ABSTRACT
Background
Powered intraosseous (IO) systems are valuable devices for emergent situations, with limited data on user preferences. A simulation/survey-based study was conducted among emergency medical service (EMS) providers to evaluate attitudes toward general powered IO system features to measure preferences/satisfaction for the most-commonly used and a novel powered IO system (with a passive safety needle, battery life indicator, and snap-securement/dressing).
Research Design and Methods
Forty-two EMS providers completed a simulated activity using both powered IO systems and a 30-item questionnaire, including multiple choice, free-text, ranking, and Likert-like questions. Ranking scores were reported using a scale of 0 (least important/satisfactory) to 100 (most important/satisfactory). Statistical significances were evaluated via Wilcoxon signed-rank sum test.
Results
Providers indicated driver performance (mean score ± SD; 77.8 ± 27.5) and IO needle safety mechanism (63.1 ± 27.9) as the most important features. Participants reported significantly higher (p < 0.001) satisfaction with the novel IO system overall, and its needle safety, battery life indicator, securement/dressing, and ease-of-use. Powered driver performance satisfaction was similar and favorable for the novel (88.1 ± 18.2) and traditional (87.1 ± 15.3) systems.
Conclusions
These findings highlight the value of clinician/user input and demonstrate EMS providers are more satisfied with a powered IO system featuring design elements intended to enhance safety and ease-of-use.
1. Introduction
Intraosseous (IO) access is an important method for achieving rapid vascular access during emergent situations and is recommended by the American Heart Association and European Resuscitation Council when intravenous (IV) access is unobtainable [Citation1,Citation2]. IO access is achieved using a manual, semiautomatic (i.e. spring-loaded), or battery-powered IO system, and studies comparing these systems suggest powered IO devices are superior with respect to ease-of-use, first-attempt success rates, and user preference [Citation3]. Despite the suggested superiority of powered IO systems, these instruments have been subject to minimal innovation since their introduction to address common challenges experienced during emergent situations.
Emergency medical service (EMS) providers are exposed to many occupational hazards due to the unpredictable and uncontrolled nature of emergent cases [Citation4], which can be further exacerbated by equipment failure. Common issues with emergency medical (EM) equipment are associated with safety, ease-of-use, and reliability [Citation5] and often consist of needlestick injuries (NSIs) [Citation6], disregard for the impact of ergonomics [Citation7], and poor battery reliability [Citation5]. Innovations addressing safety, ease-of-use, and reliability issues in EM equipment could potentially reduce occupational hazards for EMS providers and improve clinical practice.
A novel powered IO system was recently introduced and differs from the most-commonly used (traditional) powered IO system by featuring design elements intended to enhance safety, ease-of-use, and reliability. The differences between the novel and traditional IO systems are presented in . Although previous reports have suggested technical complications with IO systems are infrequent [Citation8,Citation9], the risk for NSIs or inadequate battery power leading to a delayed/failed procedure are inherent issues associated with powered IO systems, especially in the absence of product features designed to address these risks. Additionally, data regarding the utility of innovations applied to powered IO system product features are scarce. Therefore, a better understanding of the benefits gained from powered IO system innovations intended to improve safety, ease-of-use, and reliability could be useful for EMS providers when selecting a powered IO system for their clinical practice.
Table 1. Traditional and novel IO system features.
Considering the vital role of clinician input in advancing the innovation of medical devices [Citation10], a simulation-based study was recently conducted to evaluate emergency physicians’ preferences related to the novel IO system [Citation11]. The findings from this preliminary study suggested that emergency physicians preferred the novel IO system and particularly valued its features related to safety and ease-of-use. Furthermore, these findings highlighted the need for more extensive research with a larger sample size, including a diverse set of clinicians who may utilize powered IO systems in a variety of emergency settings.
Researchers developed the present study to represent a more comprehensive simulation-based assessment of provider preferences of the novel IO system and the traditional/most-commonly used powered IO system. This study thus enrolled a larger, more diverse population of EMS providers, whose predominantly prehospital setting fosters an environment associated with many potential occupational hazards, potentially contributing to their professional opinion of IO technology [Citation4,Citation12]. The goal of this investigation was to identify which powered IO features EMS providers find most important, and to evaluate if the novel IO system’s innovative design elements, intended to assist with challenges experienced in EM settings, impacted their preferences and satisfaction levels toward the traditional and novel IO devices.
2. Participants & methods
2.1. Study design
EMS providers were enrolled to participate in a simulation and cross-sectional survey-based study (October of 2021 at the EMS World Expo in Atlanta, GA, USA) using the most commonly utilized powered IO system (traditional IO system; EZ-IO Intraosseous Vascular Access System; Teleflex Medical, NC, USA) and a novel powered IO system with added features (novel IO system; BD™ Intraosseous Vascular Access System; Becton, Dickinson and Company, NJ, USA) (). Participants were educated/trained on each device prior to the IO-simulation. Immediately after the simulated use activity, participants were asked to complete a questionnaire designed to assess the importance of IO features in general, and satisfaction levels related to the novel and traditional IO system features.
Figure 1. The traditional and novel powered intraosseous (IO) systems. (A) The traditional powered IO system features include (1) powered driver, (2) single-light battery life indicator (not shown), (3) irreplaceable/non-rechargeable lithium battery, (4) IO needles of various lengths (15, 25, and 45 mm), (5) telescoping securement/skin attachment; and (6) extension set. The manual sharps securement block included with the traditional IO system is not pictured. (B) The novel powered IO system features include (1) powered driver, (2) multilight battery life indicator, (3) rechargeable battery with power supply, (4a) passive safety mechanism for IO needle stylet, (4b) catheter that is left in place after IO insertion, (4c) IO needles of various lengths (15, 25, 35, 45, and 55 mm), (5) snap-securement/skin attachment, and (6) extension set. Abbreviations: IO, intraosseous.
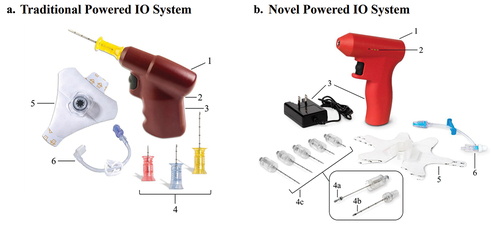
There are several differences between the novel and traditional IO systems (). The traditional IO powered driver uses a non-rechargeable and irreplaceable lithium battery with a battery life indicator featuring a single LED light. Upon placing an IO needle with the traditional IO system, the needle hub is disconnected from the driver, the needle stylet is manually unscrewed and removed, and the needle hub is left at the site of insertion. The traditional IO needle stylet can then be placed in the included NeedleVISE® (Atrion Medical Products Inc., AL, USA) manual sharps securement block for needle safety. There are three IO needle length options (15 mm, 25 mm, 45 mm) for the traditional system. The traditional IO system also uses a telescoping securement/dressing (accommodates various amounts of needle protruding from the skin) that must be placed prior to the extension set attachment. In contrast, the novel IO-powered driver uses a rechargeable battery and a multi-LED light battery life indicator that illuminates according to remaining battery power. After placement of the novel IO needle using the powered driver, the stylet is manually removed from the needle cannula. As the needle stylet is removed, an integrated passive safety mechanism automatically engages on the stylet tip, precluding access to the contaminated sharp. The novel IO system has five IO needle length options (15 mm, 25 mm, 35 mm, 45 mm, 55 mm). Moreover, the novel system uses a dynamic snap-securement/dressing system that can open and close around the catheter hub, allowing for its placement before or after the extension set is attached.
2.2. Ethical considerations
This survey/simulation-based study was completed by healthcare providers to indicate their preferences regarding IO devices based on a simulated activity using training blocks. No patient data were collected, and in accordance with the Declaration of Helsinki ethical principles for medical research and the US Office of Human Research Protections guidance [Citation13,Citation14], informed consent was obtained from participants as evidence of their agreement to voluntarily participate in this study. Participants also consented to the release of information obtained due to their participation, and were informed that the study sponsor would not reveal identifying information in any publication resulting from the study. Therefore, an ethical committee was not consulted. All data were deidentified before performing any analyses. Participants received a copy of the Journal of Infusion Nursing: Infusion Therapy Standards of Practice 8th Edition Vol 44 Number 15, but were not financially compensated for their participation in this research.
2.3. Study population
US-based EMS providers (i.e. emergency medical technicians [EMT], paramedics, military medics, air medical services) with an EM certification or license were recruited using a convenience sampling method to participate in this study. Participants were approached by de-identified researchers during the 2021 EMS World Expo in Atlanta, GA, USA, which allowed for the enrollment of a diverse participant population representative of EMS providers who may utilize IO access in their clinical practice. Interested providers were screened and excluded from the investigation if: 1.) they were employees or consultants for any medical device company, 2.) they were not familiar with the IO procedure and IO systems (self-reported), or 3.) they exhibited active symptoms of the novel coronavirus disease 2019 (COVID-19). Based on a power analysis from a previous and related study [Citation11], the study investigators attempted to enroll at least 22 participants. Participants were informed that they had the right to cease study participation at any time, and they were made aware that their identity would not be revealed in study-related publications. Finally, each participant was required to consent to the release of information regarding or obtained as a result of their participation in the study.
2.4. Measurements
Participants meeting the study criteria completed the study in three sections: 1.) participant screening and education/training, 2.) IO-simulation, and 3.) questionnaire completion. The completion of all three sections took approximately 40 minutes and began with a 15-minute training for the traditional and novel IO systems (order randomized) using promotional materials, presentations, and videos of comparable lengths, created by the manufacturers of each device. Participants then proceeded to the IO-simulation area containing five IO-simulation stations. Each station was equipped with one IO access injection training block (adult tibia reference with 20 mm skinned soft tissue; Sawbones USA, Pacific Research Laboratories, Inc., WA, USA), one traditional IO system, one novel IO system, and the respective 25 mm IO needle kits and securement/dressings for each system. Participants were randomized to start the simulation with either the traditional or novel IO system and instructed to perform a minimum of two IO insertions using each system.
Following the IO-simulation, participants completed a 30-item questionnaire (see supplemental materials for full questionnaire) on a tablet device using the Qualtrics® Platform (Qualtrics XM, Seattle, WA, USA) in an area away from the simulation activity and the study investigators. This survey was developed and based on a previous survey that was designed and implemented by the researchers [Citation11]. The survey for the current study was improved to be more comprehensive and allow more robust statistical analyses, and the survey was pre-tested on two emergency medicine physicians prior to administration to the study participants. The questionnaire contained four sections to collect information on: 1.) demographic and clinical background information, 2.) IO system experience and practice preferences, 3.) IO system satisfaction levels, and 4.) general follow-up information. A variety of question types were utilized, including multiple-choice, drop-down selection, 11-point Likert-like satisfaction ranking (0 = extremely dissatisfied, 10 = extremely satisfied), and free-text answer questions. A 7-item drag-and-drop rank-ordering question was also featured with randomized answer selections to query participants on the most important product features when choosing a powered IO system in general.
2.5. Analysis
To minimize human error in data entry, questionnaire responses were collected using the Qualtrics® Platform and exported directly to Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). Analyses were performed using the Statistical Analysis System 9.4 (SAS Institute Inc., Cary, NC, USA). Missing data were avoided by requiring all participants to answer each question before proceeding to the next question. Data collected from the 7-item drag-and-drop rank-ordering question and the 11-point Likert-like satisfaction ranking questions were linearly converted to a 0–100 scale to provide results with uniform scale values. The 0–100 scale represents less importance or satisfaction with lower scores, and more importance or satisfaction with higher scores. Acquired descriptive statistics included mean, standard deviation (SD), standard error (SE), median, and range. Statistical analyses were performed on data from the satisfaction ranking questions and p-values were calculated using the Wilcoxon signed-rank sum test. To analyze free-text answers, responses were categorized by similarity, and the frequency of participants providing a given answer category was calculated.
3. Results
3.1. Characteristics of study participants
Forty-three unique participants were enrolled in the study, and 42 participants completed both the simulation and the study survey with a 100% response rate. All providers screened as eligible agreed to join and consented to the study. One participant, who did not begin the survey portion of the study, ended the study prematurely due to a schedule conflict; no survey responses from this individual were collected or included in the analysis. Participant demographic and clinical background information for the participant population is presented in . Questionnaire results indicated that most participants were paramedics (61.9%, n = 26) or basic EMTs (14.3%, n = 6). The remaining participant population was composed of advanced EMTs (7.1%, n = 3), military medics (7.1%, n = 3), and several EMS roles represented by one participant (2.4% each; physician assistant-certified, phlebotomist, air medical services provider, and critical care paramedic). The average age (±SD, range) among the participants was 37.8 (±11.5, 20–63) years, and the participants’ average years of EM experience was 14.1 (±10.1, 0–36) years. Participants reported a mean of 9.5 (±8.0, 0–28) years of experience operating IO systems, and more than half of the participants reported using an IO system for patient care 2–10 times in the past year (54.8%, n = 23). Many EMS providers had been previously trained on the traditional IO system (88.1%, n = 37), while only 4.8% (n = 2) of participants had been previously trained on the novel IO system. Similarly, most individuals reported using the traditional IO system to gain IO access prior to the simulation study (85.7%, n = 36), whereas fewer participants had previously used the novel IO system to gain IO access (9.5%, n = 4). These results indicate the study participants represented a diverse group of EMS providers who primarily had previous experience/training using the traditional IO system.
Table 2. Demographic and clinical background information.
3.2. Product features considered important when choosing a powered IO system
Participating EMS providers were asked to rank the importance they place in various features when choosing a powered IO system for their clinical practice. shows the ranking results for seven powered IO system features from most important (maximum score: 100) to least important (lowest score: 0). The product features with the highest rank were powered driver performance (ranked most important by 47.6%, n = 20; mean score: 77.8) and IO needle safety mechanism (ranked most important by 16.7%, n = 7; mean score: 63.1). Ergonomics and securement/dressing were given the lowest ranking scores (mean scores: ergonomics, 35.3; securement/dressing, 36.5), with 26.2% (n = 11) and 19.1% (n = 8) of participants ranking these features as least important, respectively. The remaining product features were ranked with mean scores intermediate to the most and least important product features (means score: battery life indicator, 40.1; battery type, 46.0; variety of IO needle length options, 51.2).
Table 3. IO product features ranked by importance when choosing an IO system for clinical practice.
3.3. Satisfaction levels for traditional and novel IO system features
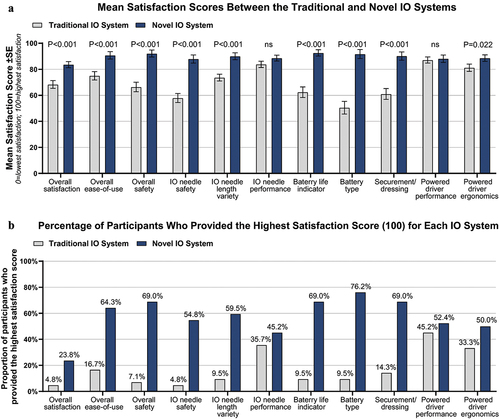
Study participants used both systems in an IO-simulation activity and provided satisfaction levels (maximum score: 100; lowest score, 0) for each powered IO system overall and individual IO system features; these results are presented in . The mean score for overall IO system satisfaction was significantly higher (p < 0.001) for the novel IO system (mean score: 83.6) compared to the traditional IO system (mean score: 68.3), with 23.8% (n = 10) and 4.8% (n = 2) of participants assigning the highest score to the novel and traditional IO systems, respectively. Satisfaction was also significantly higher for overall ease-of-use (p < 0.001) and overall system safety (p < 0.001) for the novel IO system compared to the traditional IO system. EMS providers reported significantly higher scores for multiple individual novel IO system features compared to the traditional IO system, including IO needle safety (p < 0.001), variety of IO needle length options (p < 0.001), battery life indicator (p < 0.001), battery type (p < 0.001), securement/dressing (p < 0.001), and powered driver ergonomics (p = 0.022). Similar results between the novel and traditional IO systems were reported for powered driver performance (mean score: novel IO system, 88.1; traditional IO system, 87.1) and IO needle performance (mean score: novel IO system, 88.6; traditional IO system, 83.6).
Figure 2. Satisfaction Scores for IO System Attributes Between the Traditional and Novel IO Systems. A. Mean (±SE) satisfaction scores reported by participants where 0 = lowest satisfaction and 100 = highest satisfaction between the traditional IO system (EZ-IO Intraosseous Vascular Access System; Teleflex Medical) and novel IO system (BD™ Intraosseous Vascular Access System; Becton, Dickinson and Company). P-values were calculated using the Wilcoxon signed-rank sum test. B. Percentage of participants who provided the highest satisfaction score (100 = highest satisfaction) for each IO system. N = 42 study participants. Abbreviations: IO, intraosseous; ns, not significant; SE, standard error.
4. Discussion
This report provides valuable insight into the importance EMS providers place in product features when choosing a powered IO system, and a comparative analysis of satisfaction levels between a commonly used/traditional IO system and a novel IO system featuring a passive safety needle, snap-securement/dressing, rechargeable battery, and a multi-light battery life indicator. A major strength of this investigation is represented by the diversity of the participating EMS providers who served various EM roles with a wide range of relevant experience. Study participants also reported varied frequencies of practical IO system use for patient care ranging from zero to 50 procedures in the past year. Therefore, the findings from this study are representative of the attitudes toward powered IO systems among a diverse clinician population with varied experiences related to occupational hazards.
This study found that the most important considerations for EMS providers when choosing a powered IO system for their clinical practice were powered driver performance and IO needle safety. The powered driver executes the primary function of a powered IO device in acquiring vascular access; therefore, it is expected that the participants placed a significant amount of value in its performance. The importance afforded to device safety by EMS providers in this study is consistent with the value conferred to IO needle safety by emergency physicians in our previous simulation-based investigation [Citation11]. These findings suggest that safety is a shared priority among EM clinicians and are not surprising given how frequently NSIs occur in EM settings [Citation6]. When participants were asked about their satisfaction levels regarding the safety and driver performance of the traditional and novel IO systems, we found a significantly higher satisfaction level for the novel IO system’s needle safety, while favorable but statistically similar powered driver performance results were reported for both IO systems. Despite most EMS providers having previous experience with the traditional IO system, both IO systems in this study had a satisfactory powered driver performance, and the participants found significantly more value in the safety of the novel IO system. The priority of IO needle safety has not been demonstrated in previous comparative studies of IO systems [Citation15,Citation16], suggesting our collective findings have identified an essential IO feature that has been overlooked in the past, possibly due to the lack of innovative safety features in powered IO systems.
In addition to overall safety, EMS providers conferred higher satisfaction for the novel IO system’s overall ease-of-use, an IO system component that clinicians have valued in previous research [Citation11,Citation15,Citation16]. Favorable attitude toward the ease-of-use for the novel IO system was further supported by the higher satisfaction levels provided for its battery type, battery life indicator, securement/dressing, variety of IO needle length options, and powered driver ergonomics, all of which can be considered as related to the user-friendliness of the device. Although the participants did not rank all these features as chief considerations for IO system selection, our findings illustrate that EMS providers afforded value to features that may improve ease-of-use by enhancing reliability and efficiency of the IO procedure.
Collectively, the results from our studies evaluating EM provider attitudes and satisfaction/preferences toward the traditional and novel IO systems demonstrate that clinicians favor the novel IO system’s innovations that enhance ease-of-use, reliability, and predominantly safety. While some of these features (i.e. ease-of-use) have been subject to previous comparative studies among various IO systems [Citation15,Citation16], data regarding safety, which was one of the most critical considerations indicated by participants, are surprisingly scarce. Given the prevalence of occupational hazards, especially NSIs [Citation6], among EM providers, this deficit in research and innovation regarding IO needle safety highlights the need to conduct further investigations and consider clinician safety as a factor in device design, especially in EM settings. Similarly, the implications of additional device features (e.g. dynamic snap-securement, rechargeable battery, and battery life indicator) on clinical practice and patient outcomes require further research.
This investigation had several limitations, including the use of nonobjective and opinion-based data, which limits the generalizability of the results and conclusions. The study recruited participants from a diverse clinical background; however, the majority of participants worked in the prehospital setting, indicating the population was biased toward prehospital EMS providers. The authors also note that patrons of the EMS World Expo may be relatively more interested in newer technologies; therefore, those sampled may have been more inclined to prefer or be satisfied with a novel device. Although data from the questionnaire were anonymized for analysis, the participants were asked to provide optional identifying information during their study participation, which may have created a potential source of bias. The use of an IO access training block may not have adequately replicated an actual IO insertion, and the simulation environment did not provide an imitation of an actual EM setting. Additionally, only the adult tibia variation of access training block was used, which may have limited the provider preferences assessed to this use case. While training materials were selected for equal representation, the study being sponsored and developed by employees of one of the manufacturers poses the potential for bias toward that device. Future studies with the novel IO system should consider measuring IO system performance and impact on procedures, as well as utilization of an EM setting for the evaluation of the novel IO system in a real-world application.
5. Conclusion
This report demonstrated that EMS providers identified safety and powered driver performance as the most important considerations in powered IO device selection and described high satisfaction levels for a novel IO system’s passive safety needle and the performance of its powered driver. While not ranked as critical features for IO system selection, many other attributes of the novel IO system were described as more satisfactory than a more commonly used/traditional IO system. Overall, our findings demonstrate the importance EMS providers place in various powered IO features and provide evidence that individuals working in an EM setting prefer a powered IO system with passive needle safety, an easy-to-use snap-securement/dressing, and battery features that may enhance reliability. These results highlight the need for further research regarding innovative powered IO system features (i.e. safety, reliability, ergonomics) and their impact on clinical practice.
Declaration of interest
KA reports current employment and stock ownership with Becton, Dickinson and Company, which manufactured the novel IO system (BD™ Intraosseous Vascular Access System) and sponsored this study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewers disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Geolocation
Participants completed the simulations and surveys resulting in the data for this study in Atlanta, GA, USA (EMS World Expo 2021).
Authors’ contributions
This study was conceptualized, designed, and interpreted by AL, DJ, and KA. AL, DJ, and KA provided substantial editorial input and review of the manuscript. All authors read and approved the final manuscript and agree to be accountable for all aspects of the work.
Ethics approval and consent to participate
This survey/simulation-based study was completed by healthcare providers to indicate their preferences regarding IO devices based on a simulated activity using training blocks. No patient data were collected, and in accordance with the Declaration of Helsinki ethical principles for medical research and the US Office of Human Research Protections guidance, informed consent was obtained from participants as evidence of their agreement to voluntarily participate in this study.
Availability of data and materials
All relevant summary data are provided in the manuscript text, tables, and figures.
Supplemental Material
Download MS Word (37.6 KB)Acknowledgments
Data analysis, editorial support, and writing were provided by Halit O. Yapici, Lily Arnett and Amanda Agazio of Boston Strategic Partners, Inc. (funded by Becton, Dickinson and Company). Andrea McCamant (Becton, Dickinson and Company) assisted with study design and editorial review. This work was funded by Becton, Dickinson and Company.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17434440.2023.2190019
Additional information
Funding
References
- Merchant RM, Topjian AA, Panchal AR, et al. Part 1: executive summary: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142(16_suppl_2):S337–s57.
- Perkins GD, Graesner JT, Semeraro F, et al. European resuscitation council guidelines 2021: executive summary. Resuscitation. 2021;161:1–60.
- Weiser G, Hoffmann Y, Galbraith R, et al. Current advances in intraosseous infusion - a systematic review. Resuscitation. 2012;83(1):20–26.
- Sharma RK. Occupational health hazards in emergency and triage of health care setting. Lett Health Biol Sci. 2017;2(2)68–70.
- Dyson E, Smith GB. Common faults in resuscitation equipment–guidelines for checking equipment and drugs used in adult cardiopulmonary resuscitation. Resuscitation. 2002;55(2):137–149.
- Bahat H, Hasidov-Gafni A, Youngster I, et al. The prevalence and underreporting of needlestick injuries among hospital workers: a cross-sectional study. Int J Qual Health Care. 2021;33(1):1.
- Norris B, West J, Anderson O, et al. Taking ergonomics to the bedside – a multi-disciplinary approach to designing safer healthcare. Appl Ergon. 2014;45(3):629–638.
- Petitpas F, Guenezan J, Vendeuvre T, et al. Use of intra-osseous access in adults: a systematic review. Crit Care. 2016;20(1):102.
- Gazin N, Auger H, Jabre P, et al. Efficacy and safety of the EZ-IO™ intraosseous device: out-of-hospital implementation of a management algorithm for difficult vascular access. Resuscitation. 2011;82(1):126–129.
- Chatterji AK, Fabrizio KR, Mitchell W, et al. Physician-industry cooperation in the medical device industry. Health Aff (Millwood). 2008;27(6):1532–1543.
- Little A, Alsbrooks K, Jones D. Physician preferences associated with powered intraosseous access systems: safety features, reliability, and ease-of-use. J Am Coll Emerg Physicians Open. 2022;3(3):e12710.
- Patterson P, Yearly D. Safety in the prehospital emergency medical services setting patient safety network: patient safety network; 2019 [ Available from]: https://psnet.ahrq.gov/perspective/safety-prehospital-emergency-medical-services-setting#.
- World Medical Association. World Medical Association Declaration of Helsinki: ethical Principles for Medical Research Involving Human Subjects. JAMA. 2013;310(20):2191–2194.
- U.S. Department of health and human services, office for human research protections. Subpart A of 45 CFR Part 46: Basic Health and Human Services Policy for the Protection of Human Subjects. 2018.
- Bielski K, Szarpak L, Smereka J, et al. Comparison of four different intraosseous access devices during simulated pediatric resuscitation. A randomized crossover manikin trial. Eur J Pediatr. 2017;176(7):865–871.
- Shavit I, Hoffmann Y, Galbraith R, et al. Comparison of two mechanical intraosseous infusion devices: a pilot, randomized crossover trial. Resuscitation. 2009;80(9):1029–1033.
