ABSTRACT
Introduction
Medical devices play a crucial role in healthcare, addressing the diagnosis, treatment, and monitoring of various medical conditions. This study conducts a comprehensive analysis of medical device regulations across nations, considering the economic contexts of diverse countries.
Areas Covered
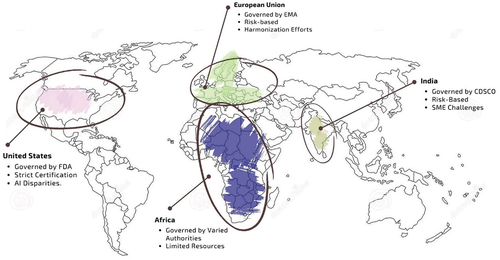
The research involves a comparative examination of medical device regulations, dissecting unique frameworks in countries like the United States (US), European Union (EU), India, and Africa. These nations were chosen based on economic significance, market influence, and regulatory structures. The study aims to achieve a nuanced understanding of global medical device regulation, develop strategies to enhance guidelines, especially in developing nations, and provide recommendations for improvements in relevant regions.
Expert Opinion
Through this study, valuable insights are gained into the diverse regulatory frameworks governing medical devices globally. The analysis identifies areas within these frameworks that require improvement, as well as strategies to enhance regulatory guidelines, particularly addressing the specific needs of developing economies. Ultimately, the research provides significant recommendations for policymakers and industry stakeholders. By offering a deeper understanding of regulatory intricacies, this study establishes pragmatic approaches to address challenges within the medical device industry and improve the regulatory landscape on a global scale.
1. Introduction
Medical devices are any equipment, machinery, implants, or other similar items used to diagnose, treat, or prevent illness or other body problems in humans. These devices might range from basic equipment as thermometers and tongue depressors to sophisticated as pacemakers, Magnetic Resonance Imaging (MRI) machines, and robotic surgical systems. The World Health Organization (WHO) defines a medical device as any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use, software, material, or another similar or related article, intended by the manufacturer to be used, alone or in combination for a medical purpose [Citation1].
Instruments has been used to address medical issues throughout history. The complexity and sophistication of medical equipment have increased from the time of the first stone blades and saws. With new tools made of bronze, the Greeks and Romans established the patterns for contemporary surgical instruments. Spectacles were created in the Middle Ages to improve vision. In the 20th century, lasers were used for surgery, kidney stone treatment, ophthalmoscopy, and cosmetic skin treatments. Electronic devices were also developed to monitor fetal heart rate. Computer technology is being employed to do robotic surgery, manage medical records, and operate tools [Citation2].
The medical device from Johnson & Johnson was one of the case-study on safety concern of medical device regulation that was observed on a broader scale. DePuy Orthopaedics, a division of Johnson & Johnson. The company voluntarily recalled its ASRTM XL Acetabular System and DePuy ASR Hip Resurfacing System product lines in 2010 as the implants had severe side effects, including pain, swelling, and trouble walking. These symptoms were brought on by flaws in the product’s design, such as loose connections to the bone, dislocation, and fracture, which led to injury to the tissues around them. In the first five years following implantation, many patients needed revision surgery, according to data from the National Joint Registry of England and Wales. DePuy sold the devices despite the dangers, which resulted in numerous product related lawsuits [Citation3,Citation4]. Such instance highlights the value of medical device regulation to protect patients’ safety and welfare. It emphasizes the requirement for manufacturers to design and rigorously test and assess the effectiveness of their medical devices.
In order to create and harmonize the international standards of medical devices, the GHTF was framed. The stakeholders include global medical device manufacturers, trade organizations, and regulatory agencies. GHTF, significantly contributed to the unification of regulatory framework to facilitate commercialization of medical devices. The IMRDF, which carries on the GHTF’s mission to further worldwide harmonization of medical device regulation, took the place of the GHTF in 2012 when it was disbanded. The main objective of GHTF was the harmonization of regulations. For international trade and to ensure the safety and effectiveness of products, particularly in sectors like healthcare, regulatory harmonization is essential. Trade barriers are decreased and businesses can more easily access global markets by harmonizing and standardizing legislation across various nations and regions.
To attain better uniformity among various national medical device regulatory systems, the GHTF was created in 1992. The two goals were to improve patient safety and broaden global access to secure, efficient, and clinically useful medical technologies. A voluntary organization of members from national medical device regulatory agencies and the regulated industry made up the GHTF. Since its formation, GHTF has composed delegates from the five primary members, divided under three geographical regions: North America, Asia-Pacific, and Europe [Citation5].
The primary means of harmonizing the requirements for establishing safety and efficacy is accomplished by implementing guidance documents on fundamental regulatory practices of medical devices. The goal of the GHTF was to encourage convergence in regulatory procedures related to encouraging technological innovation and facilitating international trade. The GHTF’s work is continued by the IMDRF [Citation6].
Medical devices are divided into four classes by the GHTF according to their intended usage and possible patient dangers. Notably, Class I devices pose the least amount of risk to customers because they are deemed low risk and governed by general standards. A moderate level of risk for Class II devices attracts more stringent regulatory scrutiny and may need compliance with particular performance standards or labeling requirements. Class III devices are considered high-risk and are subjected to extensive pre-market assessments. They frequently have implanted or invasive features or provide life-sustaining activities. Class IV, the highest risk category, including life-supporting devices, pacemakers, implants, and other cutting-edge technologies essential to public health. This categorization scheme offers a starting point for rigorous assessment processes and regulatory controls.
The Medical Device Regulation, which replaced the Medical Device Directive (MDD) in the European Union, has updated the classifications and placed more emphasis on potential injury. As an example, Class IIa and IIb devices are classified as medium-risk and medium/high risk, respectively, but Class I devices are now regarded as low-risk. The new medical device regulation still places a considerable risk on Class III devices. These classification systems aids regulatory organizations in ensuring that they go through the appropriate testing and evaluation to ensure their safety and efficacy [Citation7].
In the meantime, the Food and Drug Administration (FDA) in the United States uses a three-tier system of categorization based on risk levels. Devices classified as Class I present the least risk, Class II present a moderate risk, and Class III present the greatest risk [Citation8]. The FDA’s entire approach to ensuring the safety and efficacy of medical devices is guided by this categorization, which includes premarket assessment, postmarket surveillance, and compliance initiatives [Citation9,Citation10].
Manufacturers, healthcare professionals, patients, regulatory bodies, and organizations that advocate for patients are some of the stakeholders in the US medical device business. To ensure the security and efficacy of medical devices, stakeholders must work together and communicate. It can be challenging to release medical equipment onto the market, particularly implantable devices that are categorized as Class III by the FDA. In contrast to prior generations of medical devices that were launched without much testing, the current generation of implantable medical devices requires extensive technological and clinical assessment. The FDA, as well as other regulatory bodies have stringent controls on medical devices, particularly those with a higher risk. A Class III medical equipment must undergo extensive testing before it may be used in clinical settings, including both technical and clinical testing. Cardiovascular devices like pacemakers and cardioverter-defibrillators have prevented millions of deaths and given rise to industry behemoths [Citation11].
The global market for medical devices is anticipated to increase at a compound annual growth rate (CAGR) of 5.5% between 2022 and 2029, rising from $495.46 billion in 2022 to $718.92 billion in 2029 [Citation12]. In India, the market for medical devices is anticipated to reach US$ 12 billion by 2020 and increase at a CAGR of 15%, which is 2.5 times the rate of worldwide growth. India ranks in the top 20 medical device markets worldwide and is the fourth-largest market for medical devices in Asia after Japan, China, and South Korea [Citation13]. With a CAGR of 7.10% from 2023 to 2030, the medical devices industry in South Africa is expected to grow significantly during this time [Citation14,Citation15].
Since economies, healthcare systems, and regulatory frameworks vary significantly between nations and regions, so do the regulatory circumstances for medical devices. Although different regulatory environments can make it difficult for manufacturers to operate in different markets, it’s crucial to remember that laws exist to guarantee patient safety and device effectiveness. The regulatory process can be streamlined, and patient access to high-quality medical devices can be improved by harmonizing regulatory rules and standards across various regions and nations.
Developing and regulating medical devices is a crucial component of healthcare systems worldwide. The regulatory environment for medical devices has grown more complex due to the development of new technology and advances in medical science. With the growing economy of medical devices, new medical devices are constantly being developed and introduced into the market. Engineers and medical professionals have created a wearable ultrasound device that can evaluate the anatomy and operation of the human heart. The tiny, postage-stamp-sized device can be worn for up to 24 hours, and monitors the activities constantly. The wearable heart monitoring device employs ultrasound to continually take images of the heart’s four chambers from various angles. It then uses a specially developed AI algorithm to analyze a subset of the clinically pertinent photos in real time. Continuous and real-time cardiac imaging monitoring has the potential to radically improve and redefine the paradigm of cardiac diagnostics by giving patients and medical professionals more detailed information [Citation15].
The purpose of this study is to present a review of the legal frameworks governing medical devices in several nations, including Europe, the US, India, and Africa. The criterion to choose these nations are based on their economies, market shares, and regulatory environments. The paper’s primary objectives are 1) to comprehend the regulatory framework for medical devices across different nations based on the size of their economies, such as US, EU, India and Africa, 2) to analyze the current scenario of medical device regulation based on case studies on medical devices, and 3) recommend strategies for improving the regulatory guidelines of medical devices in developing countries. This study compares various practices in nations to regulate medical devices and illuminates on the similarities and difference. The study aims to understand approaches for potential developments in medical devices. The recommendations made in the paper are intended to assist developing nations in strengthening their regulatory environment and promoting the expansion of their medical device industries based on the practices in the developed nations.
2. Literature review
This study’s focus regions, which were chosen with careful and thorough consideration, are the US, the EU, India, and Africa. These areas exhibit various economic development trajectories and have unique healthcare environments. Developed regions like the US and the EU have established and strict regulatory regimes for medical devices. Their regulatory frameworks act as standards for the world’s medical equipment market. On the other hand, India, a developing country, offers a distinctive situation in which a regulatory framework is emerging to keep up with its expanding medical device sector. This change provides insightful information on the potential and regulatory obstacles that developing nations must navigate.
2.1. Policies implemented in medical devices regulation in the US
The regulatory affairs field, also called government affairs, deals with the contacts between manufacturers of pharmaceuticals and medical devices and the regulatory bodies of governments. The Food and Drug Administration is the principal government body overseeing these businesses in the US FDA as shown in . Companies that produce, repackage, relabel, and/or import medical devices sold in the US are subject to regulation by the FDA’s Centre for Devices and Radiological Health (CDRH). Several variables, such as technological advancements, governmental policy shifts, and the regulatory environment’s complexity, have influenced the development of regulatory affairs in the US.
Table 1. Comparison of medical device regulations in the US & EU, India and Africa.
The PMA (premarket approval) method was founded in 1976. Like new medication applications, the PMA procedure necessitates thorough testing to guarantee a device’s safety and efficacy for its intended use, especially for devices that are essential to maintaining human life or preventing health harm. But in 1976, because there were so many devices already on the market, a different, less stringent process known as the 510(k) provision was created to make it easier for subsequent versions to be sold [Citation16].
Under the 21st Century Cures Act, the FDA’s De Novo pathway reviews medical devices for which general and special controls guarantee safety and efficacy, even in the absence of comparable products that are lawfully sold. The FDA faces difficulties in establishing special controls for De Novo devices, which may involve performance standards, because it is difficult to determine safety and efficacy in the absence of comprehensive field testing. According to a recent regulatory guidance, De Novo applicants are now required to propose the special controls for their devices. This could lead to an anticompetitive patent strategy whereby core technological features and performance standards are patented, making it more difficult for subsequent developers to enter the market and raising issues for a variety of medical devices [Citation17].
With the growing need for more efficient and accurate diagnosis, there has been an increased incidence in the use of artificial intelligence (AI) in medical devices. The USFDA has released action plan on the use of AI in medical devices in response to stakeholder feedback to the April 2019 discussion paper, outlining how the organization will assess medical devices with AI capabilities based on their intended purpose, the importance of the information supplied, and the risk to patients [Citation18].
2.2. Policies implemented in medical devices regulation in the EU
The regulation of medical devices in the EU is overseen by European Commission with help from key entities such as the European Medicines Agency (EMA) and the Medical Device Coordination Group (MDCG). These regulatory bodies play pivotal roles in ensuring the compliance and uniform application of medical device regulations throughout EU member states.
The EMA and the MDCG, which are in charge of overseeing the regulation of medicines and medical devices, respectively, are in order of regulatory affairs in the EU and help in the publishing of EU medical device regulation [Citation19,Citation20]. The MDCG is a professional organization created in accordance with EU legislation to offer advice and guarantee uniform application of medical device regulations throughout the member states of the EU. In many recent papers the need to reevaluate the processes for regulatory approval of medical devices in Europe and the US even after present regulatory laws for medical devices has been discussed [Citation21–23].
In the European Union, the classification of medical devices is a vital process governed by a risk-based system that considers factors such as potential risks and intended use. Criteria including duration of body contact, invasiveness, potential toxicity, and energy source reliance are utilized to categorize devices. Regardless of class, all devices must comply with relevant obligations under the Medical Device Regulation (MDR), though specific requirements may vary. In the European Union, medical devices are categorized into the following classes: Class I, Class IIa, Class IIb, and Class III. Each class represents varying levels of risk, with Class I devices posing the lowest risk and Class III devices posing the highest risk to patients and users. The classification process is guided by Annex VIII to the MDR, which provides specific rules for determining the classification of devices based on their intended use and inherent risks.
The procedure for approving medical devices in the EU is based on conformity assessment, which comprises determining whether the device complies with all relevant laws and standards. For certain goods marketed within the European Economic Area (EEA), notably medical equipment, the ‘Conformité Européenne’ (CE) marking is a requirement for conformity. It certifies that the product conforms with all applicable EU laws and standards, allowing for its sale and marketing within the EEA. Medical device manufacturers adhere to the requirements to attain CE marking because it demonstrates that their products adhere to all relevant laws and standards, enabling them to freely promote and sell their products inside the EEA. The Notified Body conducts testing, audits, and technical documentation reviews as part of the conformity assessment procedure for CE marking.
The updated EU regulatory framework for medical devices, including in vitro diagnostic (IVD) medical devices, was released in 2017 and went into effect in May 2021. The new laws mandate further stringent certification standards for IVD tests, including lab-developed tests (LDTs), crucial in tertiary-care settings and technological advancement [Citation24]. Furthermore, the significance of drug-device combination products in medical product development is continuously increasing. However, challenges are present [Citation25].
A major difficulty encountered by small and medium-sized firms (SMEs) is illuminated by the EU policy subsection’s analysis of regulatory rules on the design and development of medical devices in the Czech Republic and other countries. In particular, the significant administrative expenses associated with implementing new medical device regulations are noted as a cause of concern for SMEs. This emphasizes how EU policies must take into account nuances in order to effectively handle the effects on enterprises of different sizes [Citation26].
The International Coalition of Medicines Regulatory Authorities (ICMRA), a division of the European Medicines Agency (EMA), offers suggestions to assist regulators in addressing the problems that the application of artificial intelligence (AI) presents for international drug regulation. There are a lot of regulatory disparities between the EU and the U.S.A. for AI-based medical devices, including the risk-based classification of medical devices, certification procedures, and the consumer’s responsibility for confirming the efficacy and integrity of the AI device. Following the release of the EU AI Act, it has become increasingly important for regulators to consider these discrepancies and align regulations to ensure the safe and effective use of AI in medical devices [Citation18,Citation27,Citation28].
With difficulties like device-operator interaction and incremental innovation in mind, the MedtecHTA project was introduced in four European countries: France, Italy, Spain, and the United Kingdom and sought to enhance health technology assessment (HTA) methodologies for evaluating medical devices. The project includes work packages on regulatory and HTA techniques, geographic diversity in medical device availability, analytical frameworks for comparative effectiveness and economic evaluation, and organizational impact [Citation29].
The In-Vitro Diagnostic Medical Devices Regulation application was partially postponed in the European Union due to the heavy burden of the COVID-19 epidemic. The phased implementation method offers a chance to get the necessary regulatory frameworks ready in all EU member states to implement In Vitro Diagnostic Regulation (IVDR) effectively [Citation30]. A new European regulatory framework for In-vitro Diagnostic Medical Devices (IVDs) called the In Vitro Diagnostics Regulation 2017/746 (IVDR) was also taken into effect in May 2022. It sought to seek companion diagnostics (CD-x), which are biomarker examinations connected to pharmaceuticals [Citation31].
There has been a rising emphasis on custom manufactured devices in order to provide medical equipment for patients who cannot use ordinary off-the-shelf equipment. Custom-made medical devices are created to fit the unique requirements and anatomy of each patient, which can improve outcomes and lower risk of complications. There have been many studies looking into medical device laws for custom made devices [Citation32,Citation33]. The new medical device regulation, which was implemented in response to controversies and injuries involving authorized medical equipment, has increased the clinical safety of medical devices and human-implant technologies offered in Europe while enhancing the credibility and reputation of the regulatory framework [Citation34,Citation35]. Additionally, the lack of financial and legal support for the development of orphan devices has been noted, highlighting the requirement for a favorable regulatory environment for orphan devices in Europe [Citation36].
The new Medical Device Regulation in the EU mandates that therapeutic benefits must be included as endpoints and establishes stringent standards for assuring accurate data from clinical trials. Manufacturers can improve claims of expected therapeutic benefits in later clinical studies by using real-world clinical data from the postmarket clinical follow-up to understand the clinical benefits of their products better [Citation37,Citation38].
2.3. Scenario in India
The regulatory system for medical devices in India is still developing. The Central Drugs Standard Control Organisation (CDSCO) is the regulatory body overseeing the regulation of medical devices in India. The regulation of medical devices in India is governed by the Ministry of Health and Family Welfare, which classifies medical devices into four categories based on their risk levels. The lowest risk is posed by Class A devices, whereas the highest risk is posed by Class D devices. The classification is based on various parameters such as of contact time with the body, how intrusive it is, and the risk of injury. Surgical equipment, wheelchairs, and bandages are examples of Class A devices. Needles, syringes, and catheters are examples of Class B devices. Orthopedic implants, implanted pacemakers, and cardiac stents are examples of Class C devices. Drug-eluting stents, implanted defibrillators, and prosthetic heart valves are examples of class D devices. The classification scheme aids in making certain that the regulatory safeguards are in place to protect patient safety and advance public health [Citation39].
The classification in India follows the GHTF framework and is implemented through a notification issued on 1 November 2017 as according to the File No: 29/Misc.l3/2017-DC(292) under the subject Classification of medical devices and IVD medical devices under the provisions of the Medical Devices Rules, 2017. The medical device industry in India is valued at USD 5.2 billion, contributing 4%–5% to the USD 96.7 billion Indian healthcare industry, with 750–800 medical device manufacturers in the country [Citation40]. Medical device regulation in India entails submitting a product registration application to the CDSCO, where it is evaluated for quality, efficacy, and safety [Citation41].
While pharmaceuticals are being produced domestically in India, 75% of medical equipment are still imported. Due to increased population, healthcare demands have increased during the past ten years. The Indian government has announced the Medical Devices Rules, 2016, for regulating the production, import, sale, clinical investigation, and other associated issues relating to medical devices to support the growth of the domestic medical device industry [Citation42].
In India, for the approval of medical devices, the CDSCO must receive an application for a medical device along with pertinent information about the product’s safety and effectiveness. The CDSCO assesses the application and decides on authorizing to be marketed. The device is given permission and is allowed to be marketed and sold in India if it complies with the necessary norms and requirements. The Medical Device Rules, 2017, which aim to enhance the regulatory framework and streamline the approval process, have recently changed how medical devices are approved in India.
In India, the regulation of medical devices also includes the implementation of materi-o-vigilance to control unfavorable consequences brought on by these devices. Materi-o-vigilance entails observing and tracking incidents brought on by medical technology [Citation43]. The Materi-o-vigilance Programme of India (Mv-PI) was established in 2015 to generate safety data, develop awareness among stakeholders, and recommend best practices for patient safety. It is still a debatable topic if India’s regulatory system is successful in terms of its capacity to keep up with changes in the global medical device regulation environment [Citation44].
The Biomedical Metrology Group of the CSIR-NPL (Council of Scientific and Industrial Research – National Physical Laboratory) is in charge of developing the National Biomedical Equipment Standards. The CSIR-NPL offers traceable biomedical equipment calibration services, enabling medical equipment quality control and substantially impacting the nation’s health policy and economy [Citation45].
There exists a potential gap in medical device Intellectual Property (IP) filings in India. Patent applications filed in medical devices contributes to 2% of the total patent applications filed. It has been established that foreign companies predominate the Indian medical device market, with most patent applications coming from the US [Citation46].
The CDSCO introduced its ‘Sugam Portal’ online licensing portal for medical device registration in November 2015 [Citation40]. In India, medical device laws have improved over the past 20 years due to the growth in the quantity, variety, and complexity of medical devices. It is believed that national medical device regulation harmonization is required to lower regulatory barriers and offer timely access to safe and effective medical equipment [Citation47,Citation48].
The Indian healthcare industry is expanding quickly, and by 2025, it is predicted that the market for medical equipment will be worth $50 billion. These Medical Device rules (2018), updated in 2020, address some regulations pertaining to devices, including categorization, registration, manufacturing and import, labeling, sales, and post-market procedures [Citation49].
2.4. Scenario in Africa
The regulations surrounding medical devices in Africa are complex and rapidly evolving, with inconsistencies and lack of clarity among various nations’ regulatory organizations. Although many nations have established regulatory bodies, there is a lack of uniformity in how they regulate medical devices. Despite these challenges, African medical device regulations share similarities with European directives, albeit with European regulations being more stringent. Some African countries have recognized the importance of regulatory control and have adopted or harmonized directives. Open design strategies, such as Open-Source Medical Devices, offer potential cost reduction but require strict supervision to ensure adherence to quality and safety standards. Furthermore, resource gaps among regulators can result in differences in the standard and scope of regulatory control. Despite these obstacles, the demand for medical devices in Africa is increasing, emphasizing the need for more efficient and unified medical device regulation to prioritize patient safety [Citation50].
In South Africa, medical device regulation has undergone significant changes through the Medicines and Related Substances Amendment Act 14 of 2015, establishing the SA Health Products Regulatory Authority as the regulatory body and prohibiting bonusing and sampling for the sale of medical devices [Citation51]. However, only half of the 14 nations within the College of Surgeons of East, Central, and Southern Africa have formal regulatory frameworks, indicating the ongoing development of medical device regulation in Africa [Citation52]. The South African medical device market faces challenges that hinder the provision of high-quality healthcare, including regulatory structure issues, a multi-channel system for purchasing medical devices, and a shortage of trained personnel. Despite the projected worth of the medical device market in South Africa, only a limited number of devices meet specific quality requirements, leading to the importation of subpar goods. Solid and effective national regulatory systems for medical products are crucial for achieving Universal Health Coverage and the Sustainable Development Goals in the Southern African Development Community (SADC) member states. While some National Regulatory Authorities for Medical Products (NMRAs) in the SADC region operate autonomously or semi-autonomously, the majority are part of the Ministries of Health. Inconsistencies in the regulation of traditional medications, biologicals, and medical devices across the region are observed despite the presence of legal frameworks for medical devices [Citation53,Citation54].
The construction of a reliable regulatory framework for medical devices is essential to ensure the quality, safety, and efficacy of medical products. The WHO developed the Global Benchmarking Tool (GBT) to enable national regulatory authorities (NRAs) to assess the effectiveness of their institutional development plans and benchmark their regulatory frameworks [Citation54,Citation55]. However, there is a lack of regulation in certain areas, particularly within the East African Community (EAC) Partner States, requiring increased access to medical devices and IVD testing across Africa. Some nations rely on laboratory-based organizations to ensure the quality of medical devices due to the limited oversight capabilities of National Regulatory Agencies for Pharmaceutical Products [Citation56].
Before 2015, South Africa lacked a comprehensive regulatory system for medical equipment, except for electro-medical devices governed by Radiation Control. The Pharmaceuticals and Related Substances Amendment Act of 2015 marked a significant step in the regulation of medical devices in South Africa [Citation57]. However, the country has faced challenges in medical device regulation, resulting in backlogs and increased lead times for product registration. The establishment of the South African Health Products Regulatory Authority (SAHPRA) as a replacement for the Medicines Control Council (MCC) through the 1965 Medicines and Related Substance Act aimed to address these challenges. To improve the regulatory process, recommendations have been made to SAHPRA, including the establishment of a quality management system, tracking regulatory performance, and adopting a risk-based approach for evaluating medical products. These initiatives not only prioritize patient safety but also seek to enhance regulatory process accountability, consistency, and transparency [Citation58].
In Africa, there is a complicated and changing environment for medical device regulation. While some nations have progressed in implementing regulatory frameworks and harmonizing with European regulations, regulatory control in Africa still has inconsistencies and inadequacies. These difficulties are a result of resource shortages among regulators and the absence of a uniform regulatory body for medical devices across the continent. However, initiatives are being made to overcome these problems through the creation of trustworthy regulatory frameworks, benchmarking tools, and regulatory organizations like SAHPRA in South Africa. To fulfill the rising demand for medical devices while assuring their quality, safety, and efficacy, it is essential to put patient safety first and work toward more effective and uniform medical device regulation throughout Africa.
3. Case studies pertaining to medical device
For the field of medical device regulation, the analysis of case studies involving negligent use of medical devices is extremely important. These case studies offer insightful information about the difficulties and flaws of the regulatory process. Regulators are able to pinpoint weaknesses in the systems for product approval, production, and post-market surveillance by looking at the underlying reasons of neglect and failures. With this information, they can improve patient safety, fill up any gaps in legislation, and refine regulations. Case studies also make it easier for manufacturers, patients, and healthcare experts to share information and evaluate the efficacy of regulatory measures.
Within the field of medical device safety, a study explores the nuances surrounding laparoscopic trocars, which are essential instruments for entering the abdominal cavity during surgical procedures. The study sheds light on 31 fatal and 1353 nonfatal instances related to these devices using data from the Manufacturer and User Facility Device Experience (MAUDE) database maintained by the US FDA between 1 January 1997, and 30 June 2002. The analysis recognizes the shortcomings of the MAUDE database while highlighting the significance of thorough adverse event reporting [Citation59].
The Mitra-Clip® Delivery System case is an example of medical device malfunction. It is a medical equipment used to treat mitral regurgitation, a condition in which every time the heart beats, blood flows backwards through the mitral valve. The mitral valve’s two flaps will be clipped together by the device, which will reduce the amount of blood that seeps backwards. The Mitra-Clip® Delivery System has been successfully used multiple times and has passed all quality testing mandated by European legislation. It has been demonstrated that a comprehensive examination of the mitral valve could be carried out with complete safety, a low rate of morbidity and death, and acceptable follow-up. However, there have been instances where the device has malfunctioned due to a clip detachment failure, which has caused severe comorbidities and problems, including patient death. This caused for recall of the Mitra-Clip® Delivery System, which was produced between July 14 and 11 August 2015, and distributed between August 28 and 3 February 2016 [Citation60].
4. Methodology
The objectives of the study were the following:
To present a comparative review of the medical device regulatory environments in several nations, including Europe, the U.S.A., India, and Africa as shown in .
To comprehend approaches for potential developments in medical devices, which will help industry experts and policymakers encourage the creation of cutting-edge, novel medical innovations.
We conducted a thorough literature analysis on the regulatory frameworks for medical devices in Europe, the U.S.A., India, and Africa. This entailed looking through electronic databases like PubMed, Google Scholar, and Scopus, as well as websites of regulatory organizations and sources from the gray literature. Furthermore, we compared the regulatory frameworks for medical devices in the chosen countries by analyzing and synthesizing the data from the literature review.
The case studies involving medical devices were analyzed in each selected nation. The case studies were selected based on their relevance to the review article’s objectives and the data availability. The analysis focused on the challenges faced in regulating medical devices and the effectiveness of the regulatory response.
Then we offered suggestions for developing nations to strengthen their regulatory environment based on the case studies and comparative review analysis. The recommendations aimed to improve the regulatory frameworks, streamline the approval processes, and enhance post-market surveillance and enforcement mechanisms.
An overview of the regulatory landscape for medical devices in four regions: Europe, U.S.A., India, and Africa is shown in the . It covers regulatory frameworks, certification standards, challenges faced in regulating medical devices, recommendations for improving regulatory frameworks, and case studies pertaining to the medical devices [Citation3,Citation4].
5. Results
There are many different methods and difficulties in the regulatory environments for medical devices in Europe, the U.S.A., India, and Africa. Although the strong CE marking system in Europe places a focus on quality and safety, there are worries about safety gaps because of the emphasis on unrestricted device circulation. Although the FDA-regulated 510(k) process, PMA, De Novo process and stringent post-market surveillance in the U.S.A. work to ensure device safety, there are still disparities between US and EU regulations for AI-based devices. India is navigating an evolving regulatory structure, with the Materi-o-vigilance Program tracking adverse occurrences and the CDSCO managing risk-based categorization. Medical device quality control in Africa is hampered by resource shortages and regulatory inequalities between countries.
Case examples highlight the value of looking into equipment problems. In response, regulators have tightened reporting and safety regulations. However, difficulties persist in both Europe and the U.S.A., highlighting the demand for continual regulatory improvements. In order to encourage the development of novel medical devices, the proposals made in this article emphasize strengthening regulatory frameworks, expediting the approval procedure, and improving post-market surveillance.
6. Discussion
6.1. Comparison of medical device regulation in the US, EU, Africa and India
The US and the EU have stringent regulations governing the approval of medical devices. The Centre for Devices and Radiological Health (CDRH) under USFDA is the body overseeing the activities of medical device. In contrast, in the EU European Commission is the main regulatory body with other bodies like the EMA and the European Medical Devices Agency (MDCG) who act as reference entities and help the European Commission to manage the regulations for medicines and medical devices, respectively.
The CDSCO, the regulatory organization in charge of regulating the regulation of medical devices, has a well-established regulatory system for medical devices in India. The Ministry of Health and Family Welfare introduced the categorization through a notification. India’s medical device market is worth USD 5.2 billion, and 750–800 medical device makers are there. A product registration application for a medical device must be submitted to the CDSCO in India, where it is assessed for quality, efficacy, and safety.
The regulation of medical devices in Africa varies greatly from country to country, and many need to be established regulatory frameworks. A separate regulatory body regulates medical devices in some nations, while the Ministry of Health does it in others. The African Union has created a model regulatory framework for medical devices. However, it has yet to be widely used. Furthermore, many African nations need more infrastructure and resources to regulate medical equipment adequately.
The US and EU have more established and strict medical device regulations than India and Africa. India and Africa have developing regulatory systems and less stringent regulations for medical device producers, unlike the US and EU, which have well-established regulatory organizations and a risk-based approach to regulation. All regions strive to improve their regulatory frameworks to maintain patient safety and foster innovation in the medical device sector.
Understanding the legal requirements and approval procedures for medical devices in various locations requires comparison of the medical device regulations in the US, EU, India, and Africa as shown in . Their diverse regulatory regimes are significantly shaped by the economies of these nations. While India and Africa are still developing their regulatory systems, the US and EU have well-established regulatory bodies.
The regulatory environment for medical devices in the US and EU, India, and Africa are outlined in along with a general summary. Through the following table we are focusing on different regulatory agencies in different countries. For instance, EMA is one of the agencies in Europe whose role is to the safety and performance of a medical device [Citation62]. Another one we can take into example is MDCG which may not directly provide laws but helps in providing guidelines for the governing of medical devices.
6.2. Recommendation of strategies to improve medical device regulations in the developing nations
Regulations for medical devices are essential to ensure their effectiveness and safety. For medical devices to be safe, efficient, and available to everyone, laws need to be improved in least developed nations like Africa and developing nations like India. Here are some suggestions for enhancing medical device laws in developing countries:
Raising awareness and education: Campaigns should target patients, manufacturers, and healthcare professionals to increase awareness and educate the public about the importance of medical device laws. Regulatory entities, governmental organizations, business associations, and healthcare organizations can organize these campaigns. Such campaigns’ substance ought to stress the value of regulations in preserving the public’s trust, protecting patient safety, and encouraging innovation.
Strengthen regulatory bodies: Regulatory bodies must be strengthened by giving them the resources and knowledge they need in order to better regulate medical devices. This entails increasing the workforce, providing in-depth training programs, and upgrading infrastructure.
Promote innovation: Innovation is essential for sophisticated medical equipment. Incentives for R&D, including tax breaks or grants, should be offered by regulatory bodies to promote innovation.
Updating Pharmaceutical Regulations: Growing use of AI and machine learning is making it clear that regulatory frameworks need to be revised to keep up with technological advancements in the medical device business. AI and machine learning are being used to improve post-market surveillance, optimize clinical trials, boost device safety monitoring, and further research and development.
Create a comprehensive medical device database for developing countries so that medical equipment may be easily tracked and monitored, ensuring that only trustworthy and safe devices are being used.
Offer rewards for compliance: Developing countries should offer rewards to manufacturers who abide by legal requirements. Tax advantages, accelerated approval procedures, and fee reductions are a few examples of these incentives.
6.3. Comprehend approaches for potential developments in medical devices
Several potential strategies for promoting the creation of innovative medical technologies. They are as follows:
Creating regulatory sandboxes: Regulatory sandboxes are controlled environments that let innovators test their goods in actual contexts without having to worry about fully complying with regulations. The creation of such sandboxes for medical devices can facilitate quicker innovation and speed up the development process.
Offering tax incentives: Governments have the option of providing tax breaks to businesses who produce cutting-edge medical equipment. These incentives can take the form of tax reductions for businesses that invest in start-up medical device companies, accelerated depreciation schedules for equipment used in research, and tax credits for research and development. One notable example of how fiscal policies can spur breakthroughs in healthcare technology is Switzerland, which offers R&D tax exemption to encourage innovation in the medical device sector [Citation63].
Promoting public-private partnerships: To develop and market novel medical devices, public-private partnerships can pool the resources and knowledge of the public and private sectors. Government financing, subsidies, and tax incentives can help to foster these partnerships, which can speed up the development process while ensuring that new technologies fulfill public health needs. Pharmaceutical companies and the National Institutes of Health (NIH) collaborate to research and develop novel medicines or cures. One instance of how this type of public-private partnership can help to advance the development of novel medications is the quick creation of COVID-19 vaccines [Citation64].
Participation of the patient: By include the demands of the patient in the creation of new medical equipment, this can help to ensure that these devices are effective. Industry professionals may develop new goods that are more efficient, user-friendly, and ultimately more successful on the market by incorporating patients in the design and testing process.
6.4. Limitations
Some of the potential limitations that may be associated with the study are-
6.4.1. Scope and generalizability
The US, EU, India, and African economies are the main subjects of the study. Nevertheless, the results could not be generally applicable to all countries, which could restrict the research’s applicability to nations with various legislative frameworks, healthcare systems, and economic systems.
6.4.2. Data availability and accuracy
The availability and dependability of data from many nations determines the comparison analysis’s precision and thoroughness. The accuracy of the study’s conclusions may be impacted by potential restrictions brought about by variations in data quality and reporting requirements.
6.4.3. Regulatory dynamics over time
Regulatory frameworks are fluid and prone to modification. The report might not accurately reflect the most recent changes or advancements in medical device laws, which could lead to an imperfect picture of the regulatory environment as it stands now. Understanding the dynamic nature of medical device laws requires addressing these constraints.
7. Conclusion
The comparative analysis of the laws and regulations for medical devices in several countries described in this article demonstrates substantial disparities in the regulatory frameworks between various nations. The most restrictive regulations are found in the U.S.A. and Europe, whereas the regulatory environments in India and Africa are comparatively laxer. The article’s analysis of case studies concerning medical devices makes recommendations for developing countries to improve their regulatory environments, including setting up regulatory agencies and strengthening enforcement practices. The article emphasizes how crucial regulatory frameworks for medical devices are, especially in underdeveloped countries where having access to reliable medical equipment is vital. To build a regulatory climate that fosters innovation while guaranteeing patient safety, it emphasizes the necessity for policymakers and business professionals to work together and implement best practices.
8. Expert opinion
The suggested improvements in awareness and education programs proposed in this paper hold the potential to revolutionize healthcare systems in low-income countries. By equipping medical practitioners with a deeper understanding of medical device laws, we hope to significantly improve diagnosis accuracy and the formulation of more effective treatment guidelines. This, in turn, could have a substantial impact on patient outcomes, ultimately improving the overall efficacy of healthcare interventions.
Moreover, one important suggestion that could be taken with increasing potential for practical implementation involves the strengthening of regulatory agencies. If executed effectively, this initiative could streamline regulatory procedures, ensuring quicker approvals and the seamless integration of state-of-the-art medical equipment into clinical practices. However, challenges may arise in the form of limited resources and resistance to changes within existing bureaucratic frameworks.
Looking toward the future, there exists immense potential for further research in this field. The overarching goal is to establish an international standard for medical device laws that can be tailored to suit diverse healthcare environments worldwide. While research in this landscape is promising, it is important to consider the broader landscape of healthcare research.
Two emerging domains, genomics, and personalized medicine, have been presenting interesting fields to explore in the future in terms of medical devices. These areas not only offer research opportunities but also hold the promise of revolutionizing healthcare practices by tailoring treatments to individual genetic profiles.
Within the medical device regulatory space, we hope to see a shift in standard operating procedures within the next five to ten years. This anticipated progress, however, comes with its own set of challenges. Balancing effectiveness with safety will remain a significant concern, particularly as processes become more streamlined. There is a risk of overlooking essential safety precautions amidst efforts to simplify procedures.
To mitigate any compromise in patient safety, it will be imperative to maintain a robust system of oversight and accountability. This includes rigorous monitoring of the implementation of regulatory changes, continuous evaluation of safety protocols, and swift responses to any identified risks or shortcomings.
The recommendations put forth in this paper have the potential to usher in transformative changes in healthcare systems globally. By enhancing awareness, education, and regulatory frameworks surrounding medical devices, we can pave the way for improved patient care, more efficient healthcare delivery, and advancements in medical technology. As we navigate this evolving landscape, it will be essential to remain vigilant, adaptable, and committed to the ultimate goal of improving healthcare outcomes for all.
As we move forward it is important to understand that the collaboration among stakeholders, including governments, regulatory bodies, healthcare providers, and industry experts, will be key to streamline regulatory practices in the medical device industry. By working together, we can address the challenges, seize the opportunities, and create a future where access to safe, effective, and innovative medical devices is a reality for all individuals, regardless of their geographical location or socioeconomic status. This collaborative approach will not only drive advancements in healthcare but also promote equity and inclusivity in healthcare delivery worldwide.
Article highlights
The paper outlines recommendations for enhancing medical device laws in developing countries, focusing on key areas such as awareness, education, regulatory body strengthening, innovation promotion, and pharmaceutical regulations update.
Emphasis is placed on the significance of benchmarking regulatory frameworks to evaluate the effectiveness of institutional development plans.
The document underscores the involvement of patients in the design and testing phases of medical devices as a crucial element to ensure their effectiveness and user-friendliness.
Specific cases, such as the Mitra-Clip® Delivery System, are discussed to emphasize the importance of comprehensive adverse event reporting and the implications of device recalls in the context of medical device malfunctions.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewers disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Medical devices. [cited 2023 Mar 28]. Available from: https://www.who.int/health-topics/medical-devices
- Medical devices and technology across the years [Internet]. [cited 2023 Apr 30]. Available from: https://medicine.yale.edu/news/yale-medicine-magazine/article/medical-devices-and-technology-across-the-years/
- DePuy ASR hip implants recall [Internet]. Levin Perconti; [cited 2023 Mar 28]. Available from: https://www.levinperconti.com/product-liability/depuy-asr-hip-implant/
- DePuy ASR Hip Recall - System Revisions & Recall Information [Internet]. Drugwatch.com. [cited 2023 Mar 28]. Available from: https://www.drugwatch.com/hip-replacement/depuy/recall/
- GHTF Archives [Internet]. Int. Med. Device Regul. Forum. [cited 2023 Mar 28]. Available from: https://www.imdrf.org/ghtf
- GHTF Mission Summary [Internet]. Int. Med. Device Regul. Forum. [cited 2023 Mar 28]. Available from: https://www.imdrf.org/ghtf/mission-summary
- Peter L, Hajek L, Maresova P, et al. Medical devices: Regulation, risk classification, and open innovation. J Open Innov Technol Mark Complex. 2020;6(2):42. doi: 10.3390/joitmc6020042
- Health C for D and R. Classify your medical device [Internet]. FDA; 2023 [cited 2024 Feb 10]. Available from: https://www.fda.gov/medical-devices/overview-device-regulation/classify-your-medical-device
- FDA Device Regulation - PMC [Internet]. [cited 2023 Apr 16]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6140070/
- Medical devices: US medical device regulation - ScienceDirect [Internet]. [cited 2023 Apr 16]. Available from: https://www.sciencedirect.com/science/article/pii/S1078143914003457
- Bergsland J, Elle OJ, Fosse E. Barriers to medical device innovation. Med Devices Auckl NZ. 2014;7:205–209. doi: 10.2147/MDER.S43369 Cited: in: PMID: 24966699.
- Medical devices market size, share & growth. Forecast Report [Internet]. [cited 2023 Mar 28]. Available from: https://www.fortunebusinessinsights.com/industry-reports/medical-devices-market-100085
- Medical Devices Industry in India – Market Share, Reports, Growth & Scope. IBEF [Internet]. India Brand Equity Found; [cited 2023 Mar 28]. Available from: https://www.ibef.org/industry/medical-devices
- MRF. Africa Medical Devices Market Report - Forecast 2030. MRFR; [cited 2023 Apr 30]. Available from: https://www.marketresearchfuture.com/reports/africa-medical-devices-market-2845
- Wearable sensor uses ultrasound to provide cardiac imaging on the go: UC San Diego engineers lead development of a powerful new ultrasound sensor system for cardiac imaging that even works during a workout [Internet]. ScienceDaily. [cited 2023 Mar 28]. Available from: https://www.sciencedaily.com/releases/2023/01/230125121552.htm
- Zuckerman DM, Brown P, Nissen SE. Medical Device Recalls and the FDA Approval Process. Arch Intern Med. 2011;171:1006–1011. doi: 10.1001/archinternmed.2011.30
- Sherkow JS, Aboy M. The FDA De Novo medical device pathway, patents and anticompetition. Nat Biotechnol. 2020;38:1028–1029. doi: 10.1038/s41587-020-0653-6
- Muehlematter UJ, Daniore P, Vokinger KN. Approval of artificial intelligence and machine learning-based medical devices in the USA and Europe (2015–20): a comparative analysis. Lancet Digit Health. 2021;3(3):e195–e203. doi: 10.1016/S2589-7500(20)30292-2
- Medical device regulation: should we care about It? | Artery Research | Full Text [Internet]. [cited 2023 Apr 16]. Available from: https://arteryresearch.biomedcentral.com/articles/10.1007/s44200-022-00014-0
- Full article: On the new regulation of medical devices in Europe [Internet]. [cited 2023 Apr 16]. Available from: https://www.tandfonline.com/doi/full/10.1080/17434440.2017.1407648
- How does medical device regulation perform in the United States and the European Union? A Systematic Review. PLOS Med. [cited 2023 Apr 16]. Available from: https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001276
- Improving Medical Device Regulation: The United States and Europe in Perspective - SORENSON - 2014 - the Milbank Quarterly. Wiley Online Library [Internet]; [cited 2023 Apr 16]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/1468-0009.12043
- The rise of rules: Will the new EU regulation of medical devices make us safer? Eur J Internal Med. [cited 2023 Apr 16]. Available from: https://www.ejinme.com/article/S0953-6205(20)30295-8/fulltext
- Vermeersch P, André E. How the European in vitro diagnostic regulation could negatively impact the European response to the next pandemic: an urgent call for action before May 2022. Clin Microbiol Infect. 2021;27:1074–1075. doi: 10.1016/j.cmi.2021.05.009 Cited: in: PMID: 33979703.
- Kapoor V, Kaushik D. A comparative study of regulatory prospects for drug-device combination products in major pharmaceutical jurisdictions. J Generic Med. 2013;10:86–96. doi: 10.1177/1741134313515665
- Maresova P, Hajek L, Krejcar O, et al. New regulations on medical devices in Europe: are they an opportunity for growth? Adm Sci. 2020;10(1):16. doi: 10.3390/admsci10010016
- Grzybowski A, Brona P. Approval and Certification of Ophthalmic AI devices in the European Union. Ophthalmol Ther. 2023;12:633–638. doi: 10.1007/s40123-023-00652-w
- EU Artificial Intelligence Act. Up-to-date developments and analyses of the EU AI Act. [cited 2024 May 17]. Available from: https://artificialintelligenceact.eu/
- Tarricone R, Torbica A, Drummond M. Challenges in the assessment of medical devices: the medtecHTA Project. Health Econ. 2017;26(S1):5–12. doi: 10.1002/hec.3469
- Vogeser M, Brüggemann M, Lennerz J, et al. Partial Postponement of the Application of the in vitro Diagnostic Medical Devices Regulation in the European Union. Clin Chem. 2022;68:856–857. doi: 10.1093/clinchem/hvac048
- Hermans AMM, Maliepaard M, Boon WPC, et al. Impact of the new European Union in vitro Diagnostics Regulation on the practice of hospital diagnostic laboratories. Expert Rev Mol Diagn. 2022;22:583–590. doi: 10.1080/14737159.2022.2087508 Cited: in: PMID: 35673983.
- Green JIJ. Medical device legislation for custom-made devices after the UK has left the EU: answers to ten important questions. Br Dent J. 2021;231:513–521. doi: 10.1038/s41415-021-3530-x
- Medical Device Regulations and custom-made device documentation: A further ten frequently asked questions and their answers - James I. J. Green, 2023 [Internet]. [cited 2023 Apr 16]. Available from: https://journals.sagepub.com/doi/full/10.1177/20501684231153906
- Byrne R. Medical device regulation in Europe – what is changing and how can I become more involved? EuroIntervention [Internet]. [cited 2023 Apr 16]. Available from: https://eurointervention.pcronline.com/article/medical-device-regulation-in-europe-what-is-changing-and-how-can-i-become-more-involved
- Kent J, Faulkner A. Regulating human implant technologies in Europe–understanding the new era in medical device regulation. Health Risk Soc. 2002;4(2):189–209. doi: 10.1080/13698570220137060
- Orphan devices: yesterday is history; tomorrow is mystery: towards a European orphan device directive? SpringerLink. [cited 2023 Apr 16]. Available from: https://link.springer.com/article/10.1186/s13023-016-0393-3
- Regulation and Clinical Investigation of Medical Device in the Eu…: Ingenta Connect. [cited 2023 Apr 16]. Available from: https://www.ingentaconnect.com/content/ben/acctra/2019/00000006/00000003/art00003
- The Medical Device Regulation of the European Union Intensifies Focus on Clinical Benefits of Devices. SpringerLink [Internet]; [cited 2023 Apr 16]. Available from: https://link.springer.com/article/10.1007/s43441-019-00094-2
- Lahiry S, Sinha R, Chatterjee S. Medical device regulation in India: What dermatologists need to know. Indian J Dermatol Venereol Leprol. 2019;85:133. doi: 10.4103/ijdvl.IJDVL_326_18 Cited: in: PMID: 30688215.
- Bhardwaj KK, Bangarurajan K, Naved T, et al. Terror of 10 MB, a cross-sectional study investigates the regulation to the prospective of medical device. J Adv Pharm Technol Res. 2020;11:89–94. doi: 10.4103/japtr.JAPTR_184_19 Cited: in: PMID: 32587823.
- Shah AR, Goyal RK. Current Status of the Regulation for Medical Devices. Indian J Pharm Sci. 2008;70(6):695–700. doi: 10.4103/0250-474X.49085 Cited: in: PMID: 21369427.
- Dang A, Sharma JK. Economics of Medical Devices in India. Value Health Reg Issues. 2019;18:14–17. doi: 10.1016/j.vhri.2018.06.004
- Joshi D, Sharma I, Gupta S, et al. A global comparison of implementation and effectiveness of materiovigilance program: overview of regulations. Environ Sci Pollut Res. 2021;28:59608–59629. doi: 10.1007/s11356-021-16345-5
- Hoda F, Verma R, Arshad M, et al. Materiovigilance: Concept, Structure and Emerging Perspective for Patient’s Safety in India. Drug Res. 2020;70(9):429–436. doi: 10.1055/a-1195-1945
- Sumana G, Rajesh, Aswal DK. Importance of Standards in Biomedical Device and Its Role in Strengthening the Healthcare Sector. Front Nanotechnol [Internet]. 2021 [cited 2023 Apr 16];3. doi: 10.3389/fnano.2021.622804
- Markan S, Verma Y. Indian medical device sector: insights from patent filing trends. BMJ Innov [Internet]. 2017 [cited 2023 Apr 16];3(3):167–175. doi: 10.1136/bmjinnov-2016-000131
- Kumar Gupta S. Medical Device Regulations: a current perspective. J Young Pharm. 2015;8:06–11. doi: 10.5530/jyp.2016.1.3
- Gupta P, Janodia MD, Jagadish PC, et al. Medical device vigilance systems: India, US, UK, and Australia. Med Devices Evidence Res. 2010;3:67–79. doi: 10.2147/MDER.S12396
- Manu M, Anand G. A review of medical device regulations in India, comparison with European Union and way-ahead. Perspect Clin Res. 2022;13:3–11. doi: 10.4103/picr.PICR_222_20 Cited: in: PMID: 35198422.
- De Maria C, Di Pietro L, Díaz Lantada A, et al. Safe innovation: On medical device legislation in Europe and Africa. Health Policy Technol. 2018;7(2):156–165. doi: 10.1016/j.hlpt.2018.01.012
- Saidi T, Douglas TS. Medical device regulation in South Africa: The Medicines and Related Substances Amendment Act 14 of 2015. SAMJ South Afr Med J. 2018;108:168–170. doi: 10.7196/samj.2018.v108i3.12820
- The evolving landscape of medical device regulation in East, Central, and Southern Africa | Global Health: Science and Practice [Internet]. [cited 2023 Apr 16]. Available from: https://www.ghspjournal.org/content/9/1/136.short
- Dube-Mwedzi S, Kniazkov S, Nikiema JB, et al. A rapid assessment of the National Regulatory Systems for medical products in the Southern African Development Community. J Pharm Policy Pract. 2020;13(1):64. doi: 10.1186/s40545-020-00255-x
- Medicines Regulation in Africa: Current State and Opportunities. SpringerLink; [cited 2023 Apr 16]. Available from: https://link.springer.com/article/10.1007/s40290-017-0210-x
- Khadem Broojerdi A, Baran Sillo H, Ostad Ali Dehaghi R, et al. The world health organization global benchmarking tool an instrument to strengthen medical products regulation and promote universal health coverage. Front Med. 2020 [cited 2023 Apr 16];7. doi: 10.3389/fmed.2020.00457
- Regulation of medical diagnostics and medical devices in the East African community partner states | SpringerLink [Internet]. [cited 2023 Apr 16]. Available from: https://link.springer.com/article/10.1186/s12913-014-0524-2
- Towards a medical device regulation in South Africa: an assessment of the medicines and related substances amendment act of 2015. SpringerLink. [cited 2023 Apr 16]. Available from: https://link.springer.com/article/10.1007/s12553-016-0135-5
- Keyter A, Banoo S, Salek S, et al. The South African Regulatory System: Past, Present, and Future. Front Pharmacol. 2018 [cited 2023 Apr 16];9. doi: 10.3389/fphar.2018.01407
- Fuller J, Ashar BS, Carey-Corrado J. Trocar-associated injuries and fatalities: An analysis of 1399 reports to the FDA. J Minim Invasive Gynecol. 2005;12(4):302–307. doi: 10.1016/j.jmig.2005.05.008
- Giordano A, Silvestre A, Pieri M, et al. On a defective Mitraclip© system: Considerations on the medical device regulation in Europe. Monaldi Arch Chest Dis Arch Monaldi Mal Torace. 2018;88(3):901. doi: 10.4081/monaldi.2018.901 Cited: in: PMID: 30183155.
- Mayer CC, Francesconi M, Grandi C, et al. Regulatory requirements for medical devices and vascular ageing: an overview. Heart Lung Circ. 2021;30:1658–1666. doi: 10.1016/j.hlc.2021.06.517 Cited: in: PMID: 34362673.
- Medical devices. European Medicines Agency; [cited 2023 Dec 5]. Available from: https://www.ema.europa.eu/en/human-regulatory-overview/medical-devices
- Innovation tax incentives in Pharma, BioTech and MedTec [Internet]. KPMG. 2022 [cited 2023 Dec 4]. Available from: https://kpmg.com/ch/en/blogs/home/posts/2022/05/innovation-tax-incentives-pharma-biotech-medtech.html
- Public Good vs. Private gain: the role of public–private partnerships in drug innovation and pricing. 2022 [cited 2023 Dec 4]. Available from: https://www.commonwealthfund.org/blog/2022/public-good-vs-private-gain-role-public-private-partnerships-drug-innovation-and-pricing