ABSTRACT
Introduction
Despite advances in heart failure therapies and percutaneous coronary interventions, survival for cardiogenic shock remains poor. Percutaneous ventricular assist devices (pVAD) are increasingly used, but current evidence remains conflicting. The Impella is an example of such a device, based on a catheter mounted micro-axial continuous flow pump, that has been rapidly adopted in routine practice. An important aspect of postimplantation care is the prevention of complications. Hemolysis is one of the most frequent complications seen with this device.
Areas covered
In this review, we discuss the pathophysiology, diagnosis and treatment of hemolysis in patients supported with a pVAD. A practical algorithm for rapid identification of hemolysis and the underlying cause is presented, allowing for early treatment and prevention of further complications.
Expert opinion
Hemolysis remains a threat to patients supported with any mechanical circulatory support device. Prevention as well as treatment demands for sufficient knowledge about the device, the optimal position, and hemodynamics. Future studies should try to clarify some of the elements that are still unclear, such as optimal anticoagulation, the location of pentoxifylline, or extracorporeal removal of free hemoglobin. This could help to optimize outcomes in clinical practice as well as future studies.
1. Introduction
Despite huge advances in heart failure therapies and percutaneous coronary interventions, survival for cardiogenic shock (CGS) remains poor [Citation1]. The Impella (Abiomed, Danvers, USA) percutaneous ventricular assist device (pVAD) is a catheter mounted micro-axial flow pump, available in different sizes and shapes. The failure of the Intra-aortic balloon pump to reduce mortality in a large randomized controlled trial of myocardial infarction-related CGS and the promising results of these newer devices in dedicated centers have led to a rapid clinical uptake of pVAD’s, despite controversial results [Citation2–5]. Particular challenges come with the use of pVAD’s and these might explain the discrepancy in results between different studies [Citation6]. Hemolysis is one of the most frequently encountered problems during mechanical circulatory support (MCS) with any device type and is associated with worse outcomes in some studies [Citation7–10]. The reported incidence is variable, depending on the definition of hemolysis, but also on patient management practices during the study (since hemolysis is a preventable complication). The pVAD is not an exception and the reported incidence of hemolysis during pVAD support ranges from 0% up to 32%, depending on the definition of hemolysis, type of pVAD, study population, as well as management practices () [Citation7,Citation8]. This is why profound knowledge of the pathophysiology, diagnosis, and treatment of hemolysis in the pVAD supported patient is important both to optimize individual patient outcomes and to allow proper evaluation in clinical studies
Table 1. Overview of hemolysis in contemporary studies on pVAD. Incidence of hemolysis in contemporary studies with pVAD. AMI = acute myocardial infarction. CS = cardiogenic shock. ECMO = extracorporeal membrane oxygenation. IABP = intra-aortic balloon pump. LDH = lactate dehydrogenase. pfHb = plasma-free hemoglobin.
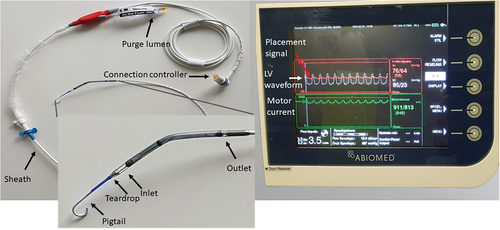
2. The pVAD system
This pVAD is essentially an axial flow pump, mounted on a long catheter, and introduced through the femoral or axillary artery. The pump works as a small Archimedes screw to lift blood from the ventricle to the aorta. It is the size of the specific pVAD that determines the maximal flow rate, ranging from 2.5 L/min up to 5.5 L/min. Specific to the system is the use of a heparinized glucose solution, termed ‘purge fluid,’ that runs in the opposite direction to that of the blood flow. This solution is used to push the blood coming from the ventricle away from the motor housing. The speed and pressure of this ‘purge’ is regulated via a cassette in the device controller. The most relevant parts of the system are shown in .
3. Pathophysiology and causes of hemolysis during pVAD support
Hemolysis during pVAD support occurs when mechanical injury to circulating red blood cells (RBCs) leads to their fragmentation and release of membrane fragments and content into the circulation. Both shear stress and flow acceleration as well as direct contact of the RBCs with the surfaces of the mechanical support device can lead to changes in the shape of erythrocytes, with subsequent stretching and ultimately rupture of the cell membrane [Citation21,Citation22].
Hemolysis is the consequence of degradation of the RBCs. The normal life span of a RBC is around 120 days. Older erythrocytes become less elastic and are more easily destroyed by mechanical stress. This occurs at a rate of around 1% of RBCs daily. The hemoglobin (Hb) content of these cells is released into the blood plasma and further degraded in the liver, where the iron atoms are recycled. In a healthy person, this normal process of destruction of older RBCs (natural hemolysis) is balanced by a compensatory release of newly formed RBCs by the bone marrow, via increased erythropoietin (EPO) secretion by the kidney. In case of intravascular hemolysis, these compensatory mechanisms are overwhelmed and a decrease of the Hb level below the normal range can ensue, termed hemolytic anemia. Furthermore, other laboratory and clinical findings (such as jaundice and dark-colored urine) can be observed [Citation23].
During pVAD support, the increased RBC shear stress is most often a result of intermittent narrowing of the device inlet or outlet opening [Citation24]. In contrast to the previous generation of durable LVAD’s (Heartmate II, Abbott, IL, USA), device thrombus seems to be a rare cause of RBC injury, although data on the role of micro-thrombosis in the context of pVAD support are lacking. Local activation of intrinsic coagulation cascade and platelet adhesion are a major issue during mechanical circulatory support using non-hemocompatible surfaces and have the potential to lead to a self-perpetuating process of thrombosis [Citation25,Citation26]. Shear stress also leads to von Willebrand and glycoprotein Ib receptor interaction, which stimulates platelet mediator release [Citation27].
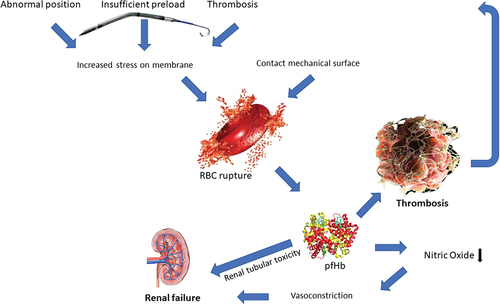
Hemolysis can lead to a self-perpetuating cycle of multi-organ thrombosis and injury (as shown in ). The mediators released by destructed RBC’s exert pro-thrombotic effects. Plasma-free hemoglobin (pfHb) plays a crucial role; it scavenges circulating nitric oxide (NO) and reduces von Willebrand factor degradation [Citation26]. Also, arginase from the RBC consumes L-arginine, which is needed for endothelial NO production [Citation28]. This leads to vasoconstriction and reduced NO-mediated platelet inhibition. In addition, small-membrane vesicles, carbon monoxide as well as iron are released in the circulation to exert pro-thrombotic effects and further stimulate the negative spiral of thrombosis, vasoconstriction, and ischemia-reperfusion [Citation26,Citation28]. Locally in the kidney, the accumulation of large amounts of hemoglobin derivatives (heme, heme proteins, and hemosiderin) in the cells of the proximal tubule can lead to acute tubular necrosis (ATN) because of direct cytotoxicity, endothelial dysfunction, and renal vasoconstriction [Citation29,Citation30]. Moreover, the formation of methemoglobin casts in the distal tubule may cause intrarenal obstruction, eventually worsening the injury to the proximal part of the tubule. These mechanisms explain how the kidney is often the first organ to suffer from failure in the case of severe intravascular hemolysis [Citation29,Citation30]
Figure 2. Pathophysiology of hemolysis during pVAD support. Abnormal position, insufficient preload and device thrombosis lead to increased red blood cell stress. When combined with artificial surface contact this can lead to red blood cell rupture (hemolysis) which releases mediators such as free hemoglobin. These mediators activate coagulation and reduce nitric oxide availability, thrombosis and renal tubular toxicity, which can be a vicious circle. RBC = red blood cell.
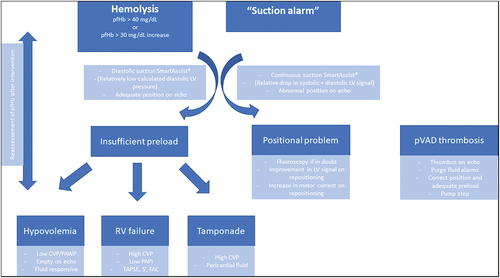
In many cases, hemolysis is associated with so-called suction alarms. In this case, the device controller detects the changes in ‘motor current’ that are the result of intermittent obstruction to the device flow and this will trigger an alarm. An approach to ‘suction alarm’ is presented in . However, it is important to realize that subtle changes can lead to hemolysis even before these suction alarms are triggered so one should ideally act proactively. The causes of RBC injury can be divided into three main categories: abnormal device position, insufficient preload and device thrombosis.
Figure 3. Algorithmic approach to pVAD with suction alarm or hemolysis. The initial question is whether it is a positional issue or an issue of device preload. Filling pressures, echocardiography and smart assist will differentiate these problems. In case of insufficient preload, one must sort out the hemodynamic culprit. CVP = central venous pressure. FAC = fractional area change. LV = left ventricle. PAPi = pulmonary artery pulsatility index. PAWP = pulmonary artery wedge pressure. pfHb = plasma free hemoglobin. pVAD = percutaneous ventricular assist device. RV = right ventricle. TAPSE = tricuspid annular plane systolic excursion.
3.1. Abnormal position
During pVAD support, one of the most frequent causes of hemolysis is abnormal device position. Overinsertion of the device in the left ventricle (LV) can lead to contact between the aortic valve and the device outlet. This has been shown to rapidly induce hemolysis in a bench study [Citation24]. Overinsertion can lead as well to contact between the device inlet and left ventricular myocardial structures, which will also increase RBC shear stress locally. Apart from insertion depth, an abnormal insertion angle can cause proximity of the device inlet to the mitral valve apparatus (leaflets, chordae), which potentially not only causes increased RBC stress but also mitral regurgitation [Citation31–33].
3.2. Insufficient preload
Another frequently encountered scenario is that of insufficient preload. As is the case for any continuous flow pump, the pVAD is preload dependent and device function will not be optimal in case of LV underfilling. The possibility that the device makes contact with ventricular structures increases in case of insufficient preload [Citation34]. The simplest scenario is that of hypovolemia but this is not the only possibility. In case of combined veno-arterial extracorporeal membrane oxygenation (VA-ECMO) and pVAD, relative LV underfilling is even more frequent and not only depends on LV diastolic function but also on VA-ECMO blood flow rate, as well as right ventricular (RV) function [Citation6,Citation34]. Exceptionally, VA-ECMO cannula migration to the left atrium can occur and induce suction events [Citation35]. Another frequent cause of LV underfilling is RV failure. One should not forget that the RV remains responsible for accepting venous return and thus LV preload. Since the RV is unsupported during treatment with an LV pVAD (except for a modest decrease in afterload), it becomes the Achilles heel of the circulation [Citation36]. Lastly, tamponade is a less frequently encountered cause of insufficient venous return and thus LV filling. For an in-depth review of hemodynamic management during pVAD support, we refer to another recently published article [Citation6].
3.3. Thrombosis
Hemolysis can also be a consequence of device thrombosis, inducing local RBC injury. Even more rare is the case of organic material (e.g. compress) engorgement in the impeller. The most common source of device clots is adherent LV thrombi after myocardial infarction, migrating into the device [Citation37,Citation38]. A recent case series describes the use of contrast enhanced computed tomography to exclude such thrombus before pVAD implantation, although patient instability will probably limit this approach to patients with slowly worsening hemodynamics or who are already on VA-ECMO before pVAD implantation [Citation39].
3.4. pVAD design and access
There is a systematic difference in the incidence of hemolysis reported between different pVAD types, favoring the larger 5.0 and 5.5 (). Part of this discrepancy could of course be the consequence of differences in hemolysis definition, patient selection, as well as post-implantation care in the different studies. However, it is also likely that the divergence in hemolysis rates reported between different pump types is the result of differences in design (smaller diameter with larger rpm, no pigtail in some, …) and access strategy. For example, the surgical axillary access that is used for the larger 5.0 and 5.5 pVAD’s might provide more stability. Also, the pigtail, that is not present in every device, might play a role in mitral valve entanglement. Hopefully, the use of a more universal definition of hemolysis in future studies will help in providing a more definite answer to this question.
4. Diagnosis and monitoring of hemolysis during pVAD support
Changes in various laboratory parameters can be observed in patients with intravascular hemolysis. Classically, lactate dehydrogenase (LDH) and phosphate as well as unconjugated bilirubin levels increase, with importantly reduced haptoglobin levels. In severe forms, hyperkalemia can be induced. It should be noted that LDH is a very unspecific parameter (levels are elevated in case of any tissue damage, which is often the case in patients under circulatory support) and that haptoglobin levels are often reduced in severely sick patients. Therefore, the level of pfHb, which is a key biologic marker, is used to estimate the presence and severity of hemolysis. It is advised to take into account earlier blood test results of the patient, if available.
Currently, a uniformly accepted definition of intravascular hemolysis based on laboratory parameters is lacking. Most studies have adopted different sets of preferred diagnostic criteria, with varying combinations of parameters and varying cutoff values for each biomarker. In the INTERMACS study, pfHb >20 mg/dl or serum LDH >2.5 times the upper limit of normal assessed 72 h after durable MCS implantation, was used [Citation33]. However, with the growing number of patients supported with a pVAD, there is a need to be able to identify hemolysis earlier in the course of circulatory support. In this setting, several published clinical studies have defined hemolysis as pfHb >40 mg/dl on more than two measurements taken 8 h apart, reflecting as well the importance of relative changes in pfHb levels during the course of treatment [Citation10,Citation40–42].
Some specific laboratory markers of hemolysis are worth discussing in more detail.
4.1. LDH (Lactate dehydrogenase)
Erythrocytes are rich in lactate dehydrogenase (LDH). Therefore, fragmentation of RBCs will lead to a rapid increase in serum LDH levels. Normal LDH values are 230–460 µ/l. In hemolysis, values above 460 µ/l are noted. In general, there is a good correlation between the degree of ongoing hemolysis and increasing LDH levels. However, one should keep in mind that LDH is a nonspecific marker for hemolysis. Any cause of cellular lysis, ranging from myocardial to kidney infarction to high-proliferative neoplasms and various forms of infectious diseases will cause LDH levels to rise. In the appropriate clinical setting, and taking into account baseline LDH levels and temporal trends in assessment, an elevated LDH >2.5 folds strongly suggests hemolysis. LDH elevation combined with decreased haptoglobin is >90% specific for hemolysis (11). LDH levels returning to normal values are a good indicator of the success of treatment measures.
4.2. Haptoglobin
Haptoglobin is an acute-phase glycoprotein produced in the liver. Its major biological function is to bind – with high affinity – free hemoglobin in plasma, in order to prevent loss of iron molecules, on the one hand, and hemoglobin-mediated renal injury, on the other hand [Citation21]. When circulating hemoglobin is bound to haptoglobin, renal excretion of hemoglobin is reduced, thereby decreasing the risk of injury to the kidney tubules. Therefore, haptoglobin levels are decreased or absent in cases of hemolysis. Normal values of the plasma range in a wide interval between 0.5 and 3.2 g/l. In cases of hemolysis, values <25 mg/dl are characteristic. However, one cannot estimate the severity of intravascular hemolysis based on haptoglobin levels, because of its limited capacity to bind free hemoglobin. Even a mild or moderate degree of RBC destruction will result in undetectable haptoglobin levels. Moreover, other confounding factors with respect to haptoglobin levels must be taken into account, such as decreased baseline levels in patients with liver disease (cirrhosis), abdominal trauma and congenital ahaptoglobinemia and increased levels in the setting of concomitant inflammatory states (role of haptoglobin as an acute-phase reactant) and the nephrotic syndrome [Citation39].
4.3. Bilirubin
Indirect bilirubin is a product of hemoglobin catabolism. The hemoglobin-haptoglobin complex is internalized and degraded to release heme. Bilirubin is generated by heme catabolism. Normal values of indirect bilirubin are 0.3–1.6 mg/dl. In hemolysis, the values usually rise above >2 mg/dl and can be even higher in cases of acute massive hemolysis. Levels >4 mg/dl in the non-acute hemolysis setting are unusual and would rather reflect a concomitant liver pathology impairing the conjugation of bilirubin or its hepatic uptake [Citation21].
4.4. Plasma free hemoglobin (pfHb)
In healthy subjects, free hemoglobin is normally released into the blood as a consequence of the intravascular destruction of senescent RBCs. There is sufficient haptoglobin in circulation to bind and clear 3 g of hemoglobin. This is enough to prevent free hemoglobin circulation in the body. In cases of intravascular hemolysis, levels of plasmatic-free hemoglobin increase when haptoglobin-binding capacity is saturated. Normal pfHb values in healthy subjects are <50 mg/l. Values between 100 and 500 mg/l are seen in cases of moderate to mild and values >500 mg/l in severe hemolysis [Citation41–43]. pfHb is an excellent marker for the early detection and monitoring of correcting actions in cases of hemolysis in patients under left ventricular support [Citation43–46]. pfHb and especially an increase in pfHb have been reported to be superior to LDH in detecting hemolysis specifically in patients with CGS treated with a pVAD [Citation40].
4.5. Direct antiglobulin test (DAT)
The direct antiglobulin test (DAT) or direct Coombs test is used to detect antibodies or complement factors in RBCs of patients. It is used for diagnosing autoimmune hemolytic anemia (AIHA), where the test shows positivity with anti-IgG and anti-C3d. In case of hemolysis, AIHA should always be excluded with a DAT. In cases of mechanical hemolysis, the DAT is negative (11).
4.6. Hemoglobinuria
Hemoglobinuria occurs only in the setting of severe and rapid intravascular hemolysis. It occurs when the binding capacity of haptoglobin for free plasma hemoglobin is exceeded. Serum haptoglobin plays an important role as a circulating buffer protecting the kidney when free hemoglobin level starts to increase. The molecular size of the haptoglobin-hemoglobin complex is too large to be filtered by the kidney [Citation21]. Dimers of alpha-beta globin that are not bound by haptoglobin are small enough to be filtered by the glomerulus. When the capacity of the proximal renal tubule to reabsorb the hemoglobin from the lumen is exceeded, the hemoglobin is freely excreted into the urine, and hemoglobinuria ensues.
5. Monitoring of pVAD position
5.1. The device controller
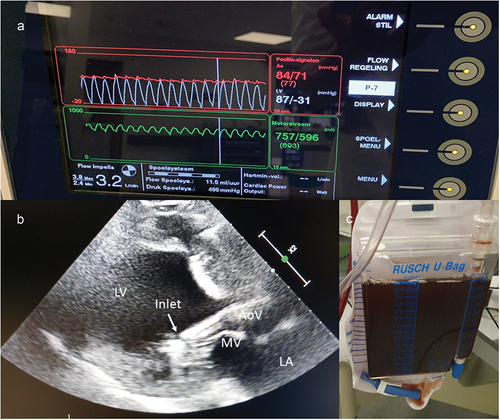
The controller () of the most commonly used devices (CP and 5.5) displays three waveforms (also shown in ):
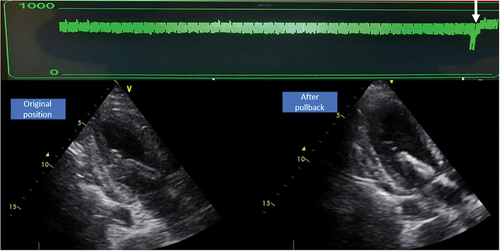
The green waveform is termed ‘motor current’ and is a measure for the flow generated by the motor throughout the cardiac cycle. This waveform should appear pulsatile because the pressure head (pressure difference between inlet and outlet) determines the amount of flow generated for a given motor speed. The pressure head decreases during systole, leading to an increase in flow through the impeller, which results in an increase in motor current. When the device dislocates, this waveform will flatten because both the inlet and outlet opening are in the same cavity and the pressure head equals zero. On dislocation motor current values will generally decrease and an increase in motor current is expected to be seen after successful repositioning of the pVAD (). This can easily be used to inform on the effect of repositioning at the bedside.
Figure 4. Effect of repositioning on motor current. The pVAD was over-inserted. On pullback you can see an immediate increase in motor current (green) and adequate position on echocardiography.
The red waveform on the top of the screen is referred to as the ‘placement signal.’ This is an aortic pressure, measured by an optical sensor just beneath the outlet. This should be an aortic waveform as long as the outlet is in the aorta. For some devices (such as the 5.0 pump) this is a differential pressure. When this pVAD is over-inserted into the LV, the red waveform will become ‘ventriculized.’ When the device is too far out of the ventricle, the placement signal will remain aortic. For pVAD’s that show a differential pressure, the placement signal will just flatten during dislocation, without highlighting the location of the outlet.
The white waveform is a calculated ventricular pressure signal, available on pVAD’s with smart assist technology. This new metric will potentially play an important role in the prevention of hemolysis in the future. The calculation is essentially based on the motor current, which informs on the pressure head (Poutlet-Pinlet). Since this pressure is calculated from the motor current, the virtual LV pressure will decrease during ‘suction’ and this phenomenon can be very useful in the diagnosis of the exact mechanism driving ‘suction.’ When the decrease in ventricular pressure is especially prominent during diastole, preload is the main cause; while continuously decreased ventricular pressure (throughout whole cardiac cycle) is most often caused by a positional problem. These changes are very sensitive and changes in the LV waveform are seen even before ‘suction alarms’ are triggered. illustrates this phenomenon of continuous suction (although, in this case, the alarm has not been triggered yet).
Figure 5. Signs of abnormal position. Example of a patient with pVAD close to the mitral valve apparatus, causing hemolysis without suction alarm. A. Subtle shift of the ventricular waveform (white) below the placement signal (red) suggests suboptimal position. B. Echocardiography shows abnormal angle of pVAD with inlet close to the mitral valve. C. Urine bag showing hemoglobinuria. AoV = aortic valve. LA = left atrium. LV = left ventricle. MV = mitral valve.
One should realize that poor native heart function can also lead to flattening of placement and motor current signals due to the small pressure differences generated in the systole. A typical scenario is that of the patient who develops ventricular tachycardia, leading to abrupt flattening of the placement signal and motor current based on the sudden reduction in left ventricular output. In this situation, echocardiography can still reliably exclude dislocation.
5.2. Echocardiography
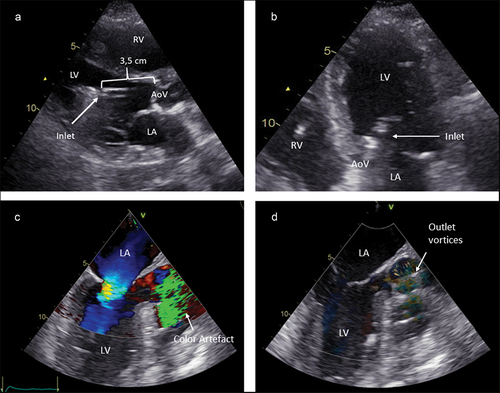
illustrates pVAD position on echocardiography. Ideally, the inlet of the CP pVAD is located ± 3.5 cm from the aortic valve. For the 5.5 pVAD this distance should be 5 cm. The pVAD should move freely in the LV without contact with any ventricular structure. The inlet is typically seen as a discontinuation in the straight line of the catheter and followed by an echogenic marker, which is referred to as the ‘teardrop’ (and should locate 4 cm from the aortic valve in the case of a CP device). A common error is to mistake the pigtail for the catheter/inlet, which can lead to erroneous pullback of the pVAD. One should also pay particular attention to the relation between the device inlet and the mitral valve. Abnormal insertion angles can lead to ‘suction’ or hemolysis based on contact with mitral valve structures [Citation31–33]. Sometimes microbubbles are a subtle sign [Citation46,Citation47]. The best windows to visualize insertion depth are the parasternal long axis on transthoracic echocardiography and the 145° aortic valve long axis view on transesophageal echocardiography (as shown in ). The outlet creates a color turbulence artifact which can be helpful in case it is impossible to have a clear view on the inlet and blood speckle tracking can also show this phenomenon. The outlet should be located widely above the aortic valve. We advise using multiple planes during echocardiographic evaluation, combining long axis views with 5-chamber views, realizing that the catheter is curved and assessment of position in one plane can be misleading.
Figure 6. pVAD position examples. A. Transthoracic parasternal long axis view showing a normal position with 3.5 cm insertion depth and free inlet. B. Transthoracic apical 5-chamber view showing the inlet in the left ventricle. C. Transoesophageal aortic valve long axis view, showing the outlet Doppler artifact in the aortic root. D. Blood speckle tracking echocardiography showing the turbulence in the aortic root at the level of the pVAD outlet. AoV = aortic valve. LA = left atrium. LV = Left ventricle. RV = right ventricle.
5.3. Fluoroscopy
Fluoroscopy has long been the gold standard and is the most reproducible method for estimating insertion depth. Looping a wire or catheter in the aortic valve can help to delineate this reference.
5.4. Chest X-ray
Ouweneel et al. developed a calculation (± 0.25 × chest width) to locate the aortic valve on chest X-ray based on data from patients with a prosthetic valve [Citation48]. This allows one to estimate pVAD insertion depth on chest X-ray. However, this is a single plane method that has not been externally validated. It is not the preferred tool for clinical decision-making.
6. Prevention of hemolysis
Maintaining the device in the correct position is one of the key issues in the prevention of ‘suction alarms’ and hemolysis. It is crucial to secure the catheter immediately after implantation by closing the Tuohy-Borst valve and to register insertion depth in the patient file. Slight rotation of the catheter within the valve is still possible. Leg movement is a typical source of frustrating catheter dislocations in awake patients with femoral pVAD’s. Every institution should develop a protocol for leg fixation. We tend to fasten the ankle in disorientated patients and recently started to use a knee brace to prevent knee jerking motions. Axillary implanted pVAD’s tend to be more stable and allow ambulation with the device [Citation48–50] so axillary implantation is probably preferred when long support duration is anticipated.
Insufficient preload should be detected early to prevent ‘suction alarms’ and hemolysis by adequate hemodynamic monitoring. Accruing data suggest that use of pulmonary artery catheters is associated with improved outcomes in severe cardiogenic shock (which are often pVAD supported) patients [Citation51,Citation52]. Hemodynamic monitoring allows following of trends in cardiac filling pressures and relates these to changes in cardiac output as well as mixed venous oxygen saturation, which might lead to earlier intervention. The ability to trend filling pressures during changes in pVAD flow as well as fluid challenges is probably the most important use of the pulmonary artery catheter. The ‘normal’ for filling pressures is variable, and depends on pVAD flow as well as patient physiology. For a more detailed discussion on hemodynamic optimization during pVAD support, we refer to recent review articles [Citation6,Citation53].
In order to prevent device thrombosis, current manufacturer’s guidelines suggest anticoagulation with unfractionated heparin (UFH). These recommendations are still based on activated clotting time, while activated partial thromboplastin time is often used in practice in the cardiac critical care unit [Citation54]. It is still unclear whether, and to what extent, the addition of systemic UFH is needed since the purge fluid already contains moderate doses of UFH. Some data suggest high-level anticoagulation might tip the balance to bleeding, without better protection against thrombosis and ischemia [Citation55].
A special situation is that of heparin-induced thrombocytopenia. In this case, the use of an agratroban as well as a bivalirudin containing purge solution is possible [Citation56–58]. Future studies are needed to compare therapeutic to intermediate-dose anticoagulation strategies and at what level the optimal balance between bleeding and prevention of thrombo-embolic events is achieved. The newest alternative is the use of bicarbonate as the purge fluid which offers the advantage of having no systemic anticoagulant effects. Preliminary data suggest excellent safety and this could become the future standard if larger studies confirm these data [Citation59].
7. Treatment of hemolysis
In case of hemolysis, the first action should be to find and correct the underlying cause (). Repositioning the device in case of dislocation can be notoriously difficult when the angle of a pVAD is orienting the inlet to the mitral valve. Also, kinking in the aorta can be the result of these bedside repositioning maneuvers. Whenever possible, correction should be undertaken under real-time imaging. Difficult situations should be addressed under fluoroscopy in combination with transoesophageal echocardiography and possible solutions are as follows: snaring of the pigtail to pull the pVAD into the LV or complete re-insertion (of a new) pVAD [Citation60,Citation61]. In case of suboptimal LV filling, a culprit-oriented approach to hemodynamic optimization can be advised () [Citation6]. In case of purge fluid thrombosis (which in itself should not automatically lead to hemolysis), local thrombolysis can be pump saving [Citation62,Citation63]. As discussed above, pump thrombosis is rare and most often related to intracardiac thrombi [Citation37,Citation38]. Sometimes ‘suction alarms’ develop before complete thrombosis occurs, but sudden pump stop can be the first presentation. Since pVAD’s are easily exchanged compared to durable LVAD’s and switching to another form of MCS is also an option, it is less appealing to try a strategy of aggressive anticoagulation to salvage the device (as is often tried in the context of a durable LVAD). Also, in some cases, cardiac function might have recovered sufficiently to allow pVAD removal. A newly described strategy is that of exchanging a CP device for a 5.5 in case of hemolysis, after excluding all reversible causes [Citation64]. Since these data are based on the experience of a single center, we are still prudent, especially since hemolysis is based on a reversible problem (as discussed earlier) in the majority of cases, but it can be considered when hemolysis persists after optimizing every aspect of pVAD function. After addressing the underlying cause, we propose to remeasure the pfHb within 2–4 h, to prevent delay (leading to prolonged patient exposure to toxic hemolysis byproducts) in the escalation of MCS strategy.
After addressing the underlying cause, we can also try to prevent the consequences of hemolysis as much as possible. Classically, hydration and urinary alkalinization (urinary pH > 6.5–7) are advised to protect the kidney in case of severe mechanical hemolysis [Citation65]. It is also possible to limit exposure to toxic pfHb and other side products of hemolysis by accelerating their clearance. This might also be a temporizing measure when the underlying cause cannot be addressed immediately and offers the possibility to slow down the self-perpetuating process of hemolysis and thrombosis. However, it remains to be proven that removing excess pfHb improves clinically significant endpoints in a pVAD supported population. In patients already receiving renal replacement therapy in the context of their cardiogenic shock, the use of an hemoadsorbant system such as Cytosorb (CytoSorbents Corporation, Monmouth Junction, NJ, USA) does offer the potential to remove excess pfHb. In this system, highly porous polyvinylpyrrolidonecoated polystyrene-divinylbenzene beads are used to bind a broad spectrum of molecules with a molecular weight <55 kDa [Citation66]. The system was originally developed for cytokine removal; but in case of cardiopulmonary bypass-induced hemolysis, Cytosorb has been shown to significantly decrease the level of pfHb [Citation67]. However, an effect on end-organ function or clinical outcome has to be addressed in future studies before recommendations are possible. Routine use of a hemoadsorbant system during LVAD implantation did not convey any benefit and might be associated with an increased incidence of respiratory failure [Citation67]. To date, there are no studies on hemoadsorbant systems in the context of pVAD support. One possible caveat is the fact that such filters also bind medications such as ticagrelor which could be of relevance in a cardiogenic shock population [Citation68]. Plasmapheresis is another very effective option to remove excess pfHb. The use of plasmapheresis was reported in isolated cases of LVAD thrombosis, but no large studies have been executed in this population yet [Citation69].
Pentoxifylline improves RBC deformability and is associated with a reduction in viscosity [Citation70]. Limited experience in the context of LVAD thrombosis and hemolysis suggests a possible role for pentoxifylline [Citation71]. A double-blinded randomized controlled trial in patients who undergo an axillary 5.0 or 5.5 pVAD insertion for acute decompensated heart failure is planned to study this treatment strategy (NCT04391231).
8. Conclusion
Over recent years, there has been a rapid increase in the use of short-term mechanical circulatory support in patients with cardiogenic shock. Hemolysis is one of the most frequently encountered problems during mechanical circulatory support. Prevention of hemolysis, and when present, its timely detection and the subsequent identification and correction of the underlying cause are vital to ensure good clinical outcomes in these critically ill patients.
9. Expert opinion
Randomized controlled trials with mechanical circulatory support devices are needed and underway. However, important aspects of device and patient management remain uncertain, and this leads to great variability in clinical practice. Differences in the incidence and treatment of complications, such as hemolysis, between different institutions pose a threat to the quality of future studies. It seems important to optimize good practice guidelines and protocols before we study these devices to enable them to reach their full potential not only in clinical practice but also in clinical trials. A structured approach to hemolysis should be part of such a practice guideline.
Future studies on percutaneous ventricular assist devices should try to clarify some of the elements that are still unclear. The optimal anticoagulation strategy during short-term mechanical circulatory support and the optimal purge fluid constitution have not been studied in a randomized fashion yet, but these are crucial elements that deserve further attention. Especially since we are evolving to longer durations of circulatory support and more patient mobility during support, further development of the SmartAssist technology in the pVAD platform should be pursued. This tool is very promising in the prevention of suction events and hemolysis. Strategies to minimize the consequences of hemolysis are of interest and studies on pentoxifylline, as well as extracorporeal removal of free hemoglobin are expected to contribute to the field as well.
We expect many steps forward in the domain of mechanical circulatory support in the next 10 years. These devices are rapidly improving, and new devices are on the horizon. For the pVAD’s, we expect to see an evolution toward smaller pumps delivering higher blood flows. The reduction in pVAD size will make it possible to use smaller sheaths, which will lead to a reduction in vascular complications and bleeding events. These pumps will be implanted in the acute phase but could remain in place for longer durations to bridge patients straight to recovery or transplantation without the need for another surgical device. Patients might even be discharged with a percutaneous device from the hospital in the future. Also, new anticoagulants that act on the contact pathway will allow for more protection with less bleeding complications, minimizing the incidence of thrombosis as the cause of hemolysis and other clinical adverse events. In these devices, new algorithms will allow for even more precise monitoring of device position and based on artificial intelligence, they can be able to predict suction events before they happen.
Article highlights
Hemolysis is an often-preventable complication during short-term mechanical circulatory support with a percutaneous ventricular assist device.
Hemolysis can result in a self-perpetuating process of progressive thrombosis and organ failure ().
Plasma-free hemoglobin is the preferred test to diagnose and monitor hemolysis in these patients (absolute value >40 mg/dL or increase by >30 mg/dL) while increases in lactate dehydrogenase and bilirubin are less specific and often confounded by organ failure
The most important causes are dislocation of the device and insufficient device preload. Both will often result in suction alarms on the device console.
A structured approach to hemolysis is shown in and identifying the specific cause is key.
Sometimes the ventricular waveform on the console can show suboptimal device function even before overt suction alarms are activated, and this can help to prevent or treat hemolysis.
Insufficient preload is caused by hypovolemia but also right ventricular failure or tamponade. In cases of combined extracorporeal membrane oxygenation (ECMO) and percutaneous ventricular assist device; high ECMO flow can be the cause.
Besides correcting the underlying problem, several strategies exist to limit further organ damage as a result of hemolysis, but high-quality data from studies are lacking.
Declaration of interest
TB and TA have received funding from Abiomed for research and presentations, but these have no influence on the content of the current manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewers disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Chioncel O, Parissis J, and Mebazaa A, et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock - a position statement from the heart failure association of the European society of cardiology. Eur J Heart Fail. 2020 Aug;22(8):1315–1341.
- Thiele H, Zeymer U, Neumann FJ, et al. IABP-SHOCK II trial investigators. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012 Oct 4;367(14):1287–1296.
- Dhruva SS, Ross JS, Mortazavi BJ, et al. Association of use of an intravascular microaxial left ventricular assist device vs intra-aortic balloon pump with in-hospital mortality and major bleeding among patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2020 Feb 25;323(8):734–745.
- Schrage B, Ibrahim K, Loehn T, et al. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation. 2019 Mar 5;139(10):1249–1258.
- Basir MB, Kapur NK, Patel K, et al. National cardiogenic shock initiative investigators. Improved outcomes associated with the use of shock protocols: updates from the national cardiogenic shock initiative. Catheter Cardiovasc Interv. 2019 Jun 1;93(7):1173–1183.
- Balthazar T, Vandenbriele C, and Verbrugge FH, et al. Managing patients with short-term mechanical circulatory support. J Am Coll Cardiol. 2021 Mar 9;77(9):1243–1256.
- Ramzy D, Anderson M, Batsides G, et al. Early outcomes of the first 200 US patients treated with Impella 5.5: a novel temporary left ventricular assist device. Innovations (Phila). 2021 Jul-Aug;16(4):365–372.
- Pieri M, Sorrentino T, Oppizzi M, et al. The role of different mechanical circulatory support devices and their timing of implantation on myocardial damage and mid-term recovery in acute myocardial infarction related cardiogenic shock. J Interv Cardiol. 2018 Dec;31(6):717–724.
- Ravichandran AK, Parker J, Novak E, et al. Hemolysis in left ventricular assist device: a retrospective analysis of outcomes. J Heart Lung Transplant. 2014 Jan;33(1):44–50.
- Katz JN, Jensen BC, Chang PP, et al. A multicenter analysis of clinical hemolysis in patients supported with durable, long-term left ventricular assist device therapy. J Heart Lung Transplant. 2015 May;34(5):701–709.
- Ouweneel DM, Eriksen E, Sjauw KD, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017 Jan 24;69(3):278–287.
- Tehrani BN, Truesdell AG, Sherwood MW, et al. Standardized team-based care for cardiogenic shock. J Am Coll Cardiol. 2019 Apr 9;73(13):1659–1669.
- Ouweneel DM, de Brabander J, Karami M, et al. Real-life use of left ventricular circulatory support with Impella in cardiogenic shock after acute myocardial infarction: 12 years AMC experience. Eur Heart J Acute Cardiovasc Care. 2019 Jun;8(4):338–349.
- Monteagudo Vela M, Simon A, Riesgo Gil F, et al. Clinical indications of Impella short-term mechanical circulatory support in a tertiary centre. Cardiovasc Revasc Med. 2020 May;21(5):629–637.
- Chieffo A, Ancona MB, Burzotta F, et al. Observational multicentre registry of patients treated with IMPella mechanical circulatory support device in Italy: the IMP-IT registry. EuroIntervention. 2020 Feb 7;15(15):e1343–e1350.
- Karami M, den Uil CA, Ouweneel DM, et al. Mechanical circulatory support in cardiogenic shock from acute myocardial infarction: impella CP/5.0 versus ECMO. Eur Heart J Acute Cardiovasc Care. 2020 Mar;9(2):164–172.
- Lemor A, Hosseini Dehkordi SH, Basir MB, et al. Impella versus extracorporeal membrane oxygenation for acute myocardial infarction cardiogenic shock. Cardiovasc Revasc Med. 2020 Dec;21(12):1465–1471.
- Loehn T, O’Neill WW, Lange B, et al. Long term survival after early unloading with Impella CP in acute myocardial infarction complicated by cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2020 Mar;9(2):149–157.
- Rock JR, Kos CA, Lemaire A, et al. Single center first year experience and outcomes with Impella 5.5 left ventricular assist device. J Cardiothorac Surg. 2022 May 23;17(1):124.
- Toda K, Ako J, Hirayama A, et al. J.-PVAD registry study investigators. Three-year experience of catheter-based micro-axial left ventricular assist device, Impella, in Japanese patients: the first interim analysis of Japan registry for percutaneous ventricular assist device (J-PVAD). J Artif Organs. 2022 Apr 25. DOI:10.1007/s10047-022-01328-1.
- Cannata A, Cantoni S, Sciortino A, et al. Mechanical hemolysis complicating transcatheter interventions for valvular heart disease: JACC state-of-the-art review. J Am Coll Cardiol. 2021 May 11;77(18):2323–2334.
- Yasuda T, Shimokasa K, Funakubo A, et al. An investigation of blood flow behavior and hemolysis in artificial organs. ASAIO J. 2000 Sep-Oct;46(5):527–531.
- Köhne I. Haemolysis induced by mechanical circulatory support devices: unsolved problems. Perfusion. 2020 Sep;35(6):474–483.
- Roberts N, Chandrasekaran U, Das S, et al. Hemolysis associated with Impella heart pump positioning: in vitro hemolysis testing and computational fluid dynamics modeling. Int J Artif Organs. 2020 Mar;4:391398820909843.
- de Biasi AR, Manning KB, Salemi A. Science for surgeons: understanding pump thrombogenesis in continuous-flow left ventricular assist devices. J Thorac Cardiovasc Surg. 2015 Mar;149(3):667–673.
- Papanastasiou CA, Kyriakoulis KG, Theochari CA, et al. Comprehensive review of hemolysis in ventricular assist devices. World J Cardiol. 2020 Jul 26; 12(7):334–341.
- Chow TW, Hellums JD, Moake JL, et al. Shear stress-induced von Willebrand factor binding to platelet glycoprotein Ib initiates calcium influx associated with aggregation. Blood. 1992 Jul 1; 80(1):113–120.
- Ataga KI. Hypercoagulability and thrombotic complications in hemolytic anemias. Haematologica. 2009 Nov;94(11):1481–1484.
- Qian Q, Nath KA, Wu Y, et al. Hemolysis and acute kidney failure. Am J Kidney Dis. 2010;56(4):780–784.
- Concepcion B, Korbet SM, Schwartz MM. Intravascular hemolysis and acute renal failure after mitral and aortic valve repair. Am J Kidney Dis. 2008 Nov;52(5):1010–1015.
- Nakamura M, Imamura T, Fukui T, et al. Impact of the angle between aortic and mitral annulus on the occurrence of hemolysis during Impella support. J Artif Organs. 2020 Sep;23(3):207–213.
- Lazicki TJ, Pagel PS. Reduced flow rate, acute hemolysis, and restricted anterior mitral leaflet opening during mechanical circulatory support for end-stage nonischemic cardiomyopathy. J Cardiothorac Vasc Anesth. 2020 Feb;34(2):562–565.
- Elhussein TA, Hutchison SJ. Acute mitral regurgitation: unforeseen new complication of the Impella LP 5.0 ventricular assist device and review of literature. Heart Lung Circ. 2014 Mar;23(3):e100–4.
- Lüsebrink E, Massberg S, Orban M. Combined extracorporeal membrane oxygenation and microaxial pump-when left ventricular preload is too low to unload in cardiogenic shock. Health Sci Rep. 2021 Jul 9;4(3):e321.
- Aoun J, Tea I, Bhimaraj A, et al. Refractory Impella suction alarms in the setting of extracorporeal membrane oxygenation. CJC Open. 2021 May 2;3(9):1186–1188.
- Whitehead EH, Thayer KL, Burkhoff D, et al. Central venous pressure and clinical outcomes during left-sided mechanical support for acute myocardial infarction and cardiogenic shock. Front Cardiovasc Med. 2020 Aug 28;7:155.
- Ranc S, Sibellas F, Green L. Acute intraventricular thrombosis of an impella LP 5.0 device in an ST-elevated myocardial infarction complicated by cardiogenic shock. J Invasive Cardiol. 2013 Jan;25(1):E1–3.
- Nguyen D, Ellison D, Ngo C, et al. Intraventricular free-floating thrombus in an Impella-supported patient: damage control in a no-win scenario. JACC Case Rep. 2020 Jun 17;2(6):886–888.
- Nakao Y, Aono J, Namiguchi K, et al. Usefulness of contrast computed tomography for diagnosing left ventricular thrombus before impella insertion. J Cardiol Cases. 2020 Aug 22; 22(6):291–293.
- Esposito ML, Morine KJ, and Annamalai SK, et al. Increased plasma-free hemoglobin levels identify hemolysis in patients with cardiogenic shock and a trans valvular micro-axial flow pump. Artif Organs. 2019 Feb;43(2):125–131.
- Pagani FD, Miller LW, Russell SD, et al. HeartMate II investigators. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009 Jul 21; 54(4):312–321.
- Wang S, Griffith BP, Wu ZJ. Device-induced hemostatic disorders in mechanically assisted circulation. Clin Appl Thromb Hemost. 2021 Jan-Dec;27:1076029620982374.
- Omar HR, Mirsaeidi M, Socias S, et al. Plasma free hemoglobin is an independent predictor of mortality among patients on extracorporeal membrane oxygenation support. PLoS One. 2015 Apr 22;10(4):e0124034.
- Pan KC, McKenzie DP, Pellegrino V, et al. The meaning of a high plasma free haemoglobin: retrospective review of the prevalence of haemolysis and circuit thrombosis in an adult ECMO centre over 5 years. Perfusion. 2016 Apr;31(3):223–231.
- Tchantchaleishvili V, Sagebin F, Ross RE, et al. Evaluation and treatment of pump thrombosis and hemolysis. Ann Cardiothorac Surg. 2014;3(5):490–495.
- Da Q, Teruya M, Guchhait P, et al. Free hemoglobin increases von Willebrand factor-mediated platelet adhesion in vitro: implications for circulatory devices. Blood. 2015 Nov 12;126(20):2338–2341.
- Quevedo HC, Abi Rafeh N. Impella-induced left ventricular microbubbles, a potential sign for hemolysis. J Invasive Cardiol. 2020 Apr;32(4):E101.
- Ouweneel DM, Sjauw KD, Wiegerinck EM, et al. Assessment of cardiac device position on supine chest radiograph in the ICU: introduction and applicability of the aortic valve location ratio. Crit Care Med. 2016 Oct;44(10):e957–63.
- Tarabichi S, Ikegami H, Russo MJ, et al. The role of the axillary Impella 5.0 device on patients with acute cardiogenic shock. J Cardiothorac Surg. 2020 Aug 14;15(1):218.
- Esposito ML, Jablonski J, Kras A, et al. Maximum level of mobility with axillary deployment of the Impella 5.0 is associated with improved survival. Int J Artif Organs. 2018 Apr;41(4):236–239.
- Garan AR, Kanwar M, Thayer KL, et al. Complete hemodynamic profiling with pulmonary artery catheters in cardiogenic shock is associated with lower in-hospital mortality. JACC Heart Fail. 2020 Nov;8(11):903–913.
- Osman M, Syed M, Patel B, et al. Invasive hemodynamic monitoring in cardiogenic shock is associated with lower in-hospital mortality. J Am Heart Assoc. 2021 Sep 21;10(18):e021808.
- Chieffo A, Dudek D, and Hassager C, et al. Joint EAPCI/ACVC expert consensus document on percutaneous ventricular assist devices. Eur Heart J Acute Cardiovasc Care. 2021 Jun 30;10(5):570–583.
- Nakamura M, Imamura T, Ueno H, et al. Impact of the whole activated clotting time during Impella support on short-term prognosis. J Artif Organs. 2022 Mar;25(1):9–15.
- Vandenbriele C, Dannenberg L, Monteagudo-Vela M, et al. Optimal antithrombotic regimen in patients with cardiogenic shock on ImpellaTM mechanical support: less might be more. Eur Heart J. 2020 Nov;41(Supplement_2):ehaa946.1843.
- Fabrizio C, Levito MN, Rivosecchi R, et al. Outcomes of systemic anticoagulation with bivalirudin for Impella 5.0. Int J Artif Organs. 2021 Oct;44(10):681–686.
- Blum EC, Martz CR, Selektor Y, et al. Anticoagulation of percutaneous ventricular assist device using argatroban-based purge solution: a case series. J Pharm Pract. 2018 Oct;31(5):514–518.
- Hohlfelder B, Militello MA, Tong MZ, et al. Anticoagulation with temporary Impella device in patients with heparin-induced thrombocytopenia: a case series. Int J Artif Organs. 2021 May;44(5):367–370.
- Al-Ayoubi AM, Bhavsar K, Hobbs RA, et al. Use of sodium bicarbonate purge solution in impella devices for heparin-induced thrombocytopenia. J Pharm Pract. 2022 April;089719002210890. DOI:10.1177/08971900221089078.
- Elsherif A, Nadir A, Ludman PF, et al. Retrieval of entrapped catheter-mounted axial flow pump from mitral subvalvular apparatus using a snare catheter. JACC: Case Reports. 2021 Oct 6;3(13):1494–1498.
- Alaiti MA, Elby MA, Lang K, et al. Percutaneous repositioning of impella mechanical circulatory support device: snare-direct-push technique. Cardiovasc Revasc Med. 2020 Nov;21(11S):103–104.
- Sorensen EN, Carla Williams P, Tabatabai A. Use of tissue plasminogen activator to resolve high purge system pressure in a catheter-based ventricular-assist device. J Heart Lung Transplant. 2014 Apr;33(4):457–458.
- Succar L, Donahue KR, Varnado S, et al. Use of tissue plasminogen activator alteplase for suspected impella thrombosis. Pharmacotherapy. 2020 Feb;40(2):169–173.
- Salas de Armas I, Bergeron A, Bhardwaj A, et al. Surgically implanted Impella device for patients on Impella CP support experiencing refractory hemolysis. ASAIO J. 2022 Mar 18. DOI:10.1097/MAT.0000000000001712.
- Zager RA, Gamelin LM. Pathogenetic mechanisms in experimental hemoglobinuric acute renal failure. Am J Physiol. 1989 Mar;256(3 Pt 2):F446–55.
- Gleason TG, Argenziano M, Bavaria JE, et al. Hemoadsorption to reduce plasma-free hemoglobin during cardiac surgery: results of refresh I pilot study. Semin Thorac Cardiovasc Surg. 2019;31(4):783–793. Winter.
- Zhigalov K, Van den Eynde J, Zubarevich A, et al. Initial experience with CytoSorb therapy in patients receiving left ventricular assist devices. Artif Organs. 2022 Jan;46(1):95–105.
- Jackson R, Trus RM, El-Diasty M. Hemadsorption for removal of ticagrelor and direct oral anticoagulants in cardiac surgery. Expert Rev Cardiovasc Ther. 2022 Feb;20(2):141–150.
- Hayes C, Shafi H, Mason H, et al. Successful reduction of plasma free-hemoglobin using therapeutic plasma exchange: a case report. Transfus Apher Sci. 2016 Apr;54(2):253–255.
- Alkhouli M, Farooq A, Go RS, et al. Cardiac prostheses-related hemolytic anemia. Clin Cardiol. 2019 Jul;42(7):692–700.
- Jennings DL, Williams CT, Morgan JA. Pentoxifylline for the treatment of hemolytic anemia in a patient who developed recurrent gastrointestinal bleeding while on continuous-flow left ventricular assist device support. ASAIO J. 2013 Sep-Oct;59(5):526–527.