ABSTRACT
Background
The American Society for Testing and Materials (ASTM), considered the gold standard worldwide, requires only testing in physiological saline solution to simulate in vivo conditions in standard testing of spinal implants.
Research design and methods
We conducted an in vitro study to identify an industrial lubricant with characteristics that are most similar to those of biologically lubricating fat, blood, and tissue fluids. The use of such a material could standardize the results of in vitro mechanical tests for better clinical applications.
Results
Our study has shown that the lubricity of physiological saline was well below that of human soft tissues and tissue fluids, and among the motor oils, Castrol GTX3 provided a testing environment similar to that of a living organism.
Conclusions
With the intention of standardizing and preventing a biological hazard, we have developed a reproducible mechanical testing proposal based on our experiments, which, in addition, would allow us to avoid many misunderstandings and contingencies.
1. Introduction
Skeletal implants inserted into a living organism can be divided into four main groups based on mechanical features: 1. moving structures (joint prostheses); 2. stand-alone implants (screws, and cerclages); 3. combined structures, montages, which predominantly exert shear forces to each other (plate-screw osteosynthesis, locked medullary nails); and 4. implants susceptible to undesired sliding along each other, where different clamping or screw mechanisms exist to prevent displacement (some spinal implants).
While adequate lubricity is desirable in the first group and is practically similar in groups 2 and 3, in group 4 it has adverse effects in the case of spine stabilization rod systems.
The American Society for Testing and Materials (ASTM) does not provide definitive guidelines for in vitro testing of mounted spinal implants with screws and hooks attached to longitudinal rods:
‘12.5 In developing this test method, significant debate revolved around the question of whether to test in saline, in a simulated body fluid, or in air. Because it was impossible to define a fluid that exactly simulates the in vivo environment and because implants must be compared to one another until performance standards are defined, the test environment is left to the individual investigator.’ [Citation1]
In this study, the research questions were directed at addressing the method of mechanical testing of spinal implants with such a system such that it could be easily reproduced by anyone, and the method to create more definite, standardized conditions for preclinical laboratory testing than the current regulation. Furthermore, from a clinical point of view, it is reported that there are still spinal implants in the market for which the manufacturer does not provide the screw tightening torque required for proper clamping.
To answer this question and find an easily accessible lubricant material that shows similar behavior of the human materials can be found during a regular surgery, we conducted systematic lubricity tests examining the relative lubricity of distilled water, human blood, human adipose tissue, synovia, simulated body fluid (SBF), physiological saline, silicone, vaseline, WD-40 industrial lubricant, turbine oil (Turbine-46 K), engine oil (CASTROL GTX3), and lubricant (Mobil Agri Grease Extra).
2. Materials and methods
We performed two series of measurements. First, we measured the relative lubricity of different substances followed by stability measurements according to a spine implant-related ASTM standard.
2.1. Measurement of relative lubricity
All experiments were performed using a special instrument (.) developed in-house at the University of Debrecen, Faculty of Engineering, Hungary. The device is suitable to measure the relative lubricity of different substances [Citation2]. We used this instrument for testing tissues and fluids present in the operative field and compared our results to readily available industrial lubricants.
Figure 1. The schematic drawing of the device developed for lubricity measurements. 1. compression force by spring. 2. moving force. 3. contact surface.
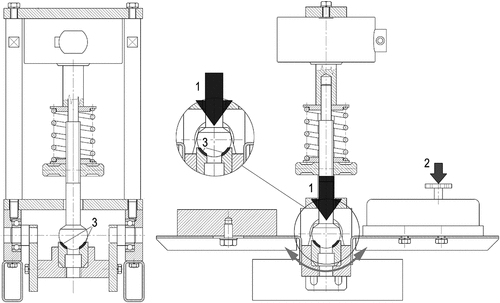
During the examination, two polished conformal metal surfaces were pressed against each other. One surface was a fixed metal sphere that fitted into the concavity of another metal hemisphere mounted on an arm shaft with bearings. The hemisphere can be displaced with respect to the fixed sphere through the movement of the arm shaft. The system also contained a controllable force spring that verified the pressure between the two surfaces. Three milliliters of the tested lubricants were introduced between these two surfaces and the device measured the force required for the displacement of the two surfaces.
The measurements were recorded as follows: before each measurement, the surfaces were cleaned, then the spring was set to the desired value, and the arm shaft was moved free-hand. The tension of the spring and the force required to move the arm shaft was measured by a dynamometer connected to a computer. This type of testing is routinely done under laboratory conditions.
The movement of the arm shaft produces torque that is resisted by the frictional force arising between the two surfaces. The surfaces are influenced by the tension of the spring. As the force increases gradually, the torque surpasses the frictional force and the system moves. Since the kinetic friction is less than the static friction, the frictional force measured during movement is less.
The device was suitable for comparative measurements; therefore, we could compare the results obtained from distilled water with values obtained from the materials listed below.
The measured materials were:
Dry state without any lubricant
Distilled water purified with a Millipore Milli-Q I. (Merck KGaA, Darmstadt, Germany) water purification system
Human blood (taken intra-operatively after prior written consent from patients undergoing elective surgery at our Department)
Human synovial fluid (taken intra-operatively after prior written consent from patients undergoing elective surgery at our Department)
Human subcutaneous fat tissue (taken intra-operatively after prior written consent from patients undergoing elective surgery at our Department)
SBF [Citation3]
Physiological saline solution (0,9%, B. Braun Melsungen AG, Melsungen, Germany)
Silicone (WOLF’S W340, Wolf Chemicals Ltd., Budapest, Hungary)
Vaseline (Vazelin Original, Vasenol, Unilever Plc., London, United Kingdom)
WD-40 (WD-40 Company, San Diego, CA, USA)
Turbine oil (Turbine-46 K, MOL Plc., Budapest, Hungary)
Engine oil (Castrol GTX3, Castrol Ltd., Pangbourne, United Kingdom)
Lubricant grease (Mobil Agri Grease Extra, Exxon Mobil Corporation, Irving, TX, USA)
The peak forces required for movement were calculated and averaged for all substances. The mean lubricity of surgical site tissues and of industrial lubricants were compared with that of distilled water. The relative lubricity of the different substances were calculated by this method. The examinations were performed with three different spring tension levels: 300 N, 500 N, and 700 N. Each measurement was repeated 100 times. This large number of measurements assures a reliable estimation of the mean of the sample.
2.2. Measurement of stability
The stability measurements were performed according to ASTM 1798–97 (2008) standard arrangements [Citation1], with an Instron 8874 material testing machine (Instron Ltd., High Wycombe, United Kingdom) using titanium (Ti6Al4V) rods (diameter: 6 mm) and transpedicular set screws (length: 45 mm) obtained from Sanatmetal Ltd., Eger, Hungary. The appliance pushed the rod after the screw was fixed to it at a rate of 10-mm/min crosshead speed for 3-mm displacement. The transpedicular set screw was tightened with the torque of 2 Nm and, based on the recommendation of most spine instrument manufacturers, with 4.5 Nm torque [Citation4]. Subsequently, a push-out test of the rods was performed with these two values (.).
Figure 2. The scheme of the setup of stability measurements (a) and using an Instron 8874 material testing machine (b) according to the ASTM 1798–97 (2008) standard setup.
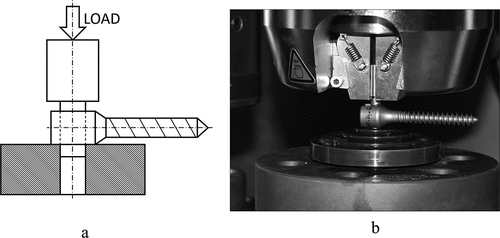
The test was performed using 10 samples, in both dry and lubricated states (both the set screw and the rod were lubricated). The measurements were repeated 3 times according to the standard [Citation1], making sure that the screws were tightened not on the same part of the rod as previously. A total of 120 measurements were recorded for stability test. Maximum load (N), displacement at maximum load (mm), and stiffness (N/mm) values were recorded for each screw.
2.3. Software and statistical analysis
The measured data were extracted and evaluated using the Catman Easy software (HBM GmBH, Darmstadt, Germany).
The normality of the population was determined using the Shapiro–Wilk test. Statistical differences between different lubricants and different spring tension levels (300 N, 500 N and 700 N) were analyzed by Two-way ANOVA supplemented with Tukey post hoc test. A p < 0.05 was considered statistically significant.
Data are presented as mean ± SD. Statistical calculations were performed using GraphPad Prism 7. (GraphPad Software Inc., San Diego, CA, USA).
3. Results
3.1. Determination of relative lubricity
For determining the relative lubricity of different substances, the peak forces required for movement were measured. The values of the mean lubricity of surgical site tissues and of industrial lubricants were compared with the results obtained with distilled water, and the relative lubricity of the different substances was determined. We assumed that the lubricity of distilled water is 100% (reference value), and the other values were compared to this one. The results obtained for the same substance at the different spring tension levels were averaged. The mean relative lubricity data were plotted on a bar graph (.).
Figure 3. The calculated relative lubricity average values and standard deviation (SD) compared to distilled water. Human samples are represented in black color and the industrial lubricants in light gray. The other conditions (SBF, saline solution, dry) are plotted in white bars.
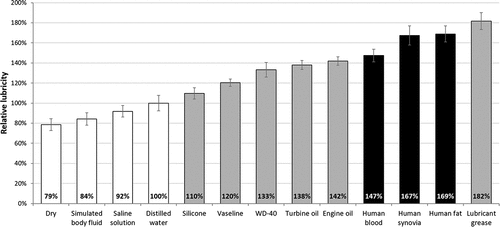
The average lubricity of the surgical sites (blood and subcutaneous fat tissue) were similar to that of the industrial lubricants. As the graph shows, the lubricity of the examined human tissue falls between those of motor oil and lubricant grease, but closer to that of the engine oil, therefore, the stability measurements were performed with CASTROL GTX-3 motor oil. In contrast, the relative lubricity of SBF, as recommended by ASTM standards, was less than that of the physiological salt solution (PSS), and was only slightly larger than the results obtained in the dry state, without any lubricant solution.
The most relevant finding of this experiment was that this motor oil’s lubricity was similar to that of human tissues found in the surgical site; therefore, stability testing of spinal implants can be easily, comparably, and inexpensively performed by using industrial oils in future, without any ethical problem.
The statistical results showed that in the case of 300 N compression force there were no significant differences between the moving force of engine oil and the three human materials applying the device showed in (Engine oil vs. Human blood: p = 0.111, Engine oil vs. Human synovia: p = 0.998, Engine oil vs. Human fat: p = 0.999). Increasing the compression force to 500 N and 700 N, the differences became significant (500 N Engine oil vs. Human blood: p = 0.036, and in all other cases with 500 N and 700 N: p < 0.001). Comparing the moving force of the selected materials to the dry state, we found that there were significant differences in each case (p < 0.0001).
3.2. Stability measurements
There are many factors that can affect stability in the case of spinal implants fixed by set screws, such as the set screw design, outer or inner configurations, and diameter, and the lubricity of surrounding soft tissues. We conducted studies on the rod-implant screw interface, in order to assess the loosening of metal implant parts.
For stability testing, based on previous experiments, Castrol GTX3 motor oil was used as a lubricant. As control, dry state was also tested at two torque variations. At 2 Nm torque, 330 N was required to push out the rod while this increased to 607 N at 4.5 Nm. When motor oil was used, this decreased to 293 N and 595 N, respectively. To push out the rod at 4.5 Nm torque, 1.84 times larger force was required in the dry state and 2.02 times in the oil-lubricated state than at 2 Nm (.).
Figure 4. Perpendicular push-out force. The results of the stability measurements; force/displacement charts: a. 2-Nm tightening torque and b. 4.5-Nm tightening torque.
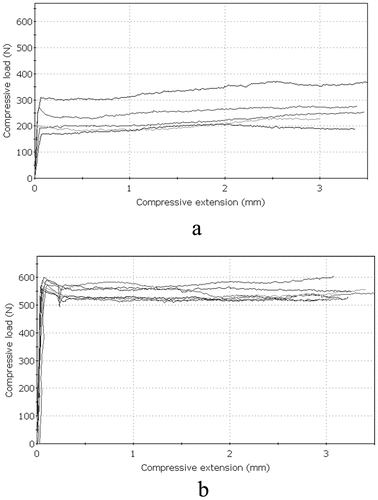
The present results showed that the use of a lubricant decreased the force required for destabilization of the montage, by 10% at 2 Nm torque and by 2% at 4.5 Nm values. Lubricated screws resulted in lower detorque which made the joint easier to loosen.
The difference was statistically significant, according to the two-sample one tailed t-test at 5% significance level (p = 0.038).
4. Discussion
This study was motivated by the need to find a synthetic material with tribological properties closest to those of materials with lubricity in the living organism, going beyond current regulations and making a consensual proposal for more realistic mechanical tests, and making it mandatory for manufacturers to provide the required placement torque in case of spinal implants mounted on a rod with screws.
Lubrication is one of the key aspects of tribology. A lubricant is usually used to keep a distance between two solid materials, consequently preventing direct mechanical contact with each other. The lower the lubricity, the greater the wear of the examined material. The lubricity of a substance cannot be measured directly, moreover it is significantly influenced by other factors such as the shape, microgeometry and extent of the surfaces, viscosity and density of the lubricant, and temperature and pressure [Citation5]. Lubricants can play a key role in the instability of assembled spinal implants in human bodies. Posterior spinal implants are composed of different pedicle screws and hooks, which are connected to two longitudinal rods. Spinal implants mounted on rods are subjected to significant strain after the correction of deformities, and this is compensated by the clamping force of the set screws securing the implant parts together. In the surgical field, both the loosening of the securing set screws and the sliding of the implants on the rods are facilitated by the undesired lubricity of the environment [Citation6] and can be associated with implant failures due to set-screw loosening [Citation7].
Implant stability is important, as it is a key to secure fixation of the implant to the bone and prevents montage failure [Citation8,Citation9]. The implant manufacturers usually specify the implant placement torque (IPT) values of set screws. The primary stability measurements are performed in standardized laboratories working mostly according to ASTM directives. However, most of the tribology tests are conducted with water or saline [Citation10,Citation11], and do not take into consideration the lubricity of surrounding human tissues.
These test methods are used to quantify the static and dynamic mechanical characteristics of different designs of spinal montages. ASTM mentions that the tests performed with SBF or PSS may cause fretting, corrosion, or lubricate the interconnections, and thereby affect the relative performance of tested devices [Citation12,Citation13]. The results obtained cannot be used directly to predict the in vivo performance, as they could significantly differ from the real values obtained in the intra-operative setting. A number of rheological, mechanical, and tribological properties of human tissues have been determined [Citation14], but the literature about the lubricity of human tissues is very limited. In orthopedic surgical practice, lubricity plays both positive and negative roles. In certain cases, increased lubricity is beneficial, especially in joint replacement surgery [Citation15,Citation16]. However, in case of assembled spinal implants, lubrication can be specifically harmful. Lubrication caused by the surrounding tissues and blood due to their high viscosity may result in loosening of assembled implants. While there is abundant information available on the lubrication of endoprosthesis [Citation17–20], we were not able to find publications about the stability of assembled spinal implants examined under physiological conditions.
Although the performed experiments were technically easy, the results showed that tissues found in the surgical field have a larger lubricant property than expected. In cases where the manufacturer does not provide the value of the torque and the screw is tightened without torque meter, the torque could be low. At low torque values (2 Nm), these lubricants showed a significant decrease in their stability (10%). We suggest the performance of stability testing under physiological conditions and not just in dry state. These examinations, based on our results obtained, can be performed with Castrol GTX-3 oil as its lubricity is similar to that of most lubricating human tissues found in the surgical field. Therefore, the stability tests can be easily performed without any human tissues, thereby avoiding any ethical, organizational, and the associated public health problems such those related to accessing and collecting the human materials and obtaining the consent to use them.
A limitation of this study is the low number of measured lubricants. Further studies are warranted to evaluate the measurements involving several other lubricants and there is a need to conduct long period in vitro testing to validate the stability testing methodology used in this research as well.
5. Conclusions
The stability performance of assembled spinal implants depends on many factors, including design and material selection. Additionally, there are other important biotribological testing parameters (such as kinematics and load, phasing, and test fluid medium), that can significantly affect the outcome of a particular stability test. The implant testing standards/guides leave many open-ended choices that will influence results. It is essential to compare the in vitro test method to that of in vivo. In addition, correct interpretation of test results will help to prevent montage loosening. Our proposal is to recommend a worst case rheological scenario of using Castrol GTX3 engine oil.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- ASTM F1798-97(2008) Standard guide for evaluating the static and fatigue properties of interconnection mechanisms and subassemblies used in spinal arthrodesis implants. West Conshohocken (PA): ASTM International; 2008. ••Contains the technical details of the mechanical test of spinal implants including the lubricity aspects
- Tiba Z, Husi G, Manó S, et al. An easy to use device for lubricity examination. Biomech Hung. 2009; 2(2):27–30.
- Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27(15):2907–2915. PubMed PMID: 16448693.
- Mellinger P. Self-limiting set screw for use with spinal implant system. United States patent US 5,697,929, Dec 16 1997.
- Wong PL, Wang R, Lingard S. Pressure and temperature dependence of the density of liquid lubricants. Wear. 1996;201(1–2):58–63. PubMed: WOS. A1996WC53100008. English.
- Okamoto T, Neo M, Fujibayashi S, et al. Mechanical implant failure in posterior cervical spine fusion. Eur Spine J. 2012;21(2):328–334. PubMed PMID: 22002474. PubMed Central PMCID: PMC3265582.
- Yao Y, Yuan H, Huang H, et al. Biomechanical design and analysis of auxetic pedicle screw to resist loosening. Comput Biol Med. 2021;133:104386. PubMed PMID: 33878515.
- Kwok AWL, Finkelstein JA, Woodside T, et al. Insertional torque and pull-out strengths of conical and cylindrical pedicle screws in cadaveric bone. Spine (Phila Pa 1976). 1996;21(21):2429–2434. doi.PubMedPMID:WOS.A1996VR73500004. English.
- Mueller TL, van Lenthe GH, Stauber M, et al. Regional, age and gender differences in architectural measures of bone quality and their correlation to bone mechanical competence in the human radius of an elderly population. Bone. 2009;45(5):882–891. PubMed PMID: WOS. 000271071500008; English.
- Trunfio-Sfarghiu A-M, Berthier Y, Meurisse M-H, et al. Multiscale analysis of the tribological role of the molecular assemblies of synovial fluid. Case of a healthy joint and implants. Tribol Int. 2007;40(10–12):1500–1515. PubMed: WOS. 000249878100012; English.
- Wang A, Essner A, Schmidig G. The effects of lubricant composition on in vitro wear testing of polymetric acetabular components. J Biomed Mater Res B Appl Biomater. 2003;689(1) :45–52. doi:10.1002/jbm.b.10077.
- ASTM F1717-15 Standard test methods for spinal implant constructs in a vertebrectomy model West Conshohocken (PA): ASTM International; 2015
- ASTM F2193-14 Standard specifications and test methods for components used in the surgical fixation of the spinal skeletal system West Conshohocken (PA): ASTM International; 2014
- Abé H, Hayashi K, Sato M. Hard Tissues. Data Book on Mechanical Properties of Living Cells, Tissues, and Organs . Tokyo: Springer. 1996;291–362.
- Stotter C, Stojanović B, Bauer C, et al. Effects of loading conditions on articular cartilage in a metal-on-cartilage pairing. J Orthop Res. 2019;37(12):2531–2539. PubMed PMID: 31334864. PubMed Central PMCID: PMC6899800.
- Seror J, Merkher Y, Kampf N, et al. Normal and shear interactions between hyaluronan-aggrecan complexes mimicking possible boundary lubricants in articular cartilage in synovial joints. Biomacromolecules. 2012;13(11):3823–3832. PubMed PMID: 23074968. PubMed Central PMCID: PMC3497856.
- Kyomoto M, Moro T, Saiga K, et al. Lubricity and stability of poly(2-methacryloyloxyethyl phosphorylcholine) polymer layer on Co-Cr-Mo surface for hemi-arthroplasty to prevent degeneration of articular cartilage. Biomaterials. 2010;31(4):658–668. PubMed PMID: 19819011.
- Gispert MP, Serro AP, Colaço R, et al. Friction and wear mechanisms in hip prosthesis: comparison of joint materials behaviour in several lubricants. Wear. 2006;260(1–2):149–158.
- Mishina H, Kojima M. Changes in human serum albumin on arthroplasty frictional surfaces. Wear. 2008;265(5–6):655–663.
- Hills BA, Crawford RW. Normal and prosthetic synovial joints are lubricated by surface-active phospholipid: a hypothesis. J Arthroplasty. 2003;18(4):499–505.
