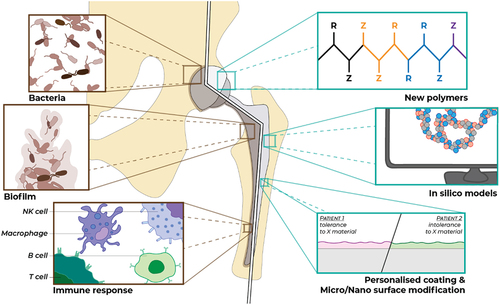
1. Introduction
The current developments in additive manufacturing, sensor technologies, and bioelectronics is leading to the development of a new generation of medical devices that can be personalized, rendered responsive to the changes in their vicinity, and can be eventually self-sustaining and fully integrable. However, there is a common barrier for all these devices to reach their full potential, the adverse immune responses, and implant-associated infections (which are also partially due to the immune-privileged conditions of the implanted devices). Although there are several technologies under development for the control of the initial innate immune response, there is still a significant gap in the temporal control of the immune responses for long-lasting devices together with the lack of precision implant surfaces optimized for the individual patients ().
2. New generation of medical implants
There is a growing convergence of technologies in the new generation of medical devices that incorporate biosensing, tissue-engineering methodologies, controlled delivery of active agents, and degradable biomaterials together with stimuli responsiveness. For example, the insertion of bioelectronic recording devices in collagen hydrogels (which are common in cell delivery and regenerative medicine applications) resulted in better tissue integration and the preservation of signal recording [Citation1]. There is a growing body of work on the incorporation of sensors for implant monitoring [Citation2] (for infection control, prevention of abrupt mechanical failure, and aseptic loosening) [Citation3]. The metamaterial research is also making its way into the medical device development, with the potential of energy-generating structures that are mechanically responding to their surroundings with self-monitoring capabilities, as demonstrated by a recent spinal fusion cage system [Citation4]. However, the additional functionalities mean devices with multiples of different materials, in some cases completely new materials, which adds to the risk of immune responses as the mounting evidence on the immune response to biomaterials in vitro, in vivo, and also clinical demonstrates that the type of the material has a strong effect on the extent and the nature of the reaction. Thus, the immune reaction needs to be controlled for such systems not only at neutrophil and macrophage level but also for adaptive immune responses (exerting control over dendritic cell maturation, antigen presentation, and T-cell differentiation, for example). The required complexity of the interaction with the immune system also resulted in an evolution of terms regarding the immune response to medical devices. The earlier terminology of ‘anti-inflammatory’ gave way to immunomodulation as simple suppression of inflammatory reaction is not sufficient for proper integration of an implanted device. This recently led to terms such as immune-instructive [Citation5] to take into account the downstream effects of the initial immunomodulation in the immune reaction cascades, from innate immunity all the way down to the exerting control over the incoming connective tissue cells such as fibroblasts. Moreover, the growing literature on the autoimmunity/inflammatory syndrome induced by adjuvants (ASIA) syndrome since its description in 2011, which is mostly related to the adjuvants in vaccines but can also be triggered by implanted materials such as silicone implants, shows that not only the local immune response to the implant but also the systemic effects of the implanted system beyond toxicity should be taken into account [Citation6].
2.1. Medical implant modifications for target organs and personalization
There are generalizable aspects of foreign body response, where the attenuation of the conditions of initial pro-inflammatory response can lead to decreased foreign body response downstream. For this end, the use of high-throughput screening methodologies resulted in the discovery of new immune-instructive topographies that can control macrophage morphology and phenotype significantly [Citation7]. This can be also achieved by other cues such as surface chemistry, mechanical properties, the presence of immunomodulatory coatings, and anti-inflammatory drug release [Citation8]. But there are also aspects that are specific to the target organ and the resident innate immunity components. The reaction to the presence of a medical device (and its material components) has both target organ-specific and patient-specific aspects. A recent study demonstrates a significant difference in immune response by RNASeq analysis between neuronal cuff implantation and a nerve injury, which can be attenuated by the inhibition of NLRP3 inflammasome activity [Citation9]. A recent study demonstrates that the foreign body response is tissue (uterine vs. subcutaneous) and species (primate vs. mice) specific with less foreign body response in uterine cavity due to its inherent immune-privileged nature [Citation10]. Another recent study demonstrates the optimal surface roughness for reduced encapsulation of breast implants (with a 4 µM surface roughness as an optimal surface) [Citation11]. Expansion of such studies will enable the better understanding of the tissue-specific immune reaction and pave the way for the selection of the optimal material type, form, and configuration for any given application beyond the basic anatomical and mechanical fit. Moreover, immunoprofiling of the patients would enable the detection of the at risk patients and appropriate modification of the implants for better clinical outcomes [Citation12]. Such risks include prior exposure to the implant biomaterial, allergies, immunogenetic predisposition [Citation13], infective disease history, exposure to other adjuvants, and susceptibility to develop autoimmune diseases.
2.2. Medical implant diagnostics
Another missing link is dedicated test systems that can enable the detection of potential adverse reactions of the patients to a given implant material and the provision of a larger scale of available materials. This would require changes in the current way of provision of implants but eventually can provide a step change in the functionality of all implant types. This will require developments in pre-implantation tests, in silico modeling-based complication prediction, discovery of new complication markers. There are current efforts in bringing the advances in single-cell analysis to the characterization of immune response to tissue-engineering scaffold [Citation14] or using advanced in vitro models for the characterization of biomaterial-induced adverse reactions such as granulomas [Citation15], further development in diagnostic systems, and in vitro models will provide the backbone for the pre-implantation diagnostics.
3. Outlook
Using new imaging technologies such as label-free tissue clearing [Citation16] and machine-learning methodologies for large-scale image analysis can significantly improve our understanding of the implant immune cell interface, which would result in better biomaterial design. The last decade has seen an explosion in the number of articles on immune cell biomaterial interactions. Harnessing this available data for the application of big data methodologies and machine learning-based in silico model development for biomaterial immune reaction correlations would also provide important insights for designing the next generation of medical devices. The advances in biorobotics and the use of biorobotic systems as medical devices (such as the recent microalgae-based autonomous systems for wound healing [Citation17]) will also pose a new set of challenges in immune response to medical devices but with new opportunities also for dynamic delivery systems with mobility in vivo. This will be aided by the recent developments in the humanized mice models, which will enable the monitoring of such devices in controlled animal models that can represent the foreign body reaction in humans very faithfully [Citation18].
Declaration of interests
NE Vrana is CEO and stockholder of SPARTHA Medical that develops coatings for medical devices. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Boys AJ, Carnicer‐Lombarte A, Güemes‐Gonzalez A, et al. 3D bioelectronics with a remodellable matrix for long‐term tissue integration and recording. Adv Mater. 2023;35(8):2207847. doi: 10.1002/adma.202207847
- Kubon M, Hartmann H, Moschallski M, et al. Multimodal chemosensor‐based, real‐time biomaterial/cell interface monitoring. Adv Biosyst. 2018;2(6):1700236. doi: 10.1002/adbi.201700236
- Veletic M, Apu EH, Simic M, et al. Implants with sensing capabilities. Chem Rev. 2022;122(21):16329–16363. doi: 10.1021/acs.chemrev.2c00005
- Barri K, Zhang Q, Swink I, et al. Patient‐specific self‐powered metamaterial implants for detecting bone healing progress. Adv Funct Mater. 2022;32(32):2203533. doi: 10.1002/adfm.202203533
- Fisher LE, Kämmerling L, Alexander MR, et al. Immune-instructive materials as new tools for immunotherapy. Curr Opin Biotechnol. 2022;74:194–203.
- Tervaert JWC, Martinez-Lavin M, Jara LJ, et al. Autoimmune/Inflammatory syndrome induced by adjuvants (ASIA) in 2023. Autoimmun Rev. 2023; 22(5):103287. doi: 10.1016/j.autrev.2023.103287
- Vassey MJ, Figueredo GP, Scurr DJ, et al. Immune modulation by design: using topography to control human monocyte attachment and macrophage differentiation. Adv Sci. 2020;7(11):1903392. doi: 10.1002/advs.201903392
- Li J, Jiang X, Li H, Gelinsky M, et al. Tailoring materials for modulation of macrophage fate. Adv Mater. 2021;33(12):2004172. doi: 10.1002/adma.202004172
- Barone DG, Carnicer-Lombarte A, Tourlomousis P, et al. Prevention of the foreign body response to implantable medical devices by inflammasome inhibition. Proc Natl Acad Sci, USA. 2022;119(12):e2115857119. doi: 10.1073/pnas.2115857119
- Hernandez JL, Park J, Yao S, et al. Effect of tissue microenvironment on fibrous capsule formation to biomaterial-coated implants. Biomaterials. 2021;273:120806. doi: 10.1016/j.biomaterials.2021.120806
- Doloff JC, Veiseh O, de Mezerville R, et al. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nat Biomed Eng. 2021;5(10):1115–1130. doi: 10.1038/s41551-021-00739-4
- Vrana NE, Palm K, Lavalle P. Personalization of medical device interfaces: decreasing implant-related complications by modular coatings and immunoprofiling. Future Sci OA. 2020 Jul 30;6(8):FSO607. doi: 10.2144/fsoa-2020-0074
- Borba V, Malkova A, Basantsova N, et al. Classical examples of the concept of the ASIA syndrome. Biomolecules. 2020;10(10):1436. doi: 10.3390/biom10101436
- Bian N, Chu C, Rung S, et al. Immunomodulatory biomaterials and emerging analytical techniques for probing the immune micro-environment. Tissue Eng Regen Med. 2023;20(1):11–24. doi: 10.1007/s13770-022-00491-z
- Antmen E, Muller CB, Calligaro C, et al. In vitro two-step granuloma formation model for testing innate immune response to implants and coatings. Biomater Sci. 2022;138:212872. doi: 10.1016/j.bioadv.2022.212872
- Ngo TB, DeStefano S, Liu J, et al. Label‐free cleared tissue microscopy and machine learning for 3D histopathology of biomaterial implants. J Biomed Mater Res Part A. 2023; 111(6):840–850. doi: 10.1002/jbm.a.37515
- Choi H, Kim B, Jeong SH, et al. Microalgae‐based biohybrid microrobot for accelerated diabetic wound healing. Small. 2023;19(1):2204617. doi: 10.1002/smll.202204617
- Doloff JC, Ma M, Sadraei A, et al. Identification of a humanized mouse model for functional testing of immune-mediated biomaterial foreign body response. Sci Adv. 2023;9(24):eade9488. doi: 10.1126/sciadv.ade9488