ABSTRACT
Introduction
Bone biopsies have great value for the diagnosis of, amongst others, hematologic diseases. Although the bone biopsy procedure is mostly performed minimally invasive with the use of a slender cannula, the patient may still experience discomfort, especially when the procedure has to be repeated due to an unsuccessful biopsy.
Areas covered
This review presents a comprehensive overview of bone biopsy devices presented in the patent literature. The patents were obtained using a classification search combined with keywords in the Espacenet patent database and were subsequently verified using pre-set eligibility criteria. This resulted in 62 unique patents included in this review.
Expert opinion
The included patents were categorized based on the used strategies for the three steps that can be identified during a bone biopsy (1) biopsy sampling, (2) biopsy severing and (3) biopsy harvesting. Most patents described strategies for multiple steps. Insight into the used strategies and the comprehensive overview may serve as a source of inspiration for the design of novel bone biopsy devices.
1. Introduction
1.1. Bone biopsies
Bone biopsies and bone marrow aspirations have great diagnostic value and may be performed in combination to diagnose and evaluate of hematologic diseases such as leukemia [Citation1]. Bone biopsies are furthermore performed for the diagnosis of benign and malignant bone tumors, including metastatic bone diseases, lymphoma, and myeloma [Citation2]. The bone biopsy procedure can be performed during open surgery in which a relatively large incision is made to reach the bone but mostly the biopsy procedure is performed minimally invasive. Bones have a hard outer shell of cortical bone which surrounds the porous cancellous bone. The pores of the cancellous bone are filled with bone marrow, a semi-solid spongy tissue that is responsible for the production of blood cells and plays a crucial role in the immune system. During a bone marrow aspiration, only a sample of the bone marrow is aspirated for investigation. During a bone biopsy, also called trephine or core biopsy, a section of the cancellous bone and the encapsulated bone marrow is taken from the patient. Where a bone marrow aspiration is solely used to investigate the bone marrow cells, bone biopsies allow the investigation of the structure of the bone marrow within the cancellous bone. Furthermore, a bone biopsy may be performed to investigate a bone lesion. Bone biopsies and bone marrow aspirations may be performed in the same intervention. In these cases, the bone marrow aspiration is usually performed first, after which, the bone biopsy is performed through the same skin incision. It is advised to enter the bone at least 1 cm away from the aspiration location to guarantee that the obtained bone biopsy is not affected by the bone marrow aspiration [Citation3]. This review focuses on technology specifically developed for bone biopsy procedures, since performing a bone biopsy is more complex than a bone marrow aspiration and is considered more painful for the patient [Citation3].
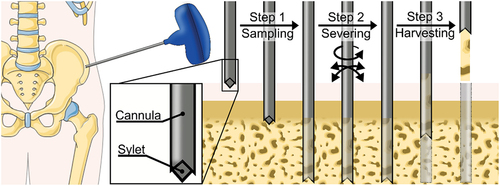
A bone biopsy can be performed in different areas of the human body depending on clinical requirements, but for suspected hematologic diseases often an area where bone can be reached with minimal damage to surrounding soft tissues is chosen, for example, the iliac crest () [Citation1]. The bone biopsy procedure begins by creating an entry hole through the skin. Subsequently, a hollow needle between 10 gauge (3.2 mm) and 8 gauge (4.1 mm), referred to as a cannula is introduced through the skin incision with a twisting motion to create the entry hole to the target location within the cancellous bone [Citation1]. During this step, a stylet is placed inside the cannula to prevent soft tissue and bone chips from entering the cannula and to avoid contamination of the biopsy [Citation4]. Furthermore, the sharp point of the stylet aids the penetration of the strong cortical bone layer. Once the distal tip of the cannula has reached the target location, the stylet is removed from the cannula and the cannula is advanced further into the cancellous bone while applying slight pressure combined with a twisting motion [Citation1]. During this process, part of the cancellous bone and encapsulated bone marrow, the biopsy, will enter the lumen of the cannula. When a biopsy sample of 1–3 cm long has been captured in the cannula, the cannula is turned and tilted to sever the biopsy from the surrounding bone [Citation1]. Subsequently, the cannula with bone biopsy sample is carefully retrieved from the patient to harvest the biopsy. A slight suction might be used to prevent the biopsy from slipping out of the cannula [Citation1]. After the cannula is successfully retrieved, the biopsy is removed from the cannula using a blunt-tipped stylet that is inserted via the distal end of the cannula as to push the biopsy out of the cannula.
1.2. Challenges in bone biopsy
Bone biopsies are generally safe procedures with very low complication rates [Citation5]. Breaking of the needle is rare with an estimated occurrence of 0.01% found in a study by Bain [Citation6] but may have serious implications as the needle fragments might need to be removed surgically resulting in a longer hospital stay. Hemorrhage is the most common complication but only accounts for an incident rate of 0.02% [Citation3]. Nevertheless, a bone biopsy procedure can cause significant pain and discomfort to patients [Citation7]. The discomfort may be caused by the heat generated by the friction between the cannula and the bone during the insertion [Citation8]. Furthermore, tilting the cannula to sever the biopsy can cause discomfort due to the creation of micro-fractures and trauma to the surrounding bone [Citation9].
Although reports on serious long-term complications for bone biopsy procedures are low, the number of unsuccessful biopsies is much higher. Cervi [Citation10] states that often two or three biopsies are performed to obtain a single useful biopsy sample. Multiple trials may be necessary due to unsuccessful severing of the biopsy, which makes it impossible to harvest the biopsy with the clinically available biopsy cannulas [Citation1]. Furthermore, the biopsy can slip out of the cannula during retrieval of the cannula [Citation1,Citation11]. Additionally, when the biopsy is successfully obtained, the biopsy might not be suitable for further investigation due to insufficient size of the biopsy or crushing artifacts in the biopsy that could be introduced during sampling or severing of the biopsy. The need to perform multiple biopsies not only results in more discomfort to the patient but also increases in costs, due to an increased procedure time and additional lab work [Citation12].
1.3. Goal of this review
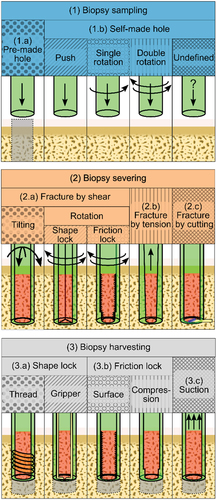
The goal of this review is to provide a comprehensive overview of bone biopsy devices in patent literature, as patent literature provides insights in the latest technical developments in this field. The bone biopsy devices described in the patent literature are categorized based on the methods used in each of three steps that are performed in a bone biopsy procedure, namely (1) biopsy sampling, (2) biopsy severing, and (3) biopsy harvesting ().
2. Method
2.1. Patent search method
A classification search was conducted in the Espacenet patent database. For this search, only patents with the Cooperative Patent Classification (CPC) of A61B10/025 or a subcategory of this class were included as this class contains patents that describe biopsy devices for bone, bone marrow, or cartilage biopsies. Patents that also had the CPC of A61B10/0283 were excluded as this class includes devices that use vacuum aspiration which is suitable for bone marrow biopsies but not for bone biopsies. Only WORLD patents (WO*), European Patents (EP*), and United States patents (US*) were included in this study.
2.2. Eligibility criteria
The scope of this review is to create an overview of devices that can be used to obtain a bone biopsy. Patents do not always specify the clinical application, hence the following eligibility criteria were used to exclude non-relevant patents. Only patents that describe a design for a device that is able to extract solid tissue such as bone, cartilage and/or tumor tissue, neoplastic or pathologic tissue within the bone, were included. Devices that merely focus on bone marrow aspiration were excluded, as strategies used for bone marrow aspiration are not applicable for bone biopsies due to substantial differences between the procedures. Furthermore, the patent was required to describe the method used to obtain the biopsy. Patents solely focusing on, for instance, handle designs were excluded. Lastly, only patents that focus on the extraction of an intact piece of tissue without damaging it were included. This means that devices that focus on the extraction of fragmented bone, for instance to acquire bone grafts, were excluded as this would make the device unfitting for the extraction of bone biopsies for diagnostic purposes.
2.3. General results
The search query resulted in the identification of 297 patents (January 2023). The patents were screened for eligibility by inspecting the title, abstract, and drawings. The description of the patent was screened for eligibility for all patents that were not excluded based on the inspection of the title, abstract and drawings. This resulted in a total of 62 patents being included in this review. Patents that had the same authors and described similar devices were regarded as duplicates although they might not be considered duplicates in the legal sense. In the case of ‘duplicates,’ only the most recent patent was included in this review.
2.4. Classification
The patents were classified based on the method used for (1) biopsy sampling, (2) biopsy severing and (3) biopsy harvesting (). In the methods used to sample the biopsy, a clear distinction was made between (1.a) devices designed for use in a pre-made hole, such that new bone is cultivated in the device over time, and (1.b) devices that create a self-made hole through the cancellous bone to sample a biopsy. The hole is in those cases made by solely pushing the cannula into the bone, or by combining the pushing with a twisting motion in one or both directions. Severing of the biopsy sample from the surrounding bone can be achieved by (2.a) inducing shear forces by tilting or rotating the cannula. Severing of the biopsy sample from the surrounding bone can also be achieved by (2.b) tension forces or by (2.c) cutting the biopsy sample from the surrounding bone. The biopsy must be harvested from the patient’s body when the cannula is retrieved without the risk of the biopsy slipping out the cannula. This can be achieved by (3.a) creating a shape lock between the biopsy and the cannula by either using a threaded section or a gripper, by (3.b) inducing friction between the biopsy and the cannula by a friction-inducing surface or by compressive forces on the biopsy or (3.c) by using suction.
3. Results
3.1. Biopsy sampling
To sample the biopsy, the first step is to penetrate the cortical bone. There are two bone conditions in which bone biopsies are performed: 1) intact cortical bone and 2) weakened or non-existent cortical bone. After penetrating the cortical bone layer, the bone biopsy device is advanced into the cancellous bone sampling the targeted tissue such as pathologic, tumorous, or neoplastic tissue.
3.1.1. Pre-made hole
Two patents describe a bone biopsy device that is intended to be placed into a pre-made hole in the bone [Citation13,Citation14]. Over time the bone will grow within the hollow section of the biopsy device and subsequently the newly cultivated bone can be removed to obtain the bone biopsy. The process of the bone growing in the implant can span multiple weeks, and over this time the device should remain fixated in the surrounding bone.
Fox [Citation14] describes an implant comprising an outer collar that is secured in the surrounding bone in which an inner structure with multiple slots is placed (). These slots allow bone ingrowth into the device. Furthermore, the sloths have sharpened edges that aid the severing of the biopsy during the removal of the inner structure. The collar may remain within the bone during harvesting of the biopsy sample. Afterward, the inner structure can be placed back into the collar to obtain another bone biopsy if required.
Figure 3. Bone biopsy devices comprising an outer cannula (green) an additional (inner) structure (yellow) and a sharp edge (blue) for biopsy (red) sampling and severing. (a) A bone biopsy device intended for implantation in a pre-made hole, figure adapted from [Citation14]. (b) A biopsy device with a wider cutting edge compared to the cannula wall, figure adapted from [Citation15]. (c) Abone biopsy device comprising two concentrically counter rotating cannulas, figure adapted from [Citation16]. (d) A bone biopsy device that severs the biopsy by rotating the inner structure, figure adapted from [Citation17]. (e) A bone biopsy device that severs the bone biopsy by pulling the bulbous inner structure upwards, figure adapted from [Citation18]. (f) A bone biopsy device that compresses the biopsy and severs the biopsy by the sharp cutting edge, figure adapted from [Citation19]. (g) A bone biopsy device with an inner needle with a snare that severs the biopsy, figure adapted from [Citation20]. (h) A bone biopsy device that cuts the biopsy by plastic deformation of the inner structure, figure adapted from [Citation21]. (i) A bone biopsy device that cuts the the biopsy by advancement of the additional outer structure, figure adapted from [Citation22]. (j) A bone biopsy device that cuts the biopsy by rotating the inner structure, figure adapted from [Citation23].
![Figure 3. Bone biopsy devices comprising an outer cannula (green) an additional (inner) structure (yellow) and a sharp edge (blue) for biopsy (red) sampling and severing. (a) A bone biopsy device intended for implantation in a pre-made hole, figure adapted from [Citation14]. (b) A biopsy device with a wider cutting edge compared to the cannula wall, figure adapted from [Citation15]. (c) Abone biopsy device comprising two concentrically counter rotating cannulas, figure adapted from [Citation16]. (d) A bone biopsy device that severs the biopsy by rotating the inner structure, figure adapted from [Citation17]. (e) A bone biopsy device that severs the bone biopsy by pulling the bulbous inner structure upwards, figure adapted from [Citation18]. (f) A bone biopsy device that compresses the biopsy and severs the biopsy by the sharp cutting edge, figure adapted from [Citation19]. (g) A bone biopsy device with an inner needle with a snare that severs the biopsy, figure adapted from [Citation20]. (h) A bone biopsy device that cuts the biopsy by plastic deformation of the inner structure, figure adapted from [Citation21]. (i) A bone biopsy device that cuts the the biopsy by advancement of the additional outer structure, figure adapted from [Citation22]. (j) A bone biopsy device that cuts the biopsy by rotating the inner structure, figure adapted from [Citation23].](/cms/asset/27bbba5f-5153-4886-9407-f22683ec826a/ierd_a_2254681_f0003_oc.jpg)
The devices that use a pre-made hole are mainly intended for use in a research setting, for instance to investigate the effects on bone growth while biopsy devices that create a self-made hole are generally used for diagnostic purposes. This difference in application area results in significantly different designs, therefore, the patents of Albrektsson [Citation13] and Fox [Citation14], that describe a biopsy device using a pre-made hole will not be considered in the categorization (2) biopsy severing and (3) biopsy harvesting.
3.1.2. Self-made hole
3.1.2.1. Push
The remaining patents all describe the use of a self-made hole to sample the biopsy. All devices use an outer cannula that is advanced through the cancellous bone to sample the bone biopsy. This cannula is often used in combination with a stylet to penetrate the soft tissue surrounding the bone and the strong cortical bone layer. After reaching the target location within the softer cancellous bone, the stylet is removed and the outer cannula is advanced through the cancellous bone. During this step, the bone biopsy material enters the lumen of the cannula.
Five patents describe the use of a linear pushing motion to advance the outer cannula through the cancellous bone [Citation21,Citation24–27]. The outer cannula of the devices described in these patents have a sharpened edge to cut through the bone. Malagoli [Citation25] describes an outer cannula with a non-circular lumen and a sharpened distal end with teeth to help push the cannula through the bone. The sampled biopsy will have a non-cylindrical cross-section. Laughlin et al. [Citation24] describe the use of an impact force instead of continuous pushing force to advance the cannula through the cancellous bone.
3.1.2.2. Single rotation
Fourteen (14) patents describe the use of a single-sided rotation in combination with linear advancement of the cannula into the cancellous bone [Citation15,Citation28–40]. A rotation in a single direction may be preferred when there is a thread-cutting section at the distal end of the cannula, or because the introduction of the cannula is motorized. Spranza [Citation15] describes an outer cannula with a cutting edge that is slightly wider than the wall thickness of the cannula wall, resulting in a clearance between the outer cannula and the surrounding bone. This clearance eliminates friction and thus the generation of heat between the rotating cannula and bone (). Furthermore, the cutting edge has sections for the accumulation of bone chips. Aakerfeldt et al. [Citation8] also describe the use of a thicker cutting edge to reduce heath generation, however the cannula is not introduced with a single rotation but with an oscillating rotation.
3.1.2.3. Double rotation
Twenty-five (25) patents describe linear advancement of the cannula combined with a rotational movement in both directions (oscillatory rotation) [Citation4,Citation8–11,Citation16–18,Citation22,Citation41–56]. Marino and Elbanna [Citation49] describe the design of a cannula with a castellated teeth pattern at its distal end to reduce the accumulation of bone particles between the teeth as this would diminish the cutting ability. Matthews [Citation16] describes a cannula that consists of two concentric tubes with saw teeth (). These saw teeth are directionally oriented such that the inner cannula should be rotated in the opposite direction of the outer cannula.
3.1.2.4. Undefined
Sixteen (16) patents describe a biopsy device that is able to create a self-made hole to sample the biopsy, however the cannula motion that is used to advance through the cortical bone is not defined [Citation19,Citation20,Citation23,Citation57–69].
3.2. Biopsy severing
Once the biopsy is sampled by the cannula, the biopsy must be severed from the surrounding bone such that the biopsy can be harvested from the patient.
3.2.1. Fracture by shear
3.2.1.1. Tilting
Severing of the biopsy can be achieved by inducing shear forces. Thirteen (13) patents describe the use of a primarily tilting motion of the cannula to sever the biopsy [Citation4,Citation10,Citation24,Citation29,Citation30,Citation32,Citation34,Citation36,Citation39,Citation50,Citation51,Citation55,Citation58]. Tilting of the cannula induces shear forces in the contact plane between the biopsy and the surrounding bone. The tilting of the cannula in different directions may be combined with rotating the cannula to sever the biopsy.
3.2.1.2. Shape lock
Fourteen (14) patents describe the use of a rotational movement of the cannula to sever the biopsy from the surrounding bone [Citation9,Citation11,Citation17,Citation25–28,Citation41,Citation46–49,Citation62,Citation63]. This eliminates the need to tilt the cannula. Severing by rotating the cannula can only be successful if the biopsy rotates together with the cannula to induce shear forces in the contact plane between the biopsy and the surrounding bone. Four patents provide designs that avoid the biopsy from rotating within the cannula by using a non-circular lumen [Citation17,Citation25–27]. Malagoli [Citation25], and Sachse and Sachse [Citation26] both describe a cannula with a non-circular lumen to allow the severing of the sample by rotating the cannula. Joish [Citation27] describes a cannula with an off-center lumen in which the biopsy is captured. Rotation of the cannula around its central axis will result in the severing of the sample. Avaltroni [Citation17] has a different approach as not the cannula itself is rotated but rather a blade that is introduced into the cannula after sampling the biopsy (). The blade cuts the biopsy in two halves and rotating the blade will sever the biopsy.
3.2.1.3. Friction lock
Rotation of the biopsy with respect to the needle can also be avoided by increasing the friction between the biopsy sample and the inner surface of the cannula [Citation9,Citation11,Citation28,Citation41,Citation46–49,Citation62,Citation63]. For example, the use of a structure on the inside of the cannula such as barbs or grooves may increase the friction between the biopsy and the cannula [Citation41,Citation63]. Another way to increase the friction between the biopsy and the inside of the cannula is by compressing the biopsy against the cannula wall, for instance by using a tapered tip [Citation28], or a ridge at the distal end [Citation9,Citation11,Citation46–48]. Due to the narrowing of the lumen at the distal end of the cannula, the biopsy will be slightly compressed as it enters the lumen due to the elasticity of the bone tissue. The increased friction induced by compression prevents the rotation of the sample with respect to the cannula and eases the severing. Krueger and Clark [Citation62] and Marino and Elbanna [Citation49] use the introduction of an inner needle in the cannula to compress the biopsy such that the friction is increased.
3.2.2. Fracture by tension
Four patents describe a biopsy severing method by inducing tensional forces on the bone [Citation18,Citation35,Citation38,Citation43]. Tensioning is only possible if the biopsy is well connected to the cannula by a shape lock, for instance, due to a threaded section at the distal end of the cannula [Citation35,Citation38,Citation43]. Pulling the cannula, and thus the enclosed biopsy, out of the patient as to retrieve the cannula results in tensioning and finally fracturing of the biopsy from the surrounding bone. Baldridge [Citation18] does not use a threaded section but rather proposes the use of an inner needle that has a bulbous end that extends from the outer cannula and is slightly larger than the cannula lumen (). Once the target area is reached, the cannula with the inner needle is advanced through the cancellous bone such that the lumen of the inner needle fills with the cancellous bone. After obtaining a biopsy with the correct length, the inner needle is pulled into the outer cannula. Slits in the bulbous end allow for radial compression such that the inner needle fits within the outer cannula. This results in tensioning of the bone, and combined with the sharp edge of the bulbous end, in severing of the bone biopsy.
3.2.3. Fracture by cutting
Sixteen (16) patents describe a cannula that can sever the biopsy from the surrounding bone by means of cutting [Citation19–23,Citation33,Citation40,Citation53,Citation56,Citation57,Citation59,Citation61,Citation64–66,Citation69]. Five patents describe the use of an inner needle that consists of two or more semi-circular cutting blades that form a tweezer-like structure [Citation19,Citation64–66,Citation69]. The inner needle is located within the cannula during insertion into the bone. After the biopsy is enclosed, the inner needle is moved deeper into the cannula, such that the tweezer is at the distal end of the cannula. Since the cannula is slightly tapered, this will result in compression of the distal tips of the tweezer and thus the cutting of the biopsy from the surrounding bone (). Rubinstein [Citation65] describes a slightly different approach to compress the distal tips of the tweezer using a cannula with an oval lumen. In this design, the tweezer tips can be compressed even further by rotating the tweezer with respect to the oval lumen of outer cannula.
Goldenberg [Citation20,Citation61] describes two cannula designs with a snare at the distal end that can be constricted once the bone biopsy is enclosed such that the biopsy is cut loose from the surrounding bone (). Zambelli [Citation40,Citation56] and Miller and Ireland [Citation21] describe the use of an inner needle that plastically deforms such that the biopsy is cut loose due to the tapered tip of the outer cannula (). Peliks et al. [Citation22] describe the use of a pre-bend cutting blade that is located at the outside of the cannula (). During the insertion of the cannula, the cutting blade is forced in a straight position such that the cutting blade does not harm the advancement of the cannula through the cancellous bone. Once a biopsy with the right length is obtained, the pre-bend cutting blade is advanced through a slit at the distal end of the cannula such that the biopsy is severed from the surrounding bone. Furthermore, two patents describe the rotation of an inner needle as a means to cut the biopsy from the surrounding bone [Citation23,Citation53] ().
3.3. Biopsy harvesting
After the biopsy is sampled by the cannula and severed from the surrounding bone, the cannula must be retrieved from the patient to harvest the biopsy. Harvesting the biopsy may fail as the biopsy is not always well secured within the cannula and may slip out when retrieving the cannula.
3.3.1. Shape lock
3.3.1.1. Thread
Of the included patents, nineteen (19) describe the use of a shape lock to prevent the biopsy from exiting the cannula such that the biopsy can successfully be harvested. Four (4) patents describe a cannula with a threaded section at the distal end [Citation34,Citation35,Citation38,Citation43] (). The cannula is advanced with a single rotation and as a result, threads are cut in the bone biopsy. This creates a shape lock between the biopsy and the threaded section such that the biopsy is securely fixated. Gillespie et al. [Citation43] also describe the use of a threaded section, but in the proposed design, an inner needle with a corkscrew at the distal end is used to create a shape lock (). The biopsy can be harvested by retrieving the cannula together with the inner needle without the risk of losing the biopsy.
Figure 4. Bone biopsy devices comprising an outer cannula (green) an additional (inner) structure (yellow) and additional characteristics (orange) to retain the biopsy. (a) A bone biopsy device with a threaded section to create a shape lock between the biopsy and the cannula, figure adapted from [Citation38]. (b) A biopsy device with a corkscrew inner structure to create a shape lock to retain the biopsy, figure adapted from [Citation43]. (c) A bone biopsy device with a spring that can compress the biopsy to retain it figure adapted from [Citation61]. (d) A bone biopsy device with a tapered inner structure to compress the biopsy for successful retainment, figure adapted from [Citation45]. (e) A bone biopsy device that retains the biopsy by a sudden change in lumen diameter, figure adapted from [Citation24]. (f) A bone biopsy device that compresses the biopsy and allows for easy removal from the inner structure, figure adapted from [Citation30].
![Figure 4. Bone biopsy devices comprising an outer cannula (green) an additional (inner) structure (yellow) and additional characteristics (orange) to retain the biopsy. (a) A bone biopsy device with a threaded section to create a shape lock between the biopsy and the cannula, figure adapted from [Citation38]. (b) A biopsy device with a corkscrew inner structure to create a shape lock to retain the biopsy, figure adapted from [Citation43]. (c) A bone biopsy device with a spring that can compress the biopsy to retain it figure adapted from [Citation61]. (d) A bone biopsy device with a tapered inner structure to compress the biopsy for successful retainment, figure adapted from [Citation45]. (e) A bone biopsy device that retains the biopsy by a sudden change in lumen diameter, figure adapted from [Citation24]. (f) A bone biopsy device that compresses the biopsy and allows for easy removal from the inner structure, figure adapted from [Citation30].](/cms/asset/cf938ec1-4533-4c69-9b51-fec3c4415c66/ierd_a_2254681_f0004_oc.jpg)
3.3.1.2. Gripper
The other fifteen (15) patents describe the use of a gripper to create a shape lock to secure the biopsy within the cannula when retrieving the cannula [Citation20–23,Citation33,Citation40,Citation53,Citation56,Citation57,Citation59,Citation61,Citation64–66,Citation69]. Goldenberg [Citation61] describes an inner needle that is connected to the distal end of the cannula with a spring (). Rotation of the inner needle with respect to the cannula results in further coiling of the spring causing a decrease in the inner diameter of the spring. This secures the biopsy within the cannula.
3.3.2. Friction lock
3.3.2.1. Surface
Friction between the biopsy and the inner wall of the cannula can be used to prevent the biopsy from slipping out of the cannula during retrieval. Three patents use a friction-inducing surface on the inside of the cannula to prevent the biopsy from slipping out [Citation10,Citation41,Citation63]. The inside of the lumen has groves or barbs to increase the friction between the cannula and the biopsy. The same friction-inducing surface can be used to sever the biopsy as described in Section 3.2.1.
3.3.2.2. Compression
Eighteen (18) patents describe the use of compression of the biopsy to increase the friction between the biopsy and the cannula via an increase in normal force to prevent the biopsy sample from slipping out of the cannula during retrieval [Citation8,Citation9,Citation11,Citation17–19,Citation24,Citation28,Citation30,Citation36,Citation45–51,Citation62]. An increase in friction by using compression can be achieved by a tapered tip of the cannula [Citation8,Citation28,Citation36,Citation50,Citation51]. The cannula lumen is narrower at the distal end and as a result, the biopsy is slightly compressed at the distal end which increases the normal force and thus the friction between the biopsy and the cannula and secures the biopsy during retrieving of the cannula. Hirsch et al. [Citation45] describe a tapered tip with a slightly wider cutting edge at the distal end as compared to the rest of the lumen (). The compression of the biopsy when it is pushed into the lumen increases the friction between the cannula wall and the biopsy and thus prevents the biopsy from slipping out.
Five patents describe a sudden change in lumen diameter at the distal end of the cannula [Citation11,Citation24,Citation46–48]. Although the working principle is similar to the cannulas with a tapered tip, these patents do not have a gradual change in lumen diameter but a sudden narrowing of the lumen at the distal end (). The tissue is cut and compressed through the narrow section of the lumen, which increases the friction to retain the biopsy. Furthermore, the biopsy can expand once it reaches the wider section of the lumen, resulting in an additional shape lock to retain the biopsy.
Seven patents describe the use of an additional structure to increase the friction between the biopsy and the cannula [Citation9,Citation17–19,Citation30,Citation49,Citation62]. Examples are inner needles that are introduced within the cannula to compress the obtained biopsy. An example is the inner needle which consists of two semi-circular structures that together form a tweezer-like device described by Doppelt [Citation30] (). Advancement of the inner needle through the cannula results in compression of the two tweezer halves due to the narrowing lumen at the distal end of the cannula. Compression of the tweezer tips compresses the biopsy and prevents it from slipping out of the cannula. A similar method is proposed by Mittermeier and Halbe [Citation19].
3.3.3. Suction
Only one patent of Wiksell et al. [Citation55] describes the use of suction to harvest the biopsy. In this device a negative pressure differential (suction) is created within the cannula, which prevents the biopsy from slipping out. Suction may also be used in combination with the earlier-mentioned strategies to harvest the biopsy.
After the removal of the cannula from the patient, the biopsy material must be removed from the cannula. Often a plunger with a blunt tip is used to push the biopsy out of the lumen. The pushing force that is used to achieve this could also harm the biopsy. As an alternative to using a plunger to remove the bone biopsy from the cannula Doppelt [Citation30] proposes a design that captures the biopsy between two semi-cylindrical structures. This would not only help to retain the biopsy during the removal of the needle. It also makes it possible to easily remove the bone biopsy from the needle while maintaining the structural integrity of the biopsy.
4. Conclusion
This review provides a comprehensive overview of the different bone biopsy devices described in patent literature. The search query used in the Espacenet database returned 297 patents of which 62 were deemed eligible for inclusion in this review. The patents were categorized based on the strategies used for the three major steps that are followed during a bone biopsy procedure (1) biopsy sampling, (2) biopsy severing, and (3) biopsy harvesting. The sampling of the biopsy may be achieved by creating a hole through the bone or by cultivating new bone in a pre-made hole. The severing of the biopsy can be achieved by tension forces, shear forces, or cutting. Harvesting the biopsy can be achieved by using a shape lock, friction between the biopsy and the device, or suction. It must be noted that the strategies used in one step of the biopsy procedure influences the possible strategies that may be used in the other steps of the biopsy. The provided overview may serve as a source of inspiration for the design of novel bone biopsy devices.
5. Expert opinion
5.1. Comparative analysis
The goal of this review was to create a comprehensive overview of bone biopsy devices in patent literature. The different designs were categorized based on the strategies used for each of the three steps that are followed during a bone biopsy procedure (1) biopsy sampling, (2) biopsy severing and (3) biopsy harvesting. shows the distribution of the filed patents over the years for each of these identified steps. Between 1970 and 2010 there has been a steady increase in filed patents however, this growth dropped suddenly between 2010 and 2020. It seems plausible that the growth in filed patents will recover after 2020 based on the current amount of filed patents between 2020 and January 2023.
Figure 5. Temporal distribution of the methods presented in the included patents for biopsy sampling, severing and harvesting until January 2023.
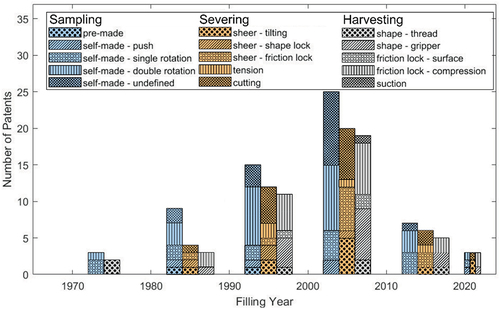
Sixty-six percent (66%) of the included patents described a method for both sampling, severing and harvesting of the bone biopsy sample. Seventy-four percent (74%) of the included patents clearly describe the introduction method of the cannula and thus the sampling of the biopsy. However, for the remaining 26% only mention that the cannula was able to cut through the bone without specifying the specific sampling method (e.g. push possibly combined with a rotating motion). Seventy-six percent (76%) of the included patents describe a method for severing the biopsy and 66% of the patents describe a method to retain the biopsy when the cannula is retrieved.
The overview with the different methods to sample, sever and harvest the bone biopsy indicated that certain methods are more frequently combined. For instance, the biopsy devices that employ a shape lock to sever the biopsy sampled the biopsy by pushing the cannula through the cancellous bone. The shape lock was often created by a non-rotational symmetric cross section of the cannula. Rotating the cannula during sampling would results in a rotational symmetric biopsy, making the use of a shape-lock to sever the biopsy more challenging. Furthermore, biopsy devices that use a threaded section are always introduced by a single rotating motion such that screw thread cuts in the biopsy to achieve the desired shape lock. Devices that severe the biopsy by tensioning or cutting use an additional structure, such as an inner needle, that is subsequently used to create a shape lock in order to retain the biopsy when the cannula is retrieved from the patient. Lastly, when friction is used to retain the biopsy during severing, the same friction inducing strategy is also used to retain the biopsy when the cannula is removed.
All patents reviewed in this study describe innovative ideas for sampling, severing and harvesting bone biopsies. While the suitability for clinical practice is often not discussed in depth in patent literature, we can evaluate the proposed biopsy device designs’ suitability for clinical practice based on the included information and current clinical practice. First of all, biopsy devices should have a small outer diameter to minimize trauma to the patient, while harvesting an as large as possible bone biopsy. It is, therefore, preferred that bone biopsy devices have a small wall thickness and thus a large lumen. The bone biopsy devices described in Group 2.c ‘Fracture by cutting’ and Group 3.a ‘Shape lock’ require an additional structure to sever and harvest the biopsy. This additional structure requires space and may increase the wall thickness and decrease the obtained biopsy which would make these devices less desirable for clinical practice. Furthermore, these structures might lack structural rigidity due to their small size, which can result in mechanical failure and inability to downsize these devices in future. The use of a friction lock, especially a friction inducing surface could, however, ease severing and harvesting of the bone biopsy without requiring an increase in wall thickness. This could increase the success-rate of bone biopsy procedures without adverse effect such as a larger outer diameter of the biopsy device.
Besides striving for a biopsy device with a small outer diameter, the usability of the biopsy device is of great importance for clinical application. Additional actions required by the user to obtain the biopsy compared to conventional biopsy needles are undesired as this will increase the procedure time and thus the associated costs [Citation12]. Furthermore, a more complex device that requires more actions might increase the workload of the user, which is associated with a higher chance of complications [Citation70]. Biopsy devices in Group 2.c ‘Fracture by cutting’ and biopsy devices in Group 3.a ‘Shape lock’ that use a gripper are expected to require an additional action to sever the biopsy with the inner needle and are, therefore, less desirable. Other solutions presented in Group 2.a ‘Fracture by shear’ do not require an addition action as compared to the current procedure and are therefore easier to implement in the current workflow. Finally, bone biopsy devices requiring implantation for bone ingrowth are only feasible if obtaining multiple samples over a longer time frame is required. These devices could be of use in specific applications but are not a substitute for the currently used biopsy needles.
5.2. Limitations and future research
This patent review focuses on the different mechanical solutions for the sampling, severing and harvesting of bone biopsies. Often a bone biopsy is combined with a bone marrow aspiration during one intervention with similar instrumentation. Even so, patents focusing on bone marrow aspiration were excluded as this is a different procedure with different challenges. Furthermore, this review only included patent literature and excluded scientific literature, as patents are generally a good way to gain insight into the future development of devices. It could be of interest for future research to extend this patent review with a review of the scientific literature to emphasize the suitability for clinical practice of different bone biopsy designs more extensively.
This review provides a comprehensive overview of bone biopsy devices which can provide insights into the future development of the devices. The overview may also serve as a source of inspiration for the development of novel bone biopsy devices.
5.3. Five-year view
Over the years there has been an increasing focus on the severing and harvesting of bone biopsies. The successful severing of the biopsy and subsequently the harvesting will increase the success rate of the biopsy procedure. Fewer trials are needed to obtain one useful biopsy which will significantly decrease the discomfort to the patient. We expect that this trend will continue in the coming years. Attention should be directed to the discomfort that patients will experience with the use of these new devices that can sever and harvest the biopsies successfully. The use of an additional inner needle or structure in the cannula may result in a larger outer diameter of the cannula which may increase the discomfort of the patient. Furthermore, the use of the bone biopsy devices in the clinical workflow should not be forgotten. Ease of operation with a limited number of steps is a necessity to ensure usability of newly designed bone biopsy devices in practice. It is expected that in the next five years more focus will be on easily obtaining bone biopsies of high quality with as little discomfort to the patient as possible.
Article highlights
Bone biopsy have great diagnostic value for hematologic diseases, benign and malignant bone tumors, but cause discomfort to patients.
The preferred method for sampling of the biopsy is by advancing the needle with a single of double rotating motion.
The preferred method for severing the biopsy from the surrounding bone is by sheer or cutting the biopsy loose.
The preferred method for harvesting the bone biopsy is by using friction between the needle and the biopsy or by utilizing a shape lock.
There are opportunities to develop novel biopsy needles that increases the success rate of the bone biopsy procedure without increasing the diameter of the biopsy needle and the complexity in use.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Riley RS, Hogan TF, Pavot DR, et al. A pathologist’s perspective on bone marrow aspiration and biopsy: I. performing a bone marrow examination. J Clin Lab Analysis. 2004;18(2):70–90. doi: 10.1002/jcla.20008
- Mavrogenis AF, Angelini A, Errani C, et al. How should musculoskeletal biopsies be performed? Orthopedics. 2014 Sep;37(9):585–588. doi: 10.3928/01477447-20140825-03
- Bain BJ. Bone marrow trephine biopsy. J Clin Pathol. 2001 Oct;54(10):737–742. doi: 10.1136/jcp.54.10.737
- Byrne JR, Rodriguez JLC, Gregory VD, et al. Biopsy needle assembly. WO9603081A1. 1996 Feb. 8.
- Tomasian A, Jennings JW. Bone marrow aspiration and biopsy: techniques and practice implications. Skeletal Radiol. 2022 Jan;51(1):81–88. doi: 10.1007/s00256-021-03882-w
- Bain BJ. Bone marrow biopsy morbidity and mortality: short report. Br J Haematol. 2003 Jun;121(6):949–951. doi: 10.1046/j.1365-2141.2003.04329.x
- Hibbs S. This is going to hurt: revisiting the patient experience of bone marrow biopsies. Hemasphere. 2022 Mar;6(4):e710. doi: 10.1097/HS9.0000000000000710
- Aakerfeldt D, Aastroem G, Ahlstroem H. Device for biopsy sampling. WO9517126A1. 1995 Jun. 29
- Avaltroni P. Improved needle instrument for taking osteomedullary bioptical samples. EP1396230A1. 2004 Mar 10.
- Cervi P. Biopsy needle. WO0056220A1. 2000 Sep 28.
- Zambelli FA. ‘Bone biopsy device and process for making the same’, EP1277440A1. 2003 Jan 22.
- Bishop PW, McNally K, Harris M. Audit of bone marrow trephines. J Clin Pathol. 1992 Dec;45(12):1105–1108. doi: 10.1136/jcp.45.12.1105
- Albrektsson T, Aspenberg P. A bone ingrowth chamber. EP0269175A2. 1988 Jun 1.
- Fox WC. Bone biopsy implant. WO9624309A1. 1996 Aug 15.
- Spranza JJ. Hardware for cutting bone cores. US2003199879A1. 2003 Oct 23.
- Matthews LS. Counter rotating biopsy needle. US4306570A. 1981 Dec 22.
- Avaltroni P. Biopsy device. EP0992218A1. 2000 Apr 12.
- Baldridge DJ. Bone marrow biopsy instrument. US5357974A. 1994 Oct 25.
- Mittermeier M, Hable AM. Bone marrow biopsy needle. WO0010465A1. 2000 Mar 2.
- Goldenberg AS. Bone marrow biopsy needle-1. US2009082697A1. 2009 Mar 26.
- Miller ME, Ireland D. Biopsy extractor. EP1136039A2. 2001 Sep 26.
- Peliks RB, Snow J, Ollerenshaw J. Bone biopsy device and related methods. WO2021178490A1. 2021 Sep 10.
- Slama B, Zerazhi H. Osteomedullar biopsy trocar. WO2006030078A1. 2006 Mar 23.
- Laughlin T, Saladino J, Fisher D, et al. Tissue coring device. WO2020185961A1. 2020 Sep 17.
- Malagoli E. Needle instrument for transcutaneous biopsy of bone marrow tissues. WO2005009246A1. 2005 Feb 3.
- Sachse H, Sachse R. Oscillating bone harvesting device. US6179853B1. 2001 Jan 30.
- Joishy SK. Two in one bone marrow surgical needle. US5012818A. 1991 May 7.
- Bianchini C. Device for bone marrow biopsy. WO2004082484A1. 2004 Sep 30.
- Cook J. Apparatus and method for harvesting bone. US10485558B1. 2019 Nov 26.
- Doppelt SH. Bone biopsy apparatus. US4798213A. 1989 Jan 17
- A Einhorn T, Valenti A, Alves M. Bone boring instrument. US4782833A. 1988 Nov 8.
- Elias E, Elias Y. Bone biopsy instrument and method. US3850158A. 1974 Nov 26.
- Gray N. Bone biopsy needle. US5040542A. 1991 Aug 20.
- Hoffmann H-R, Matusch R. Biopsy needle for the histological examination of body tissue. WO2006081947A1. 2006 Aug 10.
- Lee CK. Tissue sampling apparatus. US2014213931A1. 2014 Jul 31.
- Madhumathi R, Larkin C, Lima F. Bone biopsy System and method. WO2021003335A1. 2021 Jan 7.
- Masseglia T, Fumex L. Perforating trocar. US2008306405A1. 2008 Dec 11.
- Turkel DH. Coaxial bone marrow biopsy and aspirating needle. WO9405210A1. 1994 Mar 17.
- Vilaghy MI, Zellerman G. Bone biopsy instrument kit. US4010737A. 1977 Mar 8.
- Zambelli R. Improved bone biopsy device. EP3326540A1. 2018 May 30.
- Andelin JB, White MT. Bone marrow biopsy needle and method for using the same. US6110128A. 2000 Aug 29.
- Contreras GDS, Stavropoulos SM. Apparatus for extracting bone marrow specimens. US4142517A. 1979 Mar 6.
- Gillespie WD, Matsuura DG, Marino JF, et al. Bone graft harvester. US6764452B1. 2004 Jul 20.
- Gordon CR, Deaton JE, Harbold C. Apparatus and method for bone harvesting. US2015025534A1. 2015 Jan 22.
- A Hirsch J, Mcintyre SH, Arramon YP. Cannula for extracting and implanting material. US2004073139A1. 2004 Apr 15.
- A Islam ABM, Bevan DR. Biopsy needle. US4543966A. 1985 Oct 1.
- Islam ABMA. Bone marrow biopsy needle. US2004249306A1. 2004 Dec 9.
- Islam ABMA. Biopsy needle. US10307142B2. 2019 Jun 4.
- Marino JF, Elbanna J. Removable bone penetrating device and methods. US10292688B2. 2019 May 21.
- Negroni C. Biopsy assembly. US2004127814A1. 2004 Jul 1.
- O’neill MJ. ‘Bone harvesting method and apparatus’. US5954671A. 1999 Sep 21
- Strasser RK, Netsch RL. Bone biopsy needle assembly. US4838282A. 1989 Jun 13.
- Swaim WR. Biopsy hand tool for capturing tissue sample. WO9722299A1. 1997 Jun 26.
- Wadhwani S, Smith G. Bone marrow biopsy, aspiration and transplant needles. US5331972A. 1994 Jul 26.
- Wiksell H, Auer G, Ekstrand V, et al. Arrangement for taking a sample of bone marrow and/or evacuating the sinuses. US2007270712A1. 2007 Nov 22.
- Zambelli R. Bone biopsy device. EP2215971A1. 2010 Aug 11.
- Ackroyd RK. Dual needle core biopsy instrument. US2016074020A1. 2016 Mar 17.
- Cortes RJA, Gallegos CS, Borbolla EJR, et al. Bone biopsy and bone marrow aspiration device. WO2009031880A1. 2009 Mar 12.
- Entrekin DA, Paris MW, Bagga CS, et al. Bone harvest System. WO2007149302A2. 2007 Dec 27.
- Globerman O, Beyar M. Integrated bone biopsy and therapy apparatus. WO2008001385A2. 2008 Jan 3.
- Goldenberg AS. Bone marrow biopsy needle. WO2009085389A2. 2009 Jul 9.
- A Krueger J, Clark GA. bone marrow biopsy needle. US6443910B1. 2002 Sep 3.
- Krueger J. ‘Bone biopsy instrument having improved sample retention’. US2003050574A1. 2003 Mar 13.
- Rubinstein A, Olah AM, Olah E. Bone marrow biopsy needle. WO9627330A1. 1996 Sep 12.
- Rubinstein AI. Bone marrow biopsy needle. WO0200109A1. 2002 Jan 3.
- Rubinstein DB, Rubinstein AI. Bone marrow biopsy needle with cutting and/or retaining device at distal end. WO9319675A1. 1993 Oct 14.
- Schofield E, Lim RK, Sherman MC. Dual outside diameter cannula for insertion into bone. WO2004000127A1. 2003 Dec 31
- Tretinyak CW. ‘Biopsy needle’. US4403617A. 1983 Sep 13.
- Ward JL. ‘Biopsy instrument’. US4785826A. 1988 Nov 22.
- Lin Q-L, Wang D-J, Lin W-G, et al. Human reliability assessment for medical devices based on failure mode and effects analysis and fuzzy linguistic theory. Saf Sci. 2014 Feb;62:248–256. doi: 10.1016/j.ssci.2013.08.022.