to work was ascertained by asking employed patients when they had returned to work in any capacity, a definition that is commonly used among CTS studies [Citation16,Citation17]. Patient satisfaction with the procedure was reported on a 5-point Likert scale ranging from 1 (very dissatisfied) to 5 (very satisfied); a score of 4 or 5 indicated that a patient was satisfied with the procedure.
Postoperative complications were recorded via chart review performed by the treating physician. A pre-specified list of major complications following CTR-US were identified including superficial infection, deep infection, arterial laceration, permanent nerve injury, and reoperation for incomplete release.
2.5. Statistical methods
The current analysis included all patients with 6-month postoperative data. Categorical variables were reported with percentages and counts. Continuous variables were reported as mean and standard deviation for normally distributed data, or median and interquartile range (IQR) for non-normally distributed data. Longitudinal outcomes were analyzed with mixed-model analysis of variance which provides unbiased estimates for data which are missing at random using maximum likelihood estimation [Citation18]. Mean change values relative to baseline were reported as the mean and 95% confidence interval (95% CI). Longitudinal changes in patient-reported outcomes were also reported with Cohen’s d statistic, which is a standardized effect size measure where values of 0.2, 0.5, 0.8, and 1.0 are considered small, medium, large, and very large effect sizes, respectively [Citation19]. Subgroup analyses were performed with mixed-model analysis of variance adjusted for the baseline value to determine the influence of key patient and surgical characteristics (age, sex, body mass index, diabetes, tobacco use, worker compensation status, and unilateral/bilateral simultaneous surgery) on main outcomes. Analyses were performed on a per-patient basis. Missing data imputation was not performed. Two-sided p-values of less than 0.05 were considered statistically significant. Data were analyzed by an independent biostatistician using Stata v16 (StataCorp, College Station, TX, United States).
3. Results
Among 535 patients who enrolled in the registry and provided postoperative follow-up, data were available on 499 (93%) patients at 2 weeks, 475 (89%) at 1 month, 446 (83%) at 3 months, and 373 (70%) at 6 months. The cohort of 373 patients with 6-month follow-up form the basis for this report. Between November 2019 and July 2021, 373 patients (427 hands, mean age 55 years, 71% female, 62% employed) underwent CTR-US at 24 sites in the United States. The medical history, symptom severity, and functional status of patients were consistent with previous CTS studies [Citation20]. The specialties of the participating physicians included sports medicine (non-surgical) (12), orthopedic surgery (8), anesthesiology (2), radiology (1), and plastic surgery (1). Most (63%) physicians had performed less than 20 CTR-US procedures prior to their first patients enrolling in the registry. A total of 329 (88.2%) procedures were performed using local anesthesia, 44 (11.8%) were performed using monitored anesthesia care, and no procedures were performed using general anesthesia. There were 217 unilateral CTR-US procedures, 51 bilateral staged CTR-US procedures, and 54 bilateral simultaneous CTR-US procedures. Bilateral simultaneous procedures consisted of 14.5% of cases and 25.3% of treated hands ().
Table 1. Characteristics of patients treated using carpal tunnel release with ultrasound guidance
Patient-reported measures of symptom severity and physical function demonstrated rapid improvement following CTR-US. Mean QDASH scores were 41.7 ± 20.1 at baseline, 21.4 ± 15.9 at 2 weeks, 17.7 ± 15.1 at 1 month, 13.3 ± 15.0 at 3 months, and 11.0 ± 15.2 at 6 months. QDASH scores decreased by 20.3 (95% CI: 17.5 to 23.0) points at 2 weeks and 30.8 (95% CI: 28.1 to 33.4) points at 6 months (p < 0.001 at each follow-up interval) (). Mean BCTQ-SSS scores were 3.0 ± 0.7 at baseline, 1.7 ± 0.6 at 2 weeks, 1.7 ± 0.6 at 1 month, 1.5 ± 0.6 at 3 months, and 1.4 ± 0.6 at 6 months. BCTQ-SSS scores decreased by 1.3 (95% CI: 1.2–1.4) points at 2 weeks and 1.6 (95% CI: 1.5–1.7) points at 6 months (p < 0.001 at each follow-up interval) (). Mean BCTQ-FSS scores were 2.4 ± 0.8 at baseline, 1.7 ± 0.6 at 2 weeks, 1.6 ± 0.5 at 1 month, 1.4 ± 0.5 at 3 months, and 1.3 ± 0.5 at 6 months. BCTQ-FSS scores decreased by 0.7 (95% CI: 0.5–0.8) points and 1.0 (95% CI: 0.9–1.1) points at 2 weeks and 6 months, respectively (p < 0.001 at each follow-up interval) (). The standardized effect size at 6 months was 1.55 for QDASH, 2.12 for BCTQ-SSS, and 1.39 for BCTQ-FSS, all of which were above the threshold of 1.0 for defining a very large treatment effect ().
Figure 2. Change in QDASH score over 6 months following carpal tunnel release with ultrasound guidance. plotted values are absolute mean change and 95% confidence interval. asterisk denotes p < 0.001 for change relative to baseline. the minimal clinically important difference (MCID) for postoperative change was 15 points [Citation14]. QDASH = Quick Disabilities of the Arm, Shoulder, and Hand Questionnaire.
![Figure 2. Change in QDASH score over 6 months following carpal tunnel release with ultrasound guidance. plotted values are absolute mean change and 95% confidence interval. asterisk denotes p < 0.001 for change relative to baseline. the minimal clinically important difference (MCID) for postoperative change was 15 points [Citation14]. QDASH = Quick Disabilities of the Arm, Shoulder, and Hand Questionnaire.](/cms/asset/7cba9150-d2a1-4747-ae22-c02a9c398b97/ierd_a_2048816_f0002_b.gif)
Figure 3. Change in BCTQ-SSS and BCTQ-FSS score over 6 months following carpal tunnel release with ultrasound guidance. plotted values are the absolute mean change and 95% confidence interval. asterisk denotes p < 0.001 for change relative to baseline. the minimal clinically important difference (MCID) for postoperative change was 0.74 for BCTQ-FSS and 1.14 points for BCTQ-SSS [Citation15]. BCTQ-FSS = Boston carpal tunnel questionnaire functional status scale; BCTQ-SSS = Boston Carpal Tunnel Questionnaire Functional Status Scale, and Boston Carpal Tunnel Questionnaire Symptom Severity Scale.
![Figure 3. Change in BCTQ-SSS and BCTQ-FSS score over 6 months following carpal tunnel release with ultrasound guidance. plotted values are the absolute mean change and 95% confidence interval. asterisk denotes p < 0.001 for change relative to baseline. the minimal clinically important difference (MCID) for postoperative change was 0.74 for BCTQ-FSS and 1.14 points for BCTQ-SSS [Citation15]. BCTQ-FSS = Boston carpal tunnel questionnaire functional status scale; BCTQ-SSS = Boston Carpal Tunnel Questionnaire Functional Status Scale, and Boston Carpal Tunnel Questionnaire Symptom Severity Scale.](/cms/asset/02a93d17-056a-4dfb-8bf4-fc72e70eacf0/ierd_a_2048816_f0003_b.gif)
Table 2. Change in main outcomes reported as a standardized effect size over 6 months following carpal tunnel release with ultrasound guidance.a.
The median time to return to normal activities following CTR-US was 3 days (IQR: 2–5 days), with 96.5% of patients reporting return to normal activities within 2 weeks of the procedure (). Among employed patients, the median time to return to work following CTR-US was 5 days (IQR: 3–9 days), with 92.3% of patients reporting return to work within 2 weeks of the procedure (). The median time to return to work based on employment type was 4 days (IQR: 3–6 days) for desk-based occupations, 6 days (IQR: 4–11 days) for light manual occupations, and 5 days (IQR: 3–14 days) for heavy manual occupations. Among all patient subgroups, a rapid return to normal activities and return to work was observed and the improvements in QDASH, BCTQ-SSS, and BCTQ-FSS over 6 months were statistically significant and exceeded the MCIDs (). The percentage of patients who were satisfied or very satisfied with the procedure was 91.6% at 2 weeks, 88.2% at 1 month, 88.0% at 3 months, and 89.8% at 6 months.
Figure 4. Percentage of patients who returned to normal activities following carpal tunnel release with ultrasound guidance. median time to return to normal activities was 3 days (interquartile range: 2–5 days).
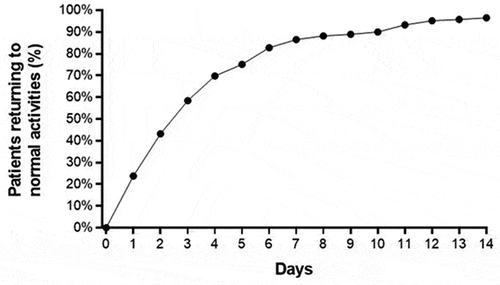
Figure 5. Percentage of employed patients who returned to work following carpal tunnel release with ultrasound guidance. median time to return to work was 5 days (interquartile range: 3–9 days).
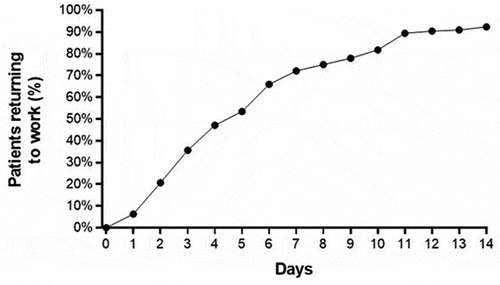
Table 3. Subgroup analysis of main outcomes following carpal tunnel release with ultrasound guidance
Among 346 (93%) patients with available complication data, no major neurovascular complications were reported. Specifically, there was 1 (0.3%) incomplete release confirmed during reoperation and no reports of superficial infection, deep infection, arterial laceration, or permanent nerve injury.
4. Discussion
Carpal tunnel release with ultrasound guidance was developed to afford patients with CTS comparable clinical outcomes relative to traditional CTR but via a less invasive technique to facilitate faster recovery [Citation21]. Although the evidence supporting CTR-US continues to evolve, it is largely limited to small randomized controlled trials and case series. To overcome this limited evidence base, this study provided data from a large sample of patients who received CTR-US in routine clinical practice and explored the influence of select patient characteristics on symptom relief, functional improvement, time to return to normal activities, work absenteeism, and neurovascular complications. The major findings from this study were: a) improvements in symptoms and function occurred rapidly and were sustained throughout 6 months of follow-up, b) the time to return to normal activities and return to work was short (even among manual laborers), c) main outcomes were consistent across patient characteristic and surgical type (i.e. unilateral vs. simultaneous bilateral) categories, and d) the procedure was safely performed in an unselected sample of patients treated in real-world conditions.
The results of this study with CTR-US compare favorably to results obtained from studies with open/mini-open CTR that measured the same outcomes at identical follow-up intervals (). This comparison was deemed relevant since open/mini-open CTR accounts for 84% of CTR procedures performed in the United States [Citation22]. Qualitatively, 6-month outcomes and major neurovascular complication rates with these techniques are comparable. However, there are notable differences in return to normal activity and return to work, as well as small differences in patient function at 2 weeks, that appear to favor CTR-US. These observations are supported by the randomized controlled trial of de la Fuente et al. who reported better functional status and faster return to work with CTR-US vs. open CTR [Citation23] as well as the study by Rojo-Manaute et al [Citation7] that reported a mean return to normal activities, including work, of 4.9 days among patients treated using CTR-US. Loss of wages from CTS and its treatment exceed those of fracture care; therefore, treatments that allow early return to work such as CTR-US may result in cost savings when considering direct and indirect costs [Citation24]. In particular, the observations of a median return to work of 4 days among worker compensation cases and 5 days among individuals engaged in heavy labor occupations provide support that CTR-US promotes rapid recovery even among the most challenging cases. Additionally, the ability to perform simultaneous bilateral procedures reduces morbidity and shortens the overall episode of care relative to staged CTR procedures among patients with bilateral CTS. Finally, patient satisfaction rates with CTR-US were comparable to the 80% to 85% rates reported with open CTR [Citation25,Citation26]. On balance, comparisons of results from this study to those from the open/mini-open CTR literature should be interpreted cautiously. Although these comparator studies are representative of the open/mini-open CTR literature, they were not selected using systematic review methodology and important differences in study design, patient characteristics, and operator experience may confound interpretation. Therefore, comparisons of CTR-US to open/mini-open CTR should be considered hypothesis-generating only.
Table 4. Comparison of results using carpal tunnel release with ultrasound guidance vs. open/mini-open carpal tunnel release
The results derived from this registry suggest broad applicability of CTR-US in a large patient sample treated in routine clinical practice without specific study eligibility criteria. Patients were enrolled from over 20 different practices, most physicians had performed less than 20 CTR-US procedures prior to patient enrollment, and surgical indications and postoperative care were left to the discretion of each practice, which are strengths of a pragmatic study. The patient group was typical of a CTR population and had a high burden of comorbidities. Nonetheless, there were several limitations of this study that warrant further discussion. First, the durability of outcomes beyond 6 months of follow-up were not reported. However, this may be of minor importance because it was observed that the majority of symptomatic and functional improvement following CTR occurs within 6 months after the procedure [Citation38]. Patient follow-up to 1 year is currently ongoing in this registry. Second, it is possible that some patients specifically electing to be treated with CTR-US may have been motivated to return to work at an earlier date based on their preoperative expectations. Return to work is a complex process that may be affected by a variety of biopsychosocial factors including, but not limited to the surgical technique, postoperative wound status, job demands, job satisfaction, patient-specific factors, and preoperative counseling [Citation39]. The specific factors that influenced return to work in the current study are unclear and beyond the scope of the current investigation. Third, specific reasons for treatment dissatisfaction were not reported in this study. Identification of determinants of treatment dissatisfaction following CTR-US is worthy of additional study. Fourth, patients did not return for in-person follow-up visits but instead reported outcomes via text and e-mail at routine intervals during follow-up. To assure high response rate and robust follow-up, remote assessment is prudent and has been applied for conditions such as CTS when outcomes questionnaire assessment is the most responsive to clinical change [Citation40,Citation41]. However, the risk of bias inherent in self-reported data must be acknowledged. Finally, outcome data were missing for a modest proportion of patients at the 6-month follow-up interval which may confound interpretation of the results.
5. Conclusions
Among patients treated with CTR-US in this multicenter pragmatic study, the results demonstrated clinically meaningful improvements in symptoms and function, rapid return to normal activities, minimal work absenteeism, and an excellent safety profile.
Declaration of interest
J Fowler reports consulting fees from Integra Life Sciences unrelated to the current study, and serves as a non-compensated advisor to Sonex Health. L Miller received financial support from Sonex Health. K Chung reports funding from Sonex Health related to the current study, and funding from the National Institutes of Health and book royalties from Wolters Kluwer and Elsevier unrelated to the current study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Conception and design: J Fowler, K Chung
Data analysis: L Miller
Data interpretation: J Fowler, K Chung, L Miller
Drafting of the paper: J Fowler, L Miller
Critical review and revision the paper: J Fowler, K Chung, L Miller
Final approval of the version to be published: J Fowler, K Chung, L Miller
Agree to be accountable for all aspects of the work: J Fowler, K Chung, L Miller
Supplemental Material
Download MS Word (12.1 KB)Supplementary material
Supplemental data for this article can be accessed here.
Data Availability Statement
Raw data will not be made available since this is an ongoing registry.
Additional information
Funding
References
- Atroshi I, Gummesson C, Johnsson R, et al. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282(2):153–158.
- American Academy of Orthopaedic Surgeons. Management of carpal tunnel syndrome evidence-based clinical practice guideline. (Ed.^(Eds). 2016. [cited Feb 2 2022]. https://www.aaos.org/globalassets/quality-and-practice-resources/carpal-tunnel/cts_cpg_4-25-19.p
- Greenfield PT, Spencer CC, Dawes A, et al. The preoperative cost of carpal tunnel syndrome. J Hand Surg Am. 2021. DOI:10.1016/j.jhsa.2021.07.027
- Vasiliadis HS, Georgoulas P, Shrier I, et al. Endoscopic release for carpal tunnel syndrome. Cochrane Database Syst Rev. 2014;1:CD008265.
- Shin EK. Endoscopic versus open carpal tunnel release. Curr Rev Musculoskelet Med. 2019;12(4):509–514.
- Leiby BM, Beckman JP, Joseph AE. Long-term clinical results of carpal tunnel release using ultrasound guidance. Hand (N Y). 2021;1558944720988080. 10.1177/1558944720988080
- Rojo-Manaute JM, Capa-Grasa A, and Chana-Rodriguez F, et al. Ultra-minimally invasive ultrasound-guided carpal tunnel release: a randomized clinical trial. J Ultrasound Med. 2016;35(6):1149–1157.
- Asserson DB, North TJ, Rhee PC, et al. Return to work following ultrasound guided thread carpal tunnel release versus open carpal tunnel release: a comparative study. J Hand Surg Eur Vol. 2021;17531934211051276.
- Joseph AE, Leiby BM, Beckman JP. Clinical results of ultrasound-guided carpal tunnel release performed by a primary care sports medicine physician. J Ultrasound Med. 2020;39(3):441–452.
- Henning PT, Yang L, Awan T, et al. Minimally invasive ultrasound-guided carpal tunnel release: preliminary clinical results. J Ultrasound Med. 2018;37(11):2699–2706.
- Kamel SI, Freid B, Pomeranz C, et al. Minimally invasive ultrasound-guided carpal tunnel release improves long-term clinical outcomes in carpal tunnel syndrome. AJR Am J Roentgenol. 2021;217(2):460–468.
- Beaton DE, Wright JG, Katz JN. Upper extremity collaborative g. development of the quickDASH: comparison of three item-reduction approaches. J Bone Joint Surg Am. 2005;87(5):1038–1046.
- Levine DW, Simmons BP, Koris MJ, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993;75(11):1585–1592.
- Franchignoni F, Vercelli S, Giordano A, et al. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (quickDASH). J Orthop Sports Phys Ther. 2014;44(1):30–39.
- Kim JK, Jeon SH. Minimal clinically important differences in the carpal tunnel questionnaire after carpal tunnel release. J Hand Surg Eur Vol. 2013;38(1):75–79.
- Ratzon N, Schejter-Margalit T, Froom P. Time to return to work and surgeons’ recommendations after carpal tunnel release. Occup Med (Lond). 2006;56(1):46–50.
- Parot-Schinkel E, Roquelaure Y, Ha C, et al. Factors affecting return to work after carpal tunnel syndrome surgery in a large French cohort. Arch Phys Med Rehabil. 2011;92(11):1863–1869.
- Ibrahim JG, Molenberghs G. Missing data methods in longitudinal studies: a review. Test (Madr). 2009;18(1):1–43
- Sullivan GM, Feinn R. Using effect size-or why the p value is not enough. J Grad Med Educ. 2012;4(3):279–282.
- Bodavula VK, Burke FD, Dubin NH, et al. A prospective, longitudinal outcome study of patients with carpal tunnel surgery and the relationship of body mass index. Hand (N Y). 2007;2(1):27–33.
- Petrover D, Hakime A, Silvera J, et al. Ultrasound-guided surgery for carpal tunnel syndrome: a new interventional procedure. Semin Intervent Radiol. 2018;35(4):248–254.
- Foster BD, Sivasundaram L, Heckmann N, et al. Surgical approach and anesthetic modality for carpal tunnel release: a nationwide database study with health care cost implications. Hand (N Y). 2017;12(2):162–167.
- de la Fuente J, Aramendi JF, and Ibanez JM, et al. Minimally invasive ultrasound-guided vs open release for carpal tunnel syndrome in working population: a randomized controlled trial. J Clin Ultrasound. 2021;49(7):693–703.
- Foley M, Silverstein B, and Polissar N. The economic burden of carpal tunnel syndrome: long-term earnings of CTS claimants in Washington State. Am J Ind Med. 2007;50(3):155–172.
- Lozano Calderon SA, Paiva A, Ring D. Patient satisfaction after open carpal tunnel release correlates with depression. J Hand Surg Am. 2008;33(3):303–307.
- Mosegaard SB, Stilling M, Hansen TB. Higher preoperative pain catastrophizing increases the risk of low patient reported satisfaction after carpal tunnel release: a prospective study. BMC Musculoskelet Disord. 2020;21(1):42.
- Capa-Grasa A, Rojo-Manaute JM, Rodriguez FC, et al. Ultra minimally invasive sonographically guided carpal tunnel release: an external pilot study. Orthop Traumatol Surg Res. 2014;100(3):287–292.
- Gurpinar T, Polat B, Polat AE, et al. Comparison of open and endoscopic carpal tunnel surgery regarding clinical outcomes, complication and return to daily life: a prospective comparative study. Pak J Med Sci. 2019;35(6):1532–1537.
- Muhammed Fazil S VV, Karuppal S, Gopinathan R, et al. Mini-open transverse flexor crease incision versus limited longitudinal palmar incision carpal tunnel release: a short term outcome study. J Orthop. 2022;29:15–21.
- Atroshi I, Larsson GU, Ornstein E, et al. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ. 2006;332(7556):1473.
- Khoshnevis J, Layegh H, Yavari N, et al. Comparing open conventional carpal tunnel release with mini-incision technique in the treatment of carpal tunnel syndrome: a non-randomized clinical trial. Ann Med Surg (Lond). 2020;55:119–123.
- Saw NL, Jones S, Shepstone L, et al. Early outcome and cost-effectiveness of endoscopic versus open carpal tunnel release: a randomized prospective trial. J Hand Surg Br. 2003;28(5):444–449.
- Aslani HR, Alizadeh K, Eajazi A, et al. Comparison of carpal tunnel release with three different techniques. Clin Neurol Neurosurg. 2012;114(7):965–968.
- Jacobsen MB, Rahme H. A prospective, randomized study with an independent observer comparing open carpal tunnel release with endoscopic carpal tunnel release. J Hand Surg Br. 1996;21(2):202–204.
- Gil JA, Weiss B, Kleiner J, et al. A prospective evaluation of the effect of supervised hand therapy after carpal tunnel surgery. Hand (N Y). 2020;15(3):315–321.
- Kim JK, Koh YD, Kim JO, et al. Changes in clinical symptoms, functions, and the median nerve cross-sectional area at the carpal tunnel inlet after open carpal tunnel release. Clin Orthop Surg. 2016;8(3):298–302.
- De Kleermaeker F, Meulstee J, Claes F, et al. Outcome after carpal tunnel release: effects of learning curve. Neurol Sci. 2019;40(9):1813–1819.
- Guyette TM, Wilgis EF. Timing of improvement after carpal tunnel release. J Surg Orthop Adv. 2004;13(4):206–209.
- Peters S, Johnston V, Hines S, et al. Prognostic factors for return-to-work following surgery for carpal tunnel syndrome: a systematic review. JBI Database System Rev Implement Rep. 2016;14(9):135–216.
- Schwartzenberger J, Presson A, Lyle A, et al. Remote collection of patient-reported outcomes following outpatient hand surgery: a randomized trial of telephone, mail, and e-mail. J Hand Surg Am. 2017;42(9):693–699.
- Anthony CA, Lawler EA, Glass NA, et al. Delivery of patient-reported outcome instruments by automated mobile phone text messaging. Hand (N Y). 2017;12(6):614–621.
