1. Introduction
Cardiac contractility modulation (CCM) is an innovative device-based therapy for the treatment of patients with heart failure (HF) with mildly reduced ejection fraction (HFmrEF) and reduced ejection fraction (HFrEF) with a narrow QRS complex (and therefore with no indication for cardiac resynchronization therapy).
The CCM therapy is based on the delivery, by an impulse pocket generator (Optimizer Smart®), of high-voltage (≈7.5 V) and long-duration (≈20 ms) biphasic electrical signals to the septal wall of the right ventricle during the absolute refractory period of the myocardium [Citation1].
Therefore, these signals do not cause a novel myocardial contraction, but induce an improvement in calcium handling and, consequently, an increase in myocardial contractility [Citation2].
Besides improving calcium handling, CCM therapy induces several positive effects () that affects the entire biology of the failing myocardium [Citation3–5].
Table 1. Summary of the effects of cardiac contractility modulation therapy on failing myocardium.
In randomized controlled trials, CCM therapy improves functional capacity (i.e. VO2 peak at cardiopulmonary exercise test) [Citation6]; also, in a real-world registry, CCM therapy is associated with a lower rate of HF-related hospitalizations [Citation7].
In addition, CCM therapy induces both left and right ventricular reverse remodeling [Citation8–10].
Based on the results of these randomized clinical trials, the Optimizer Smart® system has been approved in countries covered by CE markings since October 2016 and granted United States Food and Drug Administration (FDA) approval in March 2019.
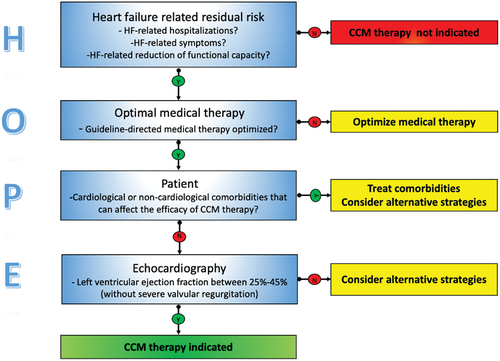
As with all device-based therapies for HF, appropriate patient selection is crucial for increasing the possibility of clinical and echocardiographic response. Because no practical guidance on proper patient selection for CCM therapy is currently provided by the European Society of Cardiology and the American College of Cardiology/American Heart Association guidelines [Citation11,Citation12], we proposed the HOPE algorithm as a simple tool for screening and selecting outpatients with HFrEF/HFmrEF in which CCM therapy is more likely to be effective ().
2. HOPE algorithm
The HOPE algorithm is based on four components; the first three are based on clinical parameters, and the latter is based on echocardiography. At every step, the physician must respond to simple questions to check the suitability for CCM therapy.
2.1. Evaluation of HF-related factors
The first component of the HOPE algorithm evaluates HF-related burdens that impact patients’ quality of life. These include HF-related hospitalizations in the previous 12 months, HF-related dyspnea and/or fatigue, and HF-related poor functional capacity as assessed by a 6-min walking distance test or a cardiopulmonary exercise test. This phase is essential for verifying that the patient can have clinical benefit from CCM therapy, as this therapy has been shown in randomized clinical trials [Citation6], clinical registries [Citation7], and metanalyses [Citation13] to reduce HF-related hospitalizations, improve quality of life, distance walking at the 6-min walking test, and the peak oxygen uptake assessed at cardiopulmonary exercise test.
2.2. Review of drugs therapy
The HOPE algorithm’s second component verifies if the patient is on Optimal Medical Treatment (OMT). Robust evidence from well-conducted randomized clinical trials has documented that target doses of disease-modifier drugs (β-blockers, mineralocorticoid receptor antagonists, angiotensin receptor neprilysin inhibitors, and sodium-glucose co-transporter-2 inhibitors) reduce HF-related mortality and HF-related hospitalizations, as well as improve functional capacity, symptoms, and quality of life. Therefore, in patients with HFrEF and HFmrEF, CCM therapy should be considered, as an add-on therapy, after 3 months of optimizing disease-modifier drug therapy [Citation14]. Of course, previous (or simultaneous) implantation of an implantable cardioverter defibrillator should be considered in patients with a left ventricular ejection fraction of <35%.
2.3. Global assessment of patients
The third component of the HOPE algorithm refers to the Patient; this is the most critical aspect of the algorithm because it relates to assessing the patient as a whole and not only as affected by HF syndrome. Cardiological and non-cardiological comorbidities that may limit the efficacy of CCM therapy must be carefully researched at this stage. For example, from a cardiological standpoint, the presence of atrial fibrillation with a high ventricular response (i.e. >110 b/min) or a high burden of premature ventricular beats (i.e. >10.000/24 h) leads to non-deliverance of CCM therapy [Citation15].
In addition, both conditions can be responsible for the HF burden of the patient related to the onset of tachycardia-induced cardiomyopathy. Therefore, these conditions must be treated, and the patients must be reevaluated at 3–6 months before performing Optimizer Smart® implant.
Also, at this stage, a chronic ischemic left ventricular dysfunction should be carefully ruled out or treated before considering CCM therapy.
Furthermore, non-cardiological conditions limiting the benefits of CCM on quality of life, symptoms, and hospitalizations (malignancies, chronic obstructive pulmonary disease, neuromuscular disease, end-stage chronic kidney disease) must be evaluated to perform a proper risk/benefit balance. In older patients, using frailty scores (as the clinical frailty scale) may be helpful for appropriate screening and selection.
2.4. Perform echocardiography
The last component is Echocardiography; in this phase is essential to confirm that the patients have a left ventricular ejection fraction ranging from 25% to 45% (that is the range of left ventricular ejection fraction used as inclusion criteria in clinical trials and indicated by international guidelines) and to exclude the presence of severe left-side and right-side valve regurgitations that represent, in our opinion, a contraindication for CCM implants.
Left ventricular volume overload and reduced stroke volume related to severe mitral and aortic regurgitation may affect the efficacy of CCM therapy. An exception to this rule is represented by the patients with functional (secondary) and proportionate (related to the increase in left-ventricle volume) mitral regurgitation in whom CCM therapy-induced left ventricular reverse remodeling can reduce the degree of valvular insufficiency.
For patients with severe tricuspid and, more rarely, pulmonary regurgitation, the right ventricular volume overload may affect the efficacy of CCM therapy, particularly if associated with pre-capillary pulmonary hypertension. For this reason, the presence of severe right valve insufficiency represents a relative contraindication to CCM therapy; however, in carefully selected cases of functional tricuspid regurgitation, CCM therapy-induced right ventricular reverse remodeling could induce a reduction of the degree of tricuspid regurgitation. In this situation, we recommend excluding an irreversible form of pre-capillary pulmonary hypertension by the right-heart catheterization before implanting Optimizer Smart®.
3. Conclusion
The HOPE algorithm is based on simple clinical and echocardiographic parameters that are easy to obtain in everyday clinical practice. Therefore, we hope it can facilitate the appropriate selection of patients for CCM therapy and promote the dissemination in the cardiology community of this device-based therapy.
Declaration of interest
Ishu Rao is the Medical Director of Impulse Dynamics and owns the stock options in Impulse Dynamics. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewers disclosure
Peer reviewers in this manuscript have no relevant financial relationships or otherwise to disclose.
Author contributions
All authors contributed equally to the conceptualization and writing of the manuscript.
Additional information
Funding
References
- Borggrefe M, Mann DL. Cardiac contractility modulation in 2018. Circulation. 2018;11:2738–2740.
- Abi-Samra F, Gutterman D. Cardiac contractility modulation: a novel approach for treating heart failure. Heart Fail Rev. 2016;21:645–660.
- Tschöpe C, Kherad B, Klein O, et al. Cardiac contractility modulation: mechanisms of action in heart failure with reduced ejection fraction and beyond. Eur J Heart Fail. 2019;21:14–22.
- Winter J, Brack KE, Ng GA. The acute inotropic effects of cardiac contractility modulation (CCM) are associated with action potential duration shortening and mediated by β1-adrenoceptor signaling. J Mol Cell Cardiol. 2011;51:252–262.
- Lyon AR, Samara MA, Feldman DS. Cardiac contractility modulation therapy in advanced systolic heart failure. Nat Rev Cardiol. 2013;10:584–598.
- Abraham WT, Kuck KH, Goldsmith RL, et al. A randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation. JACC Heart Fail. 2018;6:874–883.
- Kuschyk J, Falk P, Demming T, et al. Long-term clinical experience with cardiac contractility modulation therapy delivered by the optimizer smart system. Eur J Heart Fail. 2021;23:1160–1169.
- Contaldi C, De Vivo S, Martucci ML, et al. Effects of cardiac contractility modulation therapy on right ventricular function: an echocardiographic study. Appl Sci. 2022;12:7917–7923.
- Masarone D, Kittleson MM, De Vivo S, et al. Effects of cardiac contractility modulation electrodes on tricuspid regurgitation in patients with heart failure with reduced ejection fraction: a pilot study. J Clin Med. 2022;11:7442.
- Masarone D, Kittleson MM, De Vivo S, et al. The effects of device-based cardiac contractility modulation therapy on left ventricle global longitudinal strain and myocardial mechano-energetic efficiency in patients with heart failure with reduced ejection fraction. J Clin Med. 2022;11:5866.
- McDonagh TA, Metra M, Adamo M, et al.; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24:4–131. DOI:10.1002/ejhf.2333
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;45:8951032.
- Giallauria F, Cuomo G, Parlato A, et al. A comprehensive individual patient data meta-analysis of the effects of cardiac contractility modulation on functional capacity and heart failure-related quality of life. ESC Heart Fail. 2020;7:2922–2932.
- Fudim M, Abraham WT, von Bardeleben RS, et al. Device therapy in chronic heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78:931–956.
- Rao IV, Burkhoff D. Cardiac contractility modulation for the treatment of moderate to severe HF. Expert Rev Med Devices. 2021;18:15–21.