Abstract
Engineered nanomaterials (ENMs) are being produced for an increasing number of applications. Therefore, it is important to assess and categorize ENMs on the basis of their hazard potential. The immune system is the foremost defence against foreign bodies. Here we performed cytokine profiling of a panel of nineteen representative ENMs procured from the Joint Research Centre (JRC) and commercial sources. Physicochemical characterization was performed using dynamic light scattering. The ENMs were all shown to be endotoxin content free. The human macrophage-differentiated THP.1 cell line was employed for cytotoxicity screening and based on the calculated IC50 values, the multi-walled carbon nanotubes (MWCNTs), ZnO, Ag and SiO2 NMs were found to be the most cytotoxic while single-walled carbon nanotubes (SWCNTs), TiO2, BaSO4 and CeO2 NMs, as well as the nanocellulose materials, were non-cytotoxic (at doses up to 100 µg/mL). Multiplex profiling of cytokine and chemokine secretion indicated that the TiO2, SiO2, BaSO4, CeO2 and nanocellulose materials induced potent inflammatory responses at sub-cytotoxic doses. Hierarchical clustering of cytokine responses coupled with pathway analysis demonstrated that the panel of ENMs could be segregated into two distinct groups characterized by activation and deactivation, respectively, of PPAR (peroxisome proliferator-activated receptor)/LXR (liver X receptor/retinoid X receptor) nuclear receptor pathways (NRPs). Furthermore, using rosiglitazone, a selective PPAR-γ agonist, we could show that PPAR-γ played an important role in the activation of inflammatory responses in cells exposed to TiO2 and SiO2 NMs. These studies show that ENMs of diverse chemical compositions can be grouped according to their inflammatory potential.
Introduction
Engineered nanomaterials (ENMs) possess several unique properties as compared to their bulk counterparts such as higher size-to-surface ratio, optical, electrical and magnetic properties making them highly useful in various technologies, including in theranostic devices (Bhattacharya et al., Citation2016; Kang et al., Citation2015). Certain ENMs, such as nano-Ag, are extensively used for their antiseptic properties (Durán et al., Citation2016). Nanocellulose has also been proposed for a range of applications in industry and in the biomedical field (Abitbol et al., Citation2016). Thus, humans can be exposed to ENMs deliberately or accidentally, through respiratory, dermal and gastrointestinal routes. Close attention should therefore be paid to the safety assessment of ENMs. However, it is not realistically achievable to test all existing and emerging ENMs one-by-one to evaluate their safety. Therefore, efforts are being made to increase the knowledge concerning the association between different material properties and the resulting toxicity so as to categorize ENMs accordingly, in order to allow for risk assessment of these materials (Braakhuis et al., Citation2016; Lynch et al, Citation2014). The ‘grouping of substances’ or category approach has been recognized as an important means to avoid unnecessary testing of new chemicals. For chemicals in general, technical guidance on grouping is available, but not for nanomaterials (Fadeel et al., Citation2015). However, the European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC) recently reviewed available concepts for the grouping of nanomaterials for human health risk assessment (Arts et al., Citation2014) and this was followed by a proposal for a decision-making framework for grouping of nanomaterials (Arts et al., Citation2015). The authors noted that most nanomaterials will not be accurately grouped based solely on their intrinsic material properties as their role in toxicity is not yet completely understood. Indeed, ENMs with the same chemical composition may differ considerably with respect to other physicochemical properties (e.g. size, shape, surface charge, etc.) as well as with respect to acquired properties (as a function of the physicochemical properties) including corona formation (Arts et al., Citation2015). Therefore, the ECETOC proposal follows a functionality-driven approach. Similarly, Godwin et al. (Citation2015) argued that physicochemical properties alone are not sufficient for nanomaterial categorization and the authors proposed that ‘direct measures of biological activity and exposure potential’ are needed to facilitate categorization of ENMs in support of regulatory decision-making. Traditional approaches for chemical safety assessment often rely on animal studies. In recent years, alternative testing strategies incorporating mechanism-based on in vitro assays and in silico (computational) approaches have emerged (Nel & Malloy, Citation2017). However, there are several issues that need to be considered, including the extent to which in vitro tests mirror in vivo toxicological responses coupled to this; the selection of relevant dose metrics, dose ranges and exposure durations remains an important challenge (Landsiedel et al., Citation2014; Nel et al., Citation2013).
Inflammation is an adaptive response involving soluble mediators and specialized immune cells that is triggered in response to infection, trauma, ischemia, toxicants, or other forms of injury (Medzhitov, Citation2008). Chronic inflammation is detrimental to the organism. ENMs may act as inducers and stimulate the production of inflammatory mediators by innate immune cells (Bhattacharya et al., Citation2013). For instance, certain high-aspect ratio nanomaterials have been found to induce pulmonary inflammation in exposed animals with signs of acute inflammation along with fibrosis and granulomatous lesions (Braakhuis et al., Citation2014). Moreover, previous work on metal oxides has shown that different nanoparticles induce distinct inflammatory ‘footprints’ in the lungs of exposed animals, depending on the composition of the ENMs, as defined by the pattern of cellular infiltrates in the lungs (Cho et al., Citation2010). The authors cautioned that this variability in inflammatory responses to different ENMs ‘could never have been predicted or detected’ by in vitro assays. However, using carefully designed in vitro assays and appropriate end-points such as cytotoxicity coupled with pro-inflammatory cytokine responses, it should be possible to predict, or at least to have a first indication, of whether or not nanomaterials are potentially immunotoxic in vivo. Shaw et al. (Citation2008) could show that in vitro biological activity profiles of ENMs were predictive of in vivo responses, as assessed by the increase in monocytes in the spleen or in the peripheral blood of exposed mice. Moreover, in a recent study of a panel of inorganic ENMs, Wiemann et al. (Citation2016) reported that an in vitro alveolar macrophage assay was predictive of short-term inhalation toxicity of ENMs. Therefore, evaluating the cytotoxicity and inflammatory response of ENMs using inflammatory/immune-competent cells can be an important criterion for elucidating the primary biological response towards ENMs and could be useful in categorizing ENMs (Dekkers et al., Citation2016).
In the current study, we used the human macrophage-differentiated THP.1 cell line for analysis of the cytotoxicity and immunotoxicity (secretion of immune mediators) of a panel of nineteen representative ENMs, i.e. industrially/commercially relevant ENMs, obtained in the frame of the FP7-NANOREG project, an EC-funded project aimed at a common approach to the regulatory testing of nanomaterials (www.nanoreg.eu). The ENMs were first tested for endotoxin content. We then performed dose-response studies (up to 100 µg/mL) to assess for cytotoxicity at 24 and 48 h and later assessed for cytokine-chemokine secretion at two sub-cytotoxic doses (10 and 25 µg/mL) at 24 h. These studies have provided information on the biological activity of a large panel of ENMs and suggest that diverse ENMs can be grouped based on inflammatory potential.
Methods
Engineered nanomaterials
ENMs were procured through the nanomaterial repository of the Joint Research Centre (JRC) of the European Commission, OECD Working Party on Manufactured Nanomaterials (WPMN), Fraunhofer Institute for Molecular Biology and Applied Ecology (IME), and commercial sources – UPM, Finland and Stora Enso, Sweden (Suppl. Table 1).
Dispersion protocol
For dispersion, 15.36 mg equivalent of the ENMs were weighed into Scint-Burk glass vials (WHEA986581; Wheaton Industries Inc.) under inert conditions. The ENMs were made wet by adding 30 μL EtOH drop-by-drop. The final volume of the ENMs suspension was adjusted to 6 mL by adding additional 0.05% endotoxin-free bovine serum albumin (BSA) (Sigma; Cat # A2058). Sonication of the ENMs suspension was performed in ice water using Soniprep 150 (MSE, UK) for 16 min.
Size distribution and zeta potential
Particle size distribution and zeta potential of the ENM suspensions was measured by dynamic light scattering (DLS) technique using Malvern Zetasizer Nano ZS. Ten measurements with no pause were taken for particle size distribution and ∼10–20 measurements were taken for the zeta potential values of each test material at 0, 24 and 48 h at a temperature of 25 °C for batch dispersions and 37 °C for in vitro exposure media.
Endotoxin content
Lipopolysaccharide (LPS) contamination was assessed for ENMs (100 µg/mL) using the chromogenic endpoint limulus amebocyte lysate (LAL) assay (QCL-1000™ Assay, Lonza, Walkersville, MD). As internal interference control for the assay, 0.5 EU/mL of LPS (Sigma) (Cat # L6529) and 10 µM of phorbol 12-myristate 13-acetate (PMA) (Sigma) (Cat # P1585) was applied. Additionally, a macrophage activation test was performed for ENMs showing particle interference in the LAL assay (Mukherjee et al., Citation2016). Briefly, CD14 + monocytes obtained from healthy human blood donors were cultured in RPMI-1640 medium supplemented with 2 mM L-glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin and 10% heat-inactivated fetal bovine serum (FBS), supplemented with 50 ng/mL recombinant M-CSF (R&D Systems) for 3 days in 96-well plates. Then, human monocyte-derived macrophages (HMDMs) were incubated with ENMs at a nontoxic concentration (25 µg/mL) (determined by Alamar Blue assay) for 24 h in RPMI-1640 medium supplemented with 10% FBS, in the presence or absence of polymyxin B sulfate (10 μM). LPS (100 ng/ml) was included as a positive control and its dose response was measured down to 10 pg/mL. Following exposure, cell supernatants were collected and the secretion of TNF-α was determined by ELISA (Abcam) according to the manufacturer’s protocol. A standard curve was generated based on LPS-induced TNF-α secretion.
THP.1 cell culture and differentiation
Human monocytic (THP.1) cells were provided by GAIKER-IK4 Technological Center, Spain, under the framework of the FP7-NANOREG project. Cells were grown in RPMI 1640-Glutamax™-I media containing HEPES (Gibco, Sweden) and supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin and 10% heat-inactivated FBS (Sigma) (Cat # SH30071.03; Lot # AZK195950). The cells were mycoplasma tested regularly using MycoAlert® mycoplasma detection kit (Lonza) and passaged at a cell density of up to maximum 8.0 × 105/ml every 3–4 days. For the experiments, the THP.1 cells were plated at the cell density of 60,000 cells/well and pre-incubated for 24 h in the presence of 0.5 μM PMA. After 24 h the differentiated THP.1 cells were washed with luke-warm sterile PBS and exposed to ENMs. The cells were only used for up to 20 passages.
Alamar blue cell viability assay
ENMs were added to the PMA-differentiated THP.1 cells at the indicated concentrations. As negative and positive controls, sterile PBS (in volume equivalent to 100 µg/mL) and dimethyl sulfoxide (DMSO) (50 μL/mL) were applied. After exposure, the cell medium was carefully aspirated, aliquoted and was stored at −80 °C for later cytokine analysis. Loss of cell viability was determined using Alamar blue® reagent (ThermoFisher Scientific) as described before (Naha et al., Citation2010) and the 50% inhibitory concentration (IC50) per ENM was calculated using Graphpad prism software.
Multiplex analysis based cytokine profiling
Quantification of pro- and anti-inflammatory mediator release by the PMA-differentiated THP.1 cells following 24 h exposure to ENMs was performed by LUMINEX® assay. The cell culture media previously collected from cell viability assay and stored at −80 °C was used. The thawed medium was routinely centrifuged for 5 min to pellet cell debris and ENMs and samples were then analyzed using BioPlex Pro™ human cytokine standard 27-plex, Group I kit (BioRad, Solna, Sweden) for IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12(p70), IL-13, IL-15, IL-17 A, eotaxin, Basic FGF, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α and VEGF, using Luminex 200 running Bio-Plex Software V.6.0 (BioRad). Statistical significance of the result was tested using One-way ANOVA with Tukey post-hoc test using SigmaPlot® analysis software.
Bioinformatics analysis
Cytokine-chemokine expression data retrieved from the multiplex assay were analyzed using hierarchical clustering analysis following quantile-normalization of the data. Complete linkage and Euclidean distances were employed as metrics to draw association dendrograms between cytokines and the different ENMs. Cluster analysis and heatmaps were obtained using R 3.2.× (Ihaka & Gentleman, Citation1995). Pathway analysis was done using the ingenuity pathway analysis (IPA) software (license from Ingenuity Systems, Redwood City, CA) on normalized cytokine expression data, by a comparative approach between ENMs as experimental treatments using a causal analysis approach (Krämer et al., Citation2014), complemented by hierarchical cluster analysis, as above. Significant pathways were filtered by p-value (α) < .05 and activation Z-score > -2 or >2, representing a significant deactivation or activation, respectively.
IL-1β detection
ENMs were added to PMA-differentiated THP.1 cells at the concentration of 10 µg/mL for 24 h in the presence or absence of the PPAR-γ agonist, rosiglitazone (Sigma) (Cat # R2408). After exposure, the cell medium was carefully aspirated, aliquoted and was stored at −80 °C for subsequent cytokine analysis. Production of IL-1β was analyzed using the Human IL-1β ELISA kit (Invitrogen) (Cat # KHC0011). Absorbance was measured at 450 nm using a plate reader (Infinite F200, Tecan, Switzerland). The experiment was performed twice in duplicates and statistical significance of the result was tested using One-way ANOVA with Tukey post-hoc test using SigmaPlot® software.
PPAR-γ detection
ENMs were added to the PMA-differentiated THP.1 cells at the concentration of 10 µg/mL for 6 and 24 h in the presence or absence of the PPAR-γ agonist, rosiglitazone. After exposure, the cells were washed with cold PBS and were removed by scraping. Following centrifugation at 1200 rpm, cell pellets were recovered and nuclear extracts were prepared using the Nuclear Extraction Kit (Abcam) (Cat # ab113474). The presence of PPAR-γ protein was analyzed using the Human PPAR-γ ELISA kit (LSBio) (Cat # LS-F12376). The experiment was performed twice in duplicates and statistical significance of the result was tested using One-way ANOVA with Tukey post-hoc test.
Nuclear PPAR-γ activity assay
ENMs were added to the PMA-differentiated THP.1 cells at the concentration of 10 µg/mL for 24 h in the presence or absence of the PPAR-γ agonist, rosiglitazone. Following exposure, the cells were washed with cold PBS and were removed by scraping. Upon centrifugation at 1200 rpm, cell pellets were recovered and nuclear extracts were prepared using the Nuclear Extraction Kit (Abcam) (Cat # ab113474). PPAR-γ activity was analyzed using the PPAR-γ transcription factor assay kit (Abcam) (Cat # ab133101) based on specific peroxisome proliferator response element (PPRE) binding activity. The experiment was performed twice in duplicates and statistical significance of the result was tested using One-way ANOVA with Tukey post-hoc test.
Results
Nanomaterial characterization
The JRC has established a nanomaterial repository that hosts so-called representative test materials, defined as a material from a single batch, that is sufficiently homogeneous and stable with respect to one or more specified properties and is implicitly assumed to be fit for its intended use in the development of test methods which target properties other than the properties for which homogeneity and stability have been demonstrated (Roebben et al., Citation2013). By definition, such materials are not a reference material for the test for which it is intended to be used. However, for nanosafety research purposes, availability of ENMs from a single batch is preferable in order to enhance the comparability of data between different laboratories. Previous work conducted in the EC-funded project FP7-MARINA focused on in vitro testing of a panel of six metal oxides from the JRC repository (Farcal et al., Citation2015). Here, nineteen ENMs selected for testing in the FP7-NANOREG project were evaluated. These test materials were procured from the nanomaterial repository and from commercial sources in the case of the five nanocelluloses (refer to Suppl. Table 1).
The selected ENMs were characterized for their physicochemical properties, i.e. hydrodynamic diameter based on the particle size distribution and zeta potential, using DLS (Suppl. Table 2, Suppl. Table 3). Measurements were performed at 0, 24 and 48 h, in cell culture medium supplemented with 10% FBS, to approach a situation as similar as possible to the actual cell culture conditions. Overall, the hydrodynamic diameter of each ENM was stable during the course of these measurements (Suppl. Table 2). Furthermore, all the ENMs were found to have a negative charge when dispersed in 0.05% BSA or in culture medium supplemented with 10% FBS (Suppl. Table 3).
Endotoxin content analysis was performed using the LAL assay and a HMDM based functional assay. Using the LAL assay, all the inorganic, metal-based nanoparticles were found to be free of endotoxin contamination (Suppl. Figure S1(A)). However, the organic materials, i.e. carbon nanotubes (CNTs) and nanocelluloses (NCs) produced a positive signal in the LAL assay (Suppl. Figure S1(B)). Spiking the samples with a known concentration of LPS (0.5 EU/mL) indicated no interference with the LAL assay for the metal-based ENMs (Suppl. Figure S1A), but strong interference was observed for the CNTs and NCs (data not shown). This is in agreement with our previous work indicating that certain (carbon-based) nanomaterials cause interference with the LAL assay (Mukherjee et al., Citation2016). Therefore, we performed a macrophage activation test, with assessment of TNF-α secretion in the presence or absence of the specific LPS inhibitor, polymyxin B sulfate (10 μM) for the CNTs and NCs. LPS was included in order to generate a standard curve (Suppl. Figure S1(C)). None to extremely low levels of TNF-α secretion was found in cells exposed to the CNTs and NCs indicating absence of any endotoxin contamination (Suppl. Figure S1(D)). Therefore, based upon the combined results of the two assays, we concluded that all the ENMs were endotoxin free.
Cytotoxicity assessment
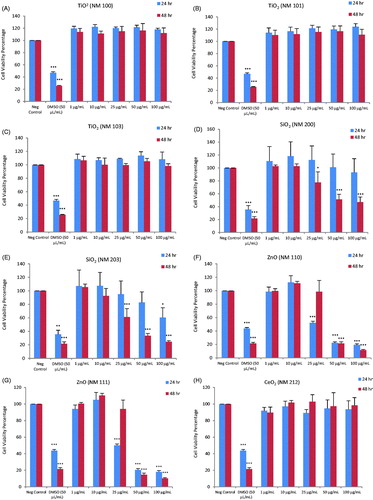
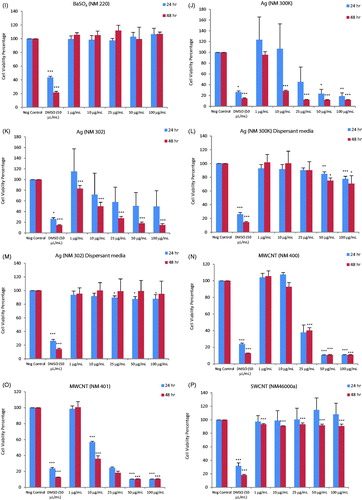
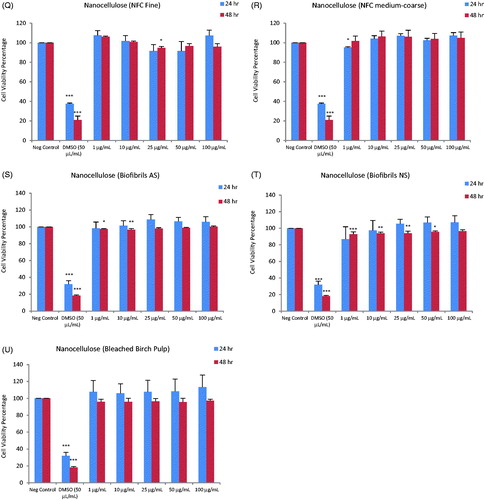
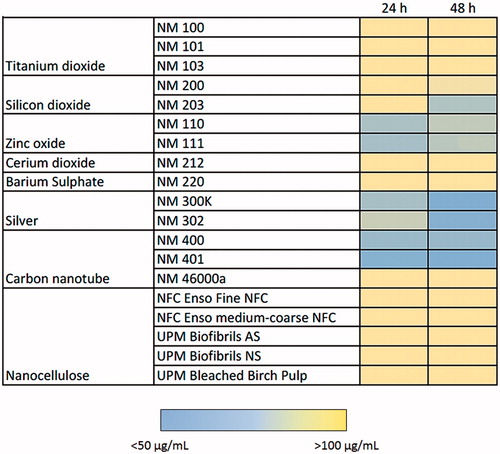
Cytotoxicity screening of the nineteen ENMs, along with two dispersants (total of 21 test materials) was performed using the PMA-differentiated THP.1 cells using the Alamar blue assay. Loss of cell viability was analyzed for a range of concentrations of the ENMs at 24 and 48 h of exposure. The results showed that the TiO2 (NM100, NM101 and NM103), CeO2 (NM212), BaSO4 (NM220), SWCNTs (NM46000a) and NCs (NFC Fine, NFC coarse-medium, Biofibrils AS, Biofibrils NS and Bleached Birch Pulp) were non-cytotoxic at all the concentrations and time-points tested (). The remaining ENMs, i.e. SiO2 (NM200 and NM203), ZnO (NM110 and NM111), nano-Ag (NM300K and NM302) and MWCNTs (NM400 and NM401) induced cytotoxicity to a varying extent (). The cytotoxicity results were summarized in a heatmap based upon IC50 values to more readily visualize the results (). Hence, the toxicity of the FP7-NANOREG panel of ENMs can be summarized accordingly: at 24 h: IC50 < 50 µg – ZnO (NM200, NM203), Ag (NM300K), MWCNTs (NM400, NM401); IC50 50–100 µg – Ag (NM302); >100 µg – TiO2 (NM100, NM101, NM103), SiO2 (NM200, NM203), CeO2 (NM212), BaSO4 (NM220), SWCNTs, NCs (NFC Enso Fine NFC, NFC Enso medium-coarse NFC, UPM Biofibrils AS, UPM Biofibrils NS, UPM Bleached Birch Pulp) and at 48 h: IC50 <50 µg – SiO2 (NM203), ZnO (NM200, NM203), Ag (NM300K, NM302), MWCNTs (NM400, NM401); IC50 50–100 µg – SiO2 (NM200); >100 µg – TiO2 (NM100, NM101, NM103), CeO2 (NM212), BaSO4 (NM220), SWCNTs, NCs (NFC Enso Fine NFC, NFC Enso medium-coarse NFC, UPM Biofibrils AS, UPM Biofibrils NS, UPM Bleached Birch Pulp) (). Since the Ag NPs were suspended in an organic dispersant (NM300K: 10% w/w and NM302: 8.6% w/w), the cells were also exposed to the equivalent weight fraction of dispersants present in the Ag samples. However, no loss of cell viability was observed in differentiated THP.1 cells exposed to the dispersants alone (). As a positive control in each experiment, we applied DMSO (50 µL/mL) which resulted in >30–40% cell viability at 24 h and >10–20% at 48 h ().
Figure 1. Cytotoxicity analysis of a panel of nineteen representative nanomaterials. Cell viability, determined by using the Alamar blue assay, of human macrophage-like THP.1 cells exposed to nanomaterials (and relevant dispersants): NM100 (A), NM101 (B), NM103 (C), NM200 (D), NM203 (E), NM110 (F), NM111 (G), NM212 (H), NM220 (I), NM300K (J), NM302 (K), NM300K Dispersant (L), NM302 Dispersant (M), NM400 (N), NM401 (O), NM46000a (P), NFC Fine (Q), NFC Medium Coarse (R), Biofibrils AS (S), Biofibrils NS (T) and Bleached birch pulp (U). The DMSO was applied to the cells as a positive control, Cells were exposed for 24 or 48 h. Data are mean values ± SD (n = 3). ***p ≤ .001, **p ≤ .01 and *p ≤ .5 by one-way ANOVA followed by Dunnett’s post-hoc test.
Figure 2. Heatmap of IC50 values for a panel of nanomaterials. Based on IC50 values calculated from the data shown in Figure 1, the ZnO NPs (NM110 and NM111), nano-Ag (NM300K and NM302) and MWCNTs (NM400 and NM401) showed the highest cytotoxicity among the tested ENMs following exposure of macrophage-differentiated THP.1 cells.
Profiling of cytokine responses
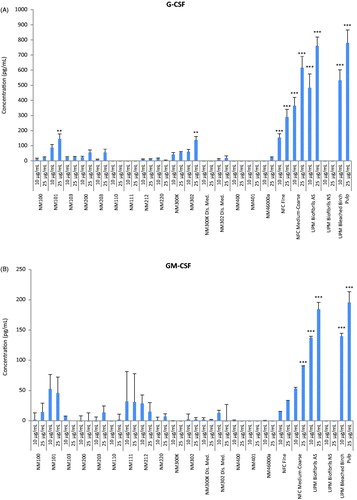
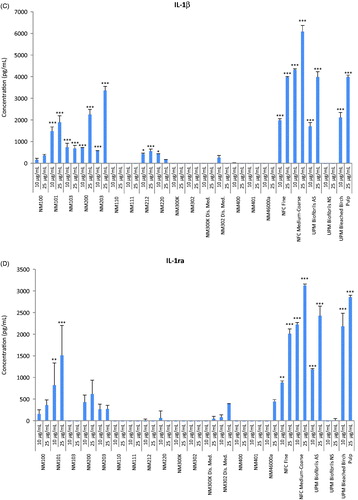
Next, inflammatory responses in macrophage-differentiated THP.1 cells were analyzed at two sub-toxic concentrations (10 and 25 µg/mL) selected on the basis of IC50 values obtained at 24 h. Quantification of a panel of human pro- and anti-inflammatory mediators was performed simultaneously using a multiplex (BioRad LUMINEX®) based assay. For some cytokines (IL-2, IL-5, IL-8, IL-13) we could not obtain results as the values were below the detection limit of the assay while for some other cytokines (MIP-1β, TNF-α) no responses were detected except for a few of the materials, but the standard deviations were very high and this precluded any reliable analysis or interpretation of these results. However, based on the profiling of the 21 remaining factors, several interesting observations could be made. For instance, we found increased levels of the two growth factors, granulocyte colony-stimulating factor (G-CSF) () and granulocyte macrophage colony-stimulating factor (GM-CSF) () in cells exposed for 24 h to NFC Fine, NFC coarse-medium, UPM Biofibrils AS and UPM Bleached Birch Pulp, but not to UPM Biofibrils NS, whereas no secretion or very low levels (for some of the TiO2 and Ag NMs) were seen for the other NMs, suggesting that this was an NC-specific response. CSFs are cytokines and growth factors that have been associated with the expression of pro-inflammatory cytokines with activation of the corresponding pathways leading to the onset of inflammatory diseases such as, rheumatoid arthritis, obesity and cancer (Hamilton, Citation2008). Notably, the same NCs also triggered significant levels of pro-inflammatory IL-1β () and its antagonist IL-1ra (IL-1 receptor antagonist) (). The balance between IL-1 and IL-1ra in tissues plays an important role in the susceptibility to and severity of many diseases (Arend, Citation2002).
Figure 3. Cytokine profiling of a panel of representative nanomaterials. Human macrophage-like THP.1 cells were exposed to nineteen representative nanomaterials at 10 or 25 µg/mL for 24 h and the secretion of cytokines, chemokines and growth factors into the supernatant was monitored by using the BioPlex Pro™ human cytokine standard 27-plex array. (A) G-CSF, (B) GM-CSF, (C) IL-1β, (D) IL-1ra, (E) IL-6, (F) RANTES, (G) IP-10 (CXCL10) and (H) MIP-1α. Results are mean values ± SD (n = 3). Selected cytokines are shown; refer to Suppl. Figure S2, for additional data. ***p ≤ .001and **p ≤ .01 by one-way ANOVA followed by Tukey’s post-hoc test.
Interestingly, some cytokines, such as IL-6 () and IP-10 () were uniquely affected by NCs (NFC Fine, NFC coarse-medium, UPM Biofibrils AS and UPM Bleached Birch Pulp) and not by other NMs, while other factors, such as the chemokines, RANTES () and MIP-1α () were uniquely affected by the metal oxides, TiO2 (NM103), SiO2 (NM200, NM203) and CeO2 (NM212), while not being affected at all by NCs, or other metal oxides, such as ZnO, nor by nano-Ag or CNTs. IL-6 is a Th1-type pro-inflammatory response that is induced through activation of macrophage surface receptors in response to pathogen-associated molecular patterns (PAMPs). IP-10 (interferon-γ induced protein 10) (also known as CXCL10) is a chemoattractant for activated T cells and its expression is seen in many Th1-type inflammatory diseases (Antonelli et al., Citation2014). RANTES and MIP-1α are associated with Th1 type responses in immune cells exposed to viruses and bacteria (Cocchi et al., Citation1995). Thus, it appears that the metal oxide-based NMs with the exception of ZnO stimulated a pathogen-type response. Furthermore, secretion of IFNγ was seen following exposure of cells to metal oxides lacking cytotoxic potential, i.e. TiO2 (NM100, NM101, NM103) as well as metal oxides displaying cytotoxicity, i.e. SiO2 (NM200, NM203) (Suppl. Figure 2I). IFNγ has important antiviral, antitumoral and immunoregulatory functions and is critical for innate and adaptive immunity against viral and certain other infections (Chow & Gale, Citation2015). IFNγ is also implicated in the formation of tissue granulomas through its activation of macrophages. The same metal oxides also triggered secretion of the pro-inflammatory cytokine, IL-17, with SiO2 being more potent than TiO2 (Suppl. Figure 2F). High levels of IL-17 are associated with many chronic inflammatory/autoimmune diseases. IL-17 also plays a role in lung diseases such as asthma and chronic obstructive pulmonary disease (COPD) (Alcorn et al., Citation2010).
Clustering and pathway analysis
To further analyze the responses to the different ENMs, we performed hierarchical cluster analysis to draw association dendrograms between cytokine responses evidenced for the different ENMs tested at the concentrations of 10 and 25 µg/mL. We observed distinct differences in the cytokine-chemokine expression patterns elicited by the different ENMs resulting in the segregation of the ENMs into obvious clusters in particular at the lower dose of 10 µg/mL () and to a lesser extent at the 25 µg/mL dose (). In general, ENMs with similar elemental compositions were clustered together at both concentrations, with certain exceptions. Thus, following exposure to 10 µg/mL, there was a clear distinction between two NCs (NFC medium-coarse and UPM Bleached Birch Pulp) inducing higher release of cytokines and chemokines by the differentiated THP.1 cells when compared to the other NCs. Indeed, these two NCs were found to induce the highest level of inflammatory cytokine and chemokine production as compared to all the other ENMs. Hierarchical cluster analysis further subdivided the remaining ENMs into distinct subclusters. Interestingly, four ENMs, namely, NM103, NM101, NM200 and NM203 were clustered together at both the doses with high induction of inflammatory mediators as compared to the NM401, NM111 and NM302 which clustered together with the lowest induction of inflammatory mediators in the differentiated THP.1 cells (). Both NFC fine and UPM Biofibrils AS were assigned to the same subcluster and caused increased expression of cytokines, thus indicating a similar pattern of response. All other ENMs were found to trigger a comparatively lower induction of inflammatory mediators in the THP.1 cell model.
Figure 4. Hierarchical cluster analysis of cytokine profiling data. The heatmaps show the hierarchical cluster analysis of cytokine, chemokine and growth factor secretion by human macrophage-like THP.1 cells exposed to (A) 10 and (B) 25 µg/mL of the indicated ENMs. Complete linkage and Euclidean distances were employed as metrics to draw association dendrograms between cytokines and ENMs. Each branch in the dendrograms shows the similarity between samples; the shorter the branch, the more similar are the samples. Association clusters for ENMs and cytokines are represented by dendrograms at the left and at the top of each heatmap, respectively.
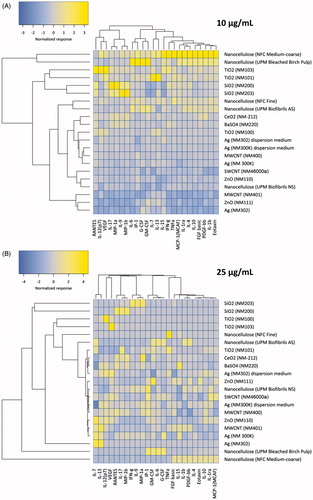
Hierarchical cluster analysis of the cytokines, chemokines and growth factors themselves showed distinct patterns of production and associations with the different ENMs. We thus identified two major clusters comprising of RANTES to MIP-1β and IL-6 to eotaxin at the 10 µg/mL dose of ENMs (). The latter cluster was found to mainly comprise of the anti-inflammatory cytokines, namely IL-13, IL-1ra, IL-4 and IL-10. From the cluster analysis of the 10 µg/mL exposure results, it was observed that the main factor segregating NFC medium-coarse and UPM Bleached Birch Pulp from the rest of the ENMs and more specifically from the other NCs was the increased expression of anti-inflammatory cytokines (). However, for the higher exposure dose of 25 µg/mL, the patterns of inflammatory mediator induction in differentiated THP.1 cells were less clear due to less pronounced differences in the release of inflammatory mediators in response to the different ENMs (). Nonetheless, as before, the NFC medium-coarse and UPM Bleached Birch Pulp NCs were segregated from the other ENMs and displayed an increased secretion of anti-inflammatory cytokines (). However, the separation between pro- and anti-inflammatory cytokines or chemokines was not as obvious as for the lower dose of the ENMs.
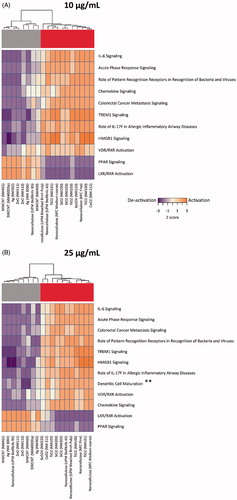
Pathway analysis using the IPA software tool yielded similar patterns of activation and deactivation of canonical pathways at the two exposure doses (10 and 25 µg/mL), with the exception of the dendritic cell maturation pathway (seen only at 25 µg/mL) (). Interestingly, at both doses, the different ENMs in the FP7-NANOREG panel were segregated into two distinct groups showing exactly opposite patterns. In one group, PPAR (peroxisome proliferator-activated receptor) and LXR/RXR (liver X receptor/retinoid X receptor) nuclear receptor pathways (NRPs) were seemingly activated, whereas the other ENMs showed the opposite trend, i.e. a tendency to deactivate these inflammation-related pathways as well as acute phase response signaling pathways (). Cluster analysis showed that the first cluster was comprised of CNTs, nano-Ag, ZnO and NCs (NM401, NM46000a, NM302, NM111, NM110, NM300K, UPM Biofibrils NS and NM400) and the second cluster comprised of NCs (with the exception of UPS Biofibrils NS), BaSO4, TiO2 and SiO2 (UPM Bleached Birch Pulp, NM101, NFC medium-coarse, NM200, NM203, NM103, NM220, NFC Fine, NM100, and NM212). Taken together, these results suggest that the segregation of ENMs into two distinct groups is largely dependent upon the activation/deactivation of NRPs and this segregation was not altered by the dose of the ENMs.
Figure 5. Pathway analysis of cytokine profiling data. Pathway analysis results using the IPA software tool integrating the normalized cytokine, chemokine and growth factor expression levels in human macrophage-like THP.1 cells exposed to (A) 10 and (B) 25 µg/mL of the indicated ENMs were transformed into interpolated networks, highlighting the grouping of ENMs into activators and deactivators of nuclear receptor (PPAR and LXR/RXR) pathways, respectively. Heatmaps show significant canonical pathways (Z > 2, adjusted p < .05 for at least one activated pathway per treatment).
Role of nuclear receptor PPAR-γ
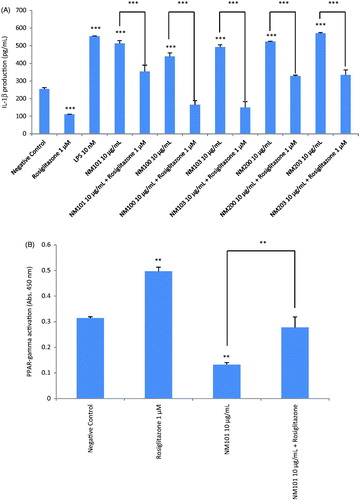
The PPARs are ligand-activated NRPs that play a major role in modulating inflammatory processes in macrophages (Rigamonti et al., Citation2008). Detection of cellular expression of PPAR-γ in macrophage-differentiated THP.1 cells exposed to a sub-toxic concentration of 10 µg/mL was analyzed by using a specific ELISA. The results showed that at 6 h the expression of PPAR-γ was reduced as compared to the negative controls when exposed to NM101, NM100, NM103, NM200, NM203, NM110, NM111, NM212, NM220, NM300k and NM302 (Suppl. Figure S3(A)). However, at 24 h the expression level of PPAR-γ was comparable to that of the negative control, while the well-documented PPAR-γ agonist, rosiglitazone, increased the level of expression of PPAR-γ (Suppl. Figure S3(B)). Having established the impact of the NMs on PPAR-γ expression, further analysis of the possible role of PPAR-γ in modulating inflammatory responses in macrophage-differentiated THP.1 cells exposed to NMs was performed by measuring IL-1β production following exposure to metal oxides in the presence or absence of rosiglitazone (1 µM). To this end, we used a specific ELISA for IL-1β. We selected TiO2 and SiO2 NMs for these experiments due to the fact that these NMs were predicted to negatively affect the PPAR signaling pathway (). As shown in , the NMs tested induced IL-1β production in THP.1 cells, thus confirming our previous multiplex based assay results (). NM-triggered production of IL-1β was significantly reduced by rosiglitazone, indicating a role for PPAR-γ. To provide further evidence for a role of PPAR-γ, we also monitored PPAR-γ activation in THP.1 cells exposed to NMs by using a transcription factor activation assay based on specific peroxisome proliferator response element (PPRE) binding activity in nuclear extracts of exposed cells. Exposure of THP.1 cells to TiO2 NMs (NM101) at 10 µg/mL for 24 h caused a significant suppression of PPAR-γ activation () while the positive control, rosiglitazone, triggered increased activation of PPAR-γ. Notably, in the presence of rosiglitazone, the ENM-induced deactivation of PPAR-γ was reversed (). Thus, we have provided evidence for the direct involvement of PPAR-γ in modulating inflammatory responses in cells exposed to metal oxides.
Figure 6. Role of PPAR-γ for cytokine responses. (A) IL-1β release from macrophage-differentiated THP.1 cells was analyzed after exposure for 24 h to TiO2 (NM101), TiO2 (NM100), TiO2 (NM103), SiO2 (NM200) and SiO2 (NM203) in the presence or absence of the PPAR-γ agonist, rosiglitazone (1 µM). (B) PPAR-γ activity was tested by analyzing specific peroxisome proliferator response element (PPRE) binding activity in nuclear extracts of exposed cells. Exposure of macrophage-differentiated THP.1 cells to TiO2 (NM101) for 24 h significantly reduced the activation of PPAR-γ while co-exposure to rosiglitazone (1 µM) reversed this effect. Data shown are mean values ± SD (n = 2). ***p ≤ .001 and **p ≤ .01 by one-way ANOVA followed by Tukey’s post-hoc test.
Discussion
In this study, a large panel of nanomaterials were screened for cytotoxicity and inflammatory responses (cytokine-chemokine secretion) using a well-established in vitro model. The experiments were performed according to standardized guidelines developed in the frame of the FP7-NANOREG project. The selected ENMs (n = 19) are representative test materials procured from the JRC repository or commercial sources. These studies allowed us to perform hazard ranking of the tested materials and we could also assign all the ENMs to two distinct groups on the basis of cytokine profiling results. These studies are the first to use cytokine-chemokine profiling for grouping of ENMs. As previously noted, it is essential to control the endotoxin contamination before conducting immunotoxicity studies of ENMs (Li & Boraschi, Citation2016). Indeed, immune cells are exquisitely sensitive to LPS and contamination of nanoparticles was shown to have a pronounced effect on cell maturation and cytokine secretion in dendritic cells (Vallhov et al., Citation2006). In the present study, endotoxin contamination was ruled out for all the ENMs, using the standard LAL assay and for the CNTs and NCs, a modified macrophage activation test based on primary human macrophages (Mukherjee et al., Citation2016).
The predictivity of in vitro assays for in vivo outcomes remains an important challenge. Focusing on pulmonary toxicity of ENMs, Landsiedel et al. (Citation2014) provided a comprehensive review of in vitro studies versus inhalation or intratracheal instillation studies. The authors identified several concerns including the lack of standardization of in vitro test methods and the application of dose ranges exceeding realistic in vivo exposure scenarios. However, the authors concluded that if in vitro assays are conducted at realistic doses and reflect adverse outcomes under in vivo conditions then such assays may be incorporated into integrated test approaches of ENMs for regulatory purposes (Landsiedel et al., Citation2014). Indeed, the same authors could show that an in vitro alveolar macrophage assay reflected the short-term inhalation toxicity of ENMs (Wiemann et al., Citation2016). Cho et al. (Citation2013) also performed a comprehensive comparison of in vitro and in vivo tests using a panel of metal oxide ENMs. The authors used acute lung inflammation as an in vivo endpoint and found that most in vitro assays showed good predictivity for ENMs that acted via soluble ions, such as ZnO and CuO NMs, but were limited in detecting ENMs that exerted their toxicity via surface reactivity. The authors noted that differentiated THP.1 cells were considerably more sensitive to ENMs with respect to both cytotoxicity and cytokine expression, compared to undifferentiated THP.1 cells. In addition to the dose range, setting the relevant dose metric is also important when attempting in vitro to in vivo correlations and a universal dose metric is unlikely; instead, a thorough understanding of the mechanism of toxicity of the selected ENMs is required (Landsiedel et al., Citation2014). In the present study, a range of different ENMs (fibrous, spherical) were tested and concentrations ranging from 1 to 100 µg/mL were applied, according to the guidelines provided by the FP7-NANOREG project. If one recalculates the concentrations as the amount of NMs per surface area of the cell culture dish, this corresponds to 0.3–31 µg/cm2. For the studies focusing on the role of PPAR-γ, we applied a concentration of 10 µg/mL, corresponding to 3.1 µg/cm2. These doses are high when compared to in vivo studies or when considered in light of suggested occupational exposure limits (OEL) for ENMs (see Mihalache et al., Citation2017, for a recent overview). It is important to note that the main objective of the present study was to conduct hazard ranking and to explore grouping of ENMs based on inflammatory responses using the human macrophage-differentiated THP.1 cell line as a model. Further studies are required to determine whether hazard ranking and grouping based on in vitro cytokine profiling is borne out in vivo.
One significant finding of this study was that ENMs may elicit inflammatory responses in the absence of overt cytotoxicity, while, conversely, some ENMs were shown to trigger cytotoxicity without any production of pro-inflammatory mediators, such as TNF-α or IL-1β; in other words, cytotoxicity and immunotoxicity (production of inflammatory mediators) do not always go hand in hand. Previous ‘round robin’ studies using the THP.1 cell model arrived at a similar conclusion insofar as ZnO was found to be cytotoxic, but did not trigger production of IL-1β (Xia et al., Citation2013). The latter study also suggested that MWCNTs were non-cytotoxic, although IL-1β secretion by LPS-primed THP.1 cells was increased, albeit in a non-significant manner, due to variability between the participating laboratories. In fact, it is notable that MWCNTs (NM400, NM401) did not elicit any cytokine or chemokine responses in macrophage-like THP.1 cells in the present study, despite strong (dose-dependent) cytotoxicity. In recent experiments performed in the frame of the FP7-MARINA project we determined that these MWCNTs did not trigger TNF-α production in primary human monocyte-derived macrophages (unpublished observations). Thus, the absence of pro-inflammatory effects of these materials cannot be explained by the fact that we have employed a cell line. It is possible that the doses used for the cytokine profiling experiments (10 and 25 µg/mL) were too high (NM401 was cytotoxic at both doses, while NM400 was cytotoxic at the higher dose, but not at 10 µg/mL). On the other hand, the NCs had significant effects on cytokine secretion in THP.1 cells, without any cytotoxicity (at doses up to 100 µg/mL).
NCs have been reported to show low or no toxicity, in studies using NIH3T3 murine embryonic fibroblasts and in the HCT116 colon adenocarcinoma cell line (Hanif et al., Citation2014), and no cytotoxicity in a recent study using primary human monocyte-derived macrophages (Catalán et al., Citation2015). In the present study, all five NCs were found to be non-cytotoxic for THP.1 cells. However, four of the NCs were shown to elicit pro-inflammatory responses and our results suggested that this could potentially be explained by the deactivation of nuclear receptor pathways. We also noted that anti-inflammatory cytokines or soluble factors were upregulated, such as IL-1ra, which could indicate an attempt by the cells to counterbalance the pro-inflammatory effects elicited by deactivation of nuclear receptor pathways. Further research is needed to clarify the inflammatory response to these materials, which have received considerable attention lately for their potential use in various biomedical applications (Abitbol et al., Citation2016).
Our downstream analysis suggested the division of the panel of tested ENMs into two major groups, one promoting inflammation through inactivation of PPAR/LXR nuclear receptor pathway(s) and the other group displaying the opposite trend, i.e. activation of PPAR/LXR. PPAR and LXR belong to the nuclear receptor superfamily and are known to be activated by free fatty acids and cholesterol, respectively and to control the expression of a host of metabolism and inflammation related genes (Hong & Tontonoz, Citation2008). PPARs thus repress NF-κB, NFAT, STAT and AP-1 target genes in response to a variety of inflammatory stimuli, including cytokines and Toll-like receptor (TLR) ligands and LXR has also been also shown to blunt the induction of inflammatory genes (Glass & Saijo, Citation2010; Ogawa et al., Citation2005). Nuclear receptors play key roles in orchestrating the internalization and degradation of apoptotic cells and cell debris by macrophages (Röszer, Citation2017) and it is intriguing to speculate that macrophages could activate or deactivate similar pathways in response to certain ENMs. There is, however, scant information on the role of nuclear receptor pathways for immune responses to ENMs. Huizar et al. (Citation2013) found that PPAR-y is downregulated in the lungs of mice exposed to MWCNTs through pharyngeal instillation leading to deactivation of nuclear receptor pathways and promotion of inflammation and granuloma formation. Moreover, in mice deficient for PPAR-?, granuloma formation was more extensive at 60 days post-exposure than in wild-type mice. The authors concluded that PPAR-? functions as a negative regulator of granulomatous inflammation. In another study, PPAR-? deficient mice did not display increased inflammation in response to carbon black (Printex 90) nanoparticles delivered by intratracheal instillation (Götz et al., Citation2011). The results obtained in our in vitro study provided further evidence related to the involvement of PPAR-γ in modulating inflammatory responses of ENMs. Specifically, we have shown that the secretion of the pro-inflammatory cytokine, IL-1β by macrophage-differentiated cells exposed to TiO2 and SiO2 NMs was counteracted by the selective PPAR-γ agonist, rosiglitazone. TiO2 and SiO2 NMs were selected for these experiments because clustering and pathway analysis of the cytokine profiling results suggested that they segregated into the group of ENMs that deactivated NRPs. We found that deactivation of PPAR-γ in macrophage-differentiated cells exposed to TiO2 NMs was reversed by rosiglitazone. Rosiglitazone, a selective PPAR-γ agonist, has been shown to possess an anti-inflammatory effect (Jiang et al.,Citation1998; Ricote et al., Citation1998) and PPAR-γ activation was found to suppress LPS and IFN-γ mediated induction of several pro-inflammatory genes in mouse macrophages (Welch et al., Citation2003). The anti-inflammatory actions of PPAR-γ are exerted, in part, through trans-repression of other transcription factors, including NF-κB and AP-1 (Rigamonti et al., Citation2008). The present results thus corroborate our pathway analysis based predictions and show that ENM-induced inflammatory responses in macrophages may be subject to modulation by specific NRPs.
Conclusions
The present studies provided information on the bioactivity of a large panel of representative ENMs and suggest that ENMs can be grouped based on their inflammatory potential. These results are fully compatible with the recent FP7-NANOREG approach to risk assessment, in which immunotoxicity is cited as one of six elements or aspects that are likely to be influenced by ‘nanospecific properties’ of the materials (Dekkers et al., Citation2016). While the present results alone cannot be used for risk assessment of ENM effects, these studies nevertheless represent a first step towards grouping of ENMs on the basis of their inflammogenic potential. The study also provides a source of data for further modeling of ENM effects. Moreover, the current results suggest that specific nuclear receptors (e.g. PPAR-γ) are modulated by ENMs.
INAN_A_1363309_Supplementary_Information.zip
Download Zip (167.4 KB)Acknowledgements
We thank our colleagues in work package 2 (WP2) of FP7-NANOREG for providing the ENMs for the present study.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Abitbol T, Rivkin A, Cao Y, Nevo Y, Abraham E, Ben-Shalom T, et al. 2016. Nanocellulose, a tiny fiber with huge applications. Curr Opin Biotechnol 39:76–88.
- Alcorn JF, Crowe CR, Kolls JK. 2010. TH17 cells in asthma and COPD. Annu Rev Physiol 72:495–516.
- Antonelli A, Ferrari SM, Corrado A, Ferrannini E, Fallahi P. 2014. CXCR3, CXCL10 and type 1 diabetes. Cytokine Growth Factor Rev 25:57–65.
- Arend WP. 2002. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev 13:323–40.
- Arts JH, Hadi M, Irfan MA, Keene AM, Kreiling R, Lyon D, et al. 2015. A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping). Regul Toxicol Pharmacol 71:S1–27.
- Arts JH, Hadi M, Keene AM, Kreiling R, Lyon D, Maier M, et al. 2014. A critical appraisal of existing concepts for the grouping of nanomaterials. Regul Toxicol Pharmacol 70:492–506.
- Bhattacharya K, Andón FT, El-Sayed R, Fadeel B. 2013. Mechanisms of carbon nanotube-induced toxicity: focus on pulmonary inflammation. Adv Drug Deliv Rev 65:2087–97.
- Bhattacharya K, Mukherjee SP, Gallud A, Burkert SC, Bistarelli S, Bellucci S, et al. 2016. Biological interactions of carbon-based nanomaterials: from coronation to degradation. Nanomedicine 12:333–51.
- Braakhuis HM, Oomen AG, Cassee FR. 2016. Grouping nanomaterials to predict their potential to induce pulmonary inflammation. Toxicol Appl Pharmacol 299:3–7.
- Braakhuis HM, Park MV, Gosens I, De Jong WH, Cassee FR. 2014. Physicochemical characteristics of nanomaterials that affect pulmonary inflammation. Part Fibre Toxicol 11:18.
- Catalán J, Ilves M, Järventaus H, Hannukainen KS, Kontturi E, Vanhala E, et al. 2015. Genotoxic and immunotoxic effects of cellulose nanocrystals in vitro. Environ MolMutagen 56:171–82.
- Cho WS, Duffin R, Bradley M, Megson IL, MacNee W, Lee JK, et al. 2013. Predictive value of in vitro assays depends on the mechanism of toxicity of metal oxide nanoparticles. Part Fibre Toxicol 10:55.
- Cho WS, Duffin R, Poland CA, Howie SE, MacNee W, Bradley M, et al. 2010. Metal oxide nanoparticles induce unique inflammatory footprints in the lung: important implications for nanoparticle testing. Environ Health tPerspect 118:1699–706.
- Chow KT, Gale M. 2015. Snapshot: interferon signaling. Cell 163:1808–e1.
- Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. 1995. Identification of RANTES, MIP-1α and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811–15.
- Dekkers S, Oomen AG, Bleeker EA, Vandebriel RJ, Micheletti C, Cabellos J, et al. 2016. Towards a nanospecific approach for risk assessment. Regul Toxicol Pharmacol 80:46–59.
- Durán N, Durán M, de Jesus MB, Seabra AB, Fávaro WJ, Nakazato G. 2016. Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomedicine 12:789–99.
- Fadeel B, Fornara A, Toprak MS, Bhattacharya K. 2015. Keeping it real: the importance of material characterization in nanotoxicology. Biochem Biophys Res Commun 468:498–503.
- Farcal L, Andón FT, Di Cristo L, Rotoli BM, Bussolati O, Bergamaschi E, et al. 2015. Comprehensive in vitro toxicity testing of a panel of representative oxide nanomaterials: first steps towards an intelligent testing strategy. PLoS One 10:e0127174.
- Glass CK, Saijo K. 2010. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat Rev Immunol 10:365–76.
- Godwin H, Nameth C, Avery D, Bergeson LL, Bernard D, Beryt E, et al. 2015. Nanomaterial categorization for assessing risk potential to facilitate regulatory decision-making. ACS Nano 9:3409–17.
- Götz AA, Vidal-Puig A, Rödel HG, de Angelis MH, Stoeger T. 2011. Carbon-nanoparticle-triggered acute lung inflammation and its resolution are not altered in PPARγ-defective (P465L) mice. Part Fibre Toxicol 8:28.
- Hamilton JA. 2008. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol 8:533–44.
- Hanif Z, Ahmed FR, Shin SW, Kim YK, Um SH. 2014. Size- and dose-dependent toxicity of cellulose nanocrystals (CNC) on human fibroblasts and colon adenocarcinoma. Colloids Surf B Biointerfaces 119:162–5.
- Hong C, Tontonoz P. 2008. Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Curr Opin Genet Dev 18:461–7.
- Huizar I, Malur A, Patel J, McPeek M, Dobbs L, Wingard C, et al. 2013. The role of PPARγ in carbon nanotube-elicited granulomatous lung inflammation. RespirRes 14:7.
- Ihaka R, Gentleman RR. 1995. A language for data analysis and graphics. J. Comput. Graph Stat 5:299–314.
- Jiang C, Ting AT, Seed B. 1998. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature 391:82–6.
- Kang H, Mintri S, Menon AV, Lee HY, Choi HS, Kim J. 2015. Pharmacokinetics, pharmacodynamics and toxicology of theranostic nanoparticles. Nanoscale 7:18848–62.
- Krämer A, Green J, Pollard J, Tugendreich S. 2014. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30:523–30.
- Landsiedel R, Sauer UG, Ma-Hock L, Schnekenburger J, Wiemann M. 2014. Pulmonary toxicity of nanomaterials: a critical comparison of published in vitro assays and in vivo inhalation or instillation studies. Nanomedicine (Lond) 9:2557–85.
- Li Y, Boraschi D. 2016. Endotoxin contamination: a key element in the interpretation of nanosafety studies. Nanomedicine (Lond) 11:269–87.
- Lynch I, Weiss C, Valsami-Jones E. 2014. A strategy for grouping of nanomaterials based on key physico-chemical descriptors as a basis for safer-by-design NMs. Nano Today 9:266–70.
- Medzhitov R. 2008. Origin and physiological roles of inflammation. Nature 454:428–35.
- Mihalache R, Verbeek J, Graczyk H, Murashov V, van Broekhuizen P. 2017. Occupational exposure limits for manufactured nanomaterials, a systematic review. Nanotoxicology 11:7– 19.
- Mukherjee SP, Lozano N, Kucki M, Del Rio-Castillo AE, Newman L, Vázquez E, et al. 2016. Detection of endotoxin contamination of graphene based materials using the TNF-α expression test and guidelines for endotoxin-free graphene oxide production. PLoS One 11:e0166816.
- Naha PC, Bhattacharya K, Tenuta T, Dawson KA, Lynch I, Gracia A, et al. 2010. Intracellular localisation, geno- and cytotoxic response of polyN-isopropylacrylamide (PNIPAM) nanoparticles to human keratinocyte (HaCaT) and colon cells (SW 480). Toxicol Lett 198:134–43.
- Nel AE, Malloy TF. 2017. Policy reforms to update chemical safety testing. Science 355:1016–18.
- Nel AE, Nasser E, Godwin H, Avery D, Bahadori T, Bergeson L, et al. 2013. A multi-stakeholder perspective on the use of alternative test strategies for nanomaterial safety assessment. ACS Nano 7:6422–33.
- Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, et al. 2005. Molecular determinants of crosstalk between nuclear receptors and Toll-like receptors. Cell 122:707– 21.
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. 1998. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391:79– 82.
- Rigamonti E, Chinetti-Gbaguidi G, Staels B. 2008. Regulation of macrophage functions by PPAR-alpha, PPAR-gamma and LXRs in mice and men. Arterioscler Thromb Vasc Biol 28:1050–9.
- Roebben G, Rasmussen K, Kestens V, Linsinger TPJ, Rauscher H, Emons H, Stamm H. 2013. Reference materials and representative test materials: the nanotechnology case. J Nanopart Res 15:1455.
- Röszer T. 2017. Transcriptional control of apoptotic cell clearance by macrophage nuclear receptors. Apoptosis 22:284–94.
- Shaw SY, Westly EC, Pittet MJ, Subramanian A, Schreiber SL, Weissleder R. 2008. Perturbational profiling of nanomaterial biologic activity. Proc Natl Acad Sci USA 105:7387–92.
- Vallhov H, Qin J, Johansson SM, Ahlborg N, Muhammed MA, Scheynius A, Gabrielsson S. 2006. The importance of an endotoxin-free environment during the production of nanoparticles used in medical applications. Nano Lett 6:1682–6
- Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, Glass CK. 2003. PPAR-γ and PPAR-δ negatively regulate specific subsets of lipopolysaccharide and IFN-γ target genes in macrophages. Proc Natl Acad Sci USA 100:6712–17.
- Wiemann M, Vennemann A, Sauer UG, Wiench K, Ma-Hock L, Landsiedel R. 2016. An in vitro alveolar macrophage assay for predicting the short-term inhalation toxicity of nanomaterials. J Nanobiotechnol 14:16.
- Xia T, Hamilton RF, Bonner JC, Crandall ED, Elder A, Fazlollahi F, et al. 2013. Interlaboratory evaluation of in vitro cytotoxicity and inflammatory responses to engineered nanomaterials: the NIEHS Nano GO Consortium. Environ Health tPerspect 121:683–90.